Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (17): 2723-2730.doi: 10.12307/2024.484
Previous Articles Next Articles
Myocardial patch: cell sources, improvement strategies, and optimal production methods
Hu Wei1, Xing Jian2, Chen Guangxin2, Chen Zee3, 4, Zhao Yi5, Qiao Dan6, Ouyang Kunfu3, Huang Wenhua1, 7, 8, 9
- 1School of Basic Medical Sciences, Fujian Medical University, Fuzhou 350122, Fujian Province, China; 2School of Medical Imaging, Mudanjiang Medical University, Mudanjiang 157011, Heilongjiang Province, China; 3Department of Cardiac Surgery, Peking University Shenzhen Hospital, Shenzhen 518036, Guangdong Province, China; 4School of Chemical Biology and Biotechnology, Shenzhen Graduate School, Peking University, Shenzhen 518055, Guangdong Province, China; 5Department of Academic Affairs, Harbin Institute of Technology (Shenzhen), Shenzhen 518055, Guangdong Province, China; 6Department of Pathology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou 310016, Zhejiang Province, China; 7State Key Discipline of Human Anatomy, Key Laboratory of Medical Biomechanics of Guangdong Province, Guangdong Medical 3D Printing Application Transformation Engineering Technology Research Center, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, Guangdong Province, China; 8Shenzhen Pingshan People’s Hospital (Pingshan General Hospital of Southern Medical University), Shenzhen 518118, Guangdong Province, China; 9Third Affiliated Hospital of Southern Medical University, Guangdong Medical 3D Printing Application Transformation and Innovation Platform, Guangzhou 510000, Guangdong Province, China
-
Received:2023-08-17Accepted:2023-09-09Online:2024-06-18Published:2023-12-15 -
Contact:Qiao Dan, MD, Department of Pathology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou 310016, Zhejiang Province, China Ouyang Kunfu, MD, Associate professor, Doctoral supervisor, Department of Cardiac Surgery, Peking University Shenzhen Hospital, Shenzhen 518036, Guangdong Province, China Huang Wenhua, MD, Professor, Doctoral supervisor, School of Basic Medical Sciences, Fujian Medical University, Fuzhou 350122, Fujian Province, China; State Key Discipline of Human Anatomy, Key Laboratory of Medical Biomechanics of Guangdong Province, Guangdong Medical 3D Printing Application Transformation Engineering Technology Research Center, School of Basic Medical Sciences, Southern Medical University, Guangzhou 510515, Guangdong Province, China; Shenzhen Pingshan People’s Hospital (Pingshan General Hospital of Southern Medical University), Shenzhen 518118, Guangdong Province, China; Third Affiliated Hospital of Southern Medical University, Guangdong Medical 3D Printing Application Transformation and Innovation Platform, Guangzhou 510000, Guangdong Province, China -
About author:Hu Wei, Master candidate, School of Basic Medical Sciences, Fujian Medical University, Fuzhou 350122, Fujian Province, China -
Supported by:National Key Research & Development Program, No. 2022YFB4600600 (to HWH); National Natural Science Foundation of China, No. 31972915, 32271181 (to HWH); General Project of National Natural Science Foundation of China, No. 81970421, 82170235 (to OYKF); China Postdoctoral Science Foundation, No. 2021M702224, 2022T150430 (to CZE); Guangdong Basic and Applied Basic Research Fund, No. 2020B1515120001 (to HWH); General Project of Guangdong Natural Science Foundation, No. 2023A1515011842 (to OYKF); Guangdong Basic and Applied Basic Research Fund Project, No. 2019A1515111178 (to ZY); Science Foundation Torch Program of Mudanjiang Medical University, No. 2022-MYHJ-004 (to XJ); Shenzhen Natural Science Foundation, No. JCYJ20190808174001746, JCYJ20210324105407019 (to OYKF); Shenzhen-Hong Kong Brain Science Innovation Institute Project, No. 2019SHIBS0004, 2023SHIBS0004 (to OYKF)
CLC Number:
Cite this article
Hu Wei, Xing Jian, Chen Guangxin, Chen Zee, Zhao Yi, Qiao Dan, Ouyang Kunfu, Huang Wenhua. Myocardial patch: cell sources, improvement strategies, and optimal production methods[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(17): 2723-2730.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

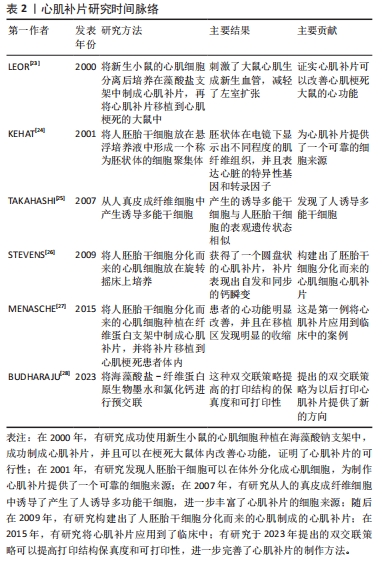
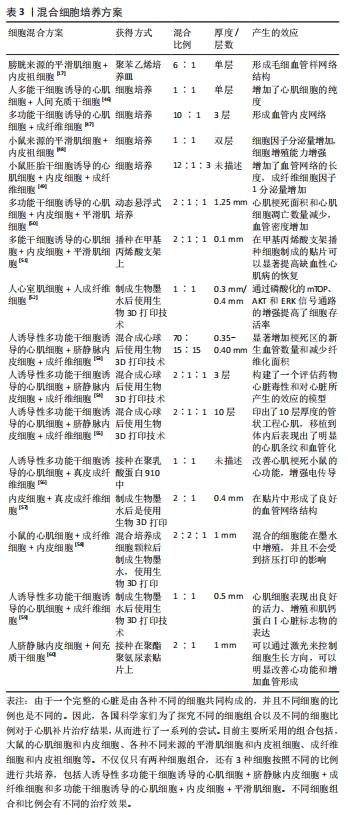
2.1.1 体细胞 心肌细胞:细胞片技术所保留的细胞外基质,可以使细胞最大程度地保留在体内发挥治疗效果。有研究使用新生大鼠的心肌细胞,对比了细胞注射法和细胞片移植两种技术的治疗效果[10],发现和注射法相比,移植心肌细胞片的大鼠体内移植细胞的存活数量明显更多,成熟的毛细血管数量也显著增加,并且显著改善梗死大鼠的心功能。细胞片不仅提高了移植细胞的存活率,还降低了受体心肌细胞的凋亡。 当细胞片移植到体内后,移植细胞存活的数量会影响其治疗效果外,与受损心肌的贴合程度也是能否发挥治疗效果的重要因素。新生大鼠心肌培养而成的单层心肌细胞片可以在体外发生自发跳动,保留了缝隙连接蛋白(connexin 43),实现了细胞片内的同步收缩,并且可以与受损心肌实现组织学和电生理学上的整合,改善心肌功能。由于保留了大鼠心肌的缝隙连接蛋白,为制作具有同步收缩活动的三维心脏组织提供了基础。 大鼠心肌细胞制成的细胞片不仅可以作为心肌细胞的移植载体,在制造功能性心管(functional cardiac tubes)方面也取得了不错的进展。将3层大鼠心肌细胞培养而成的细胞片缠绕在气管内管上,使用肠系膜为血管床来产生功能性的心管。将功能性心管移植到体内后,在移植4周后发现移植的心管产生了电刺激收缩,并且经过组织学和免疫组化后证明有心肌细胞的植入并且心管有毛细血管形成[11]。 目前,人们利用小鸡的心肌细胞和新生小鼠的心肌细胞分别构建了细胞片,并且进行了一系列的体内与体外实验。但是使用新生小鼠的心肌细胞制作细胞片最为普遍,新生小鼠的心肌细胞和人的心肌细胞在膜的形态、G蛋白的表达和电生理特性方面有相似之处。但是,单纯使用新生小鼠的心肌细胞无法获得一个完整的心肌细胞片,还需要一些非心肌物质。无论移植何种动物来源的心肌细胞片,最主要就是要克服关于异种间的免疫排斥问题。这在接下来的内容中将会谈论到。 平滑肌细胞:该细胞可以分泌多种血管因子,如血管生成因子、血管内皮细胞生长因子和肝细胞生长因子,因此可以有预期的旁分泌血管生成作用,且易于培养和维持。使用人源性的内皮祖细胞和平滑肌细胞制作的无支架的双层细胞片可以在无胸腺的小鼠中展现出了改善心室重塑的功能,并且在小鼠的心肌缺血边界区观察到了生物黏附系统和脉管发育系统基因的上调[29]。而且将白细胞介素10转导至平滑肌细胞中、增加胰岛素样生长因子在平滑肌细胞中的表达和将抗凋亡基因Bcl-2基因转染至细胞中都可以提高平滑肌细胞在体内的移植效果。 随着细胞片技术的慢慢成熟,科学家把从大鼠主动脉中分离出来的平滑肌细胞制成细胞片,移植到小鼠体内后与人真皮成纤维细胞片对比治疗效果,发现平滑肌细胞片可以更好地抑制心肌重塑,减少心肌纤维化,促进瘢痕区血管形成,并且在体外细胞片可以分泌碱性成纤维细胞生长因子[13]。并且在此之前,就已经有人使用人动脉平滑肌细胞培养成细胞片移植到老鼠体内,在缺血的老鼠体内重建了血运组织。 成纤维细胞:该细胞是非心肌细胞的主要类型。在心肌损伤后,坏死的心肌细胞释放出某些信号,促使心肌成纤维细胞的表型发生转化,使得成纤维细胞可以分泌细胞外基质成分和纤维连接蛋白,刺激细胞因子和生长因子的释放,促进损伤部位的修复[15-16]。除此之外,人成纤维细胞可以产生诱导性多能干细胞,继而衍生出心肌细胞,由衍生出的心肌细胞制成细胞片,在体内也能发挥出治疗效果[30]。血管细胞黏附分子1可以介导AKt、ERK和p38MAPK信号通路的激活,从而促进血管内皮的形成,具有明显的血管生成活性,防止细胞氧化应激死亡,对心脏有明显的保护作用。使用成纤维细胞和心肌细胞混合培养制成细胞片,发现成纤维细胞不仅可以分泌血管细胞黏附分子1,而且促进了心肌细胞的增殖[17]。但是成纤维细胞可以通过旁分泌作用和与心肌细胞之间形成电偶联,所以可能会影响心脏的电生理功能。因此成纤维细胞片植入到体内后可能会增加发生心律失常的风险[31]。 软骨细胞:该细胞可以显著刺激细胞外基质的修饰,诱导上调了弹性蛋白胶原Ⅱ,下调了基质金属蛋白酶1,基质金属蛋白酶2和内源性蛋白酶抑制剂1的表达[14]。因此,软骨细胞片可通过恢复心脏的舒张功能、刺激细胞外基质的重塑和促进血管生成来改善心功能和减弱心肌重塑。把成软骨细胞培养成的细胞片放置在梗死区,细胞片可以刺激梗死区细胞外基质的分泌、增加细胞外基质的弹性,从而减轻左室重构并增强心功能。 2.1.2 单能干细胞 心肌祖细胞:在成人心脏发生损伤后,有约15%的心肌细胞可以发生再生[32]。心肌祖细胞便是这些再生心肌细胞的细胞来源。但是,由于成人体内心肌祖细胞的数量太少,导致心肌在发生致命性的心肌梗死后无法发挥修复作用。DERGILEV等[33]使用心肌祖细胞得到了结构完整的细胞片,细胞片不仅增加了移植到体内的细胞数量,还刺激了受体心肌细胞的增殖和血管生成,极大改善了心肌重塑。移植到体内的祖细胞在梗死区释放基质衍生因子,并且强表达内源性蛋白酶抑制剂2,这些都提示SDF-1/CXCR4信号通路在细胞迁移中起到作用,使得心肌可以保留收缩功能[18]。有报道把心肌祖细胞加入到透明质酸明胶中制备成生物墨水,打印出了400 μm厚的心肌补片,移植到小鼠体内后,也可以显著减少心肌重塑,并且存活了较长时间[34]。心肌祖细胞作为心肌细胞片的可靠细胞来源之一,一些科学家也探讨了如何提高祖细胞片在体内的治疗效果。内皮祖细胞可以释放一些含有特异性microRNA的外泌体,可以促进心肌祖细胞的迁移。将心脏干细胞片与注射内皮祖细胞联合治疗可以比单独的细胞片更好地诱导瘢痕组织修复[35]。 骨骼肌母细胞:该细胞具有自体移植、抗缺血、非肌细胞谱系分化和高增殖潜力等优点,所制成的细胞片能在形态学、组织学和电生理学上与宿主的心肌整合。骨骼肌母细胞片可以修复受损的心肌,减少纤维化,并通过释放基质细胞衍生因子1和其他生长因子来阻止与造血干细胞募集相关的重塑。并且骨骼肌母细胞片在犬心力衰竭模型中也表现出良好的治疗效果[36]。 2.1.3 多能干细胞 间充质干细胞:该细胞有多种来源,包括骨髓源性、脂肪源性、脐带源性和血源性等[37],因具有低免疫原性、低心律失常风险、临床安全经验丰富、有较强的旁分泌能力和分化能力等优点,因此也被看作是最适合临床治疗的细胞来源。间充质干细胞的来源很多,不同来源的干细胞在制成细胞片移植到体内后分泌的细胞因子是不同的,因此为了达到不同的治疗目的,选择合适细胞来源的干细胞至关重要[38]。 骨髓源性的间充质干细胞片具有强大的旁分泌作用,可以通过释放细胞因子来促进血管生成,并且在心外膜存在的情况下,也能与心肌整合。但是在体内的存在时间较短,只在早期发挥旁分泌作用。脂肪源性的间充质干细胞不仅可以在体内分泌大量的细胞因子,并且相对于骨髓源性的间充质干细胞,脂肪源性间充质干细胞还可以在体内分化为心肌细胞。科学家从小鼠的腹股沟脂肪中提取间充质干细胞,并制成细胞片,移植到小鼠体内后,梗死区心肌室壁的厚度增加,生长因子和细胞因子的表达量升高,并且还发现了梗死区有血管生成,这些现象都可以表明脂肪源性的间充质干细胞改善了梗死心肌的重塑[39]。把脂肪源性间充质干细胞放在碳酸脂板中和纤维蛋白凝胶培养,制作成一个3D心肌补片,可以获得一个具有良好导电性的心肌补片[40]。 骨髓是骨髓源性间充质干细胞的主要来源,随着供者年龄的增长,骨髓的可用性也会下降,其次骨髓的获取是一种侵入性方案,而且骨髓源性间充质细胞在体外扩增时其增殖能力和分化潜力会下降。除此之外,使用骨髓间充质细胞制成的细胞片对梗死心肌的治疗作用只在早期有效,无法在体内起到长久的治疗效果。脂肪源性干细胞具有更高的分化潜力、增殖能力和各种生长因子、细胞因子和趋化因子的表达,因此可以成为一种几乎无限的成体干细胞来源,并且不会产生伦理方面的问题,但是对心肌收缩能力并没有良好的改善效果。还有一种脐带源性的间充质干细胞,此细胞作为一种新兴的细胞来源,相比于其他几种间充质干细胞,具有更高的增殖率,并且获得程序简单,伦理问题较少。 胚胎干细胞和诱导性多能干细胞:多能干细胞还包括两种干细胞,分别是胚胎干细胞和诱导性多功能干细胞。多能干细胞具有无限增殖和分化成各种细胞群的能力,移植到损伤区域的多能干细胞来源的心肌细胞可以补偿部分损伤的心肌细胞,因此,被看作是制作细胞片的细胞来源之一[22]。多功能干细胞分化而来的心肌细胞和内皮细胞混合培养以后可以制成细胞片,干细胞自身也可以制成干细胞片并且细胞本身具有向心肌分化的能力[41]。胚胎干细胞和诱导性多功能干细胞可分化为新的心肌细胞、平滑肌细胞和内皮细胞。将诱导性多功能干细胞分化而来的3种细胞共同培养所制成的细胞片比单纯一种细胞所制成的细胞片更能改善心脏的功能,并且没有增加心律失常的风险。心肌细胞、内皮细胞和平滑肌细胞之间的相互作用有效地增强了细胞片的结构整合和功能,但是可能由于缺血而导致丢失心肌细胞,因此还需要额外的策略来增加血管生成。 在2012年,MIKI等[42]使用小鼠来源的诱导性多功能干细胞制成了高心肌细胞纯度的心肌细胞片,将心肌细胞片移植到体内后,可以在移植部位的心外膜存活并且改善了心功能和心肌重塑。同年,就有科学家开发了一种多功能干细胞分化培养系统,使用此系统可以诱导纯化心肌细胞、内皮细胞和壁细胞。并且在此基础上使用细胞片技术生成了心脏贴片,得到了细胞片改善心肌的作用主要是由心肌细胞产生的结论。2年后, MASUMOTO等[43]又研发出了人诱导性多功能干细胞的分化培养方案,他们通过补充血管内皮生长因子分化诱导血管细胞,在此技术上得到的混合细胞片也明显改善了梗死心肌的功能。随着科学技术的不断发展,有研究团队用人诱导性多功能干细胞开发了一种直径约为3.5 cm的可以自发搏动的临床级心肌补片[44],移植到猪体内后,梗死区心肌的纤维化面积明显减少和血管密度显著增加。 随着分化培养方案的不断发展完善,这为以后得到大规模多能干细胞-心肌细胞成为可能。但是,多能干细胞自身的致瘤性和由于心肌细胞成熟程度不一样导致的心律失常等因素,一直干扰着多功能干细胞的应用[45]。后来人们发现可以使用间充质干细胞来促进多能干细胞-心肌细胞成熟。因为间充质干细胞可以分泌几种外泌体,而外泌体中的microRNA和蛋白质可以促进多能干细胞-心肌细胞成熟[46]。 2.2 完善心肌补片的策略 2.2.1 不同种类的细胞共同培养 一个完整的心脏不仅仅只有心肌细胞,还包括一些非心肌细胞,例如内皮细胞和平滑肌细胞,它们发挥着各自的生物学功能。因此,想要改善坏死心肌功能甚至代替坏死的心肌,心肌补片只用一种细胞往往是不够的,多细胞共同培养制成的心肌补片开始引起世界各国科学家的兴趣。文章总结了混合细胞的培养方案[17,46-60],见表3。"

有研究将心肌细胞和内皮细胞共培养后制成细胞片,发现与内皮细胞共培养的细胞片中形成了大量的血管内皮网络,可以极大地促进新生血管形成和改善心功能[47,61-62]。由于成纤维细胞高表达血管细胞黏附分子1,所以心肌细胞和成纤维细胞混合培养后,混合细胞片可以显著增加功能心肌的数量,心肌细胞可以具有更强的增殖能力。还有科学家使用心肌细胞片和成纤维细胞片一起放在一个八角形的柱状体周围,从而成功制作出功能性的管状心脏组织[63]。而内皮祖细胞和成纤维细胞共同培养或者内皮祖细胞和平滑肌细胞共同培养后[48,64-66],混合的细胞片都能表现出更强的细胞因子或血管生成因子分泌能力、新生血管数量增加和更少的纤维化程度,显著减少心脏重塑。 获得混合细胞心肌补片的方法有2种:一种是前面描述的,将两种或多种细胞混合培养后,把混合的细胞培养成心肌补片;另一种是把两种细胞分别制成细胞片,然后把两种细胞片重贴在一起,也可以获得含有多种细胞的心肌补片。把人诱导性多功能干细胞分化而来的心肌细胞和内皮细胞分别与脱细胞水凝胶混合制成生物墨水,再使用3D打印技术,打印出了约为2 mm厚的心脏贴片[67]。还有报道将血源性内皮细胞和周细胞混合到纤维蛋白凝胶中制作成心肌补片,再将人诱导性多功能干细胞分化而来的心肌细胞混合到纤维蛋白凝胶中制成心肌补片,将两种心肌补片贴合到一起,制成两层结构的心肌补片[68]。 而且,不仅仅局限于两种细胞类型细胞片,大量的科学家正在尝试进行制作具有3种细胞类型的细胞片。有科学家使用小鼠胚胎干细胞分化而来的心肌细胞、内皮细胞和成纤维细胞混合培养制成细胞片,产生了具有血管微结构的细胞片[49]。将人诱导性多功能干细胞分化而来的心肌细胞、内皮细胞和平滑肌细胞以动态悬浮式混合培养的方式制成多层心肌补片,所形成的心肌补片移植到体内后显著改善了左室壁应激、梗死面积、血管密度和细胞凋亡,并且未增加心律失常的风险。 2.2.2 生物3D打印 生物3D打印把传统打印技术中所使用的油墨或者树脂替换成了细胞或生物材料与细胞混合制成的生物墨水。使用生物3D打印技术打印心脏贴片主要有两种方法:一种是将细胞种植在可降解的三维支架上;另一种是将细胞或类器官与生物材料混合制成生物墨水,打印出无支架的三维结构。因此现在许多科学家将目光放在如何使用3D生物打印技术构建一个三维心脏结构。 天然的细胞外基质是一种器官特异性材料,它们可以提供天然理化性质,因此器官来源的脱细胞外基质具有最佳的仿生性能[69]。将人心球源性基质细胞(cardiosphere-derived stromal cells)混合至纤维蛋白凝胶中,然后在37 ℃的条件下培育固化,可以形成大约1 mm厚的心肌补片;并且与仿生微血管结合,构建出了一种血管化的心肌补片[70]。细胞外基质不仅可以作为心肌补片的原材料,还可以作为承载细胞的介质。将心肌祖细胞添加到甲基丙烯酸凝胶和脱细胞外基质的混合液中,制成生物墨水,打印出了厚度为0.6 μm的贴片[71]。除了脱细胞外基质,还有许多其他生物活性材料可以作为承载细胞的介质,制成生物墨水。将新生大鼠的心室肌细胞与海藻酸盐-纤维蛋白原生物墨水组合,再与氯化钙进行预交联,经研究发现心室肌细胞在此墨水中有更强的细胞活力和增殖能力,并且表达肌动蛋白和连接蛋白[28]。在糠酰明胶中加入透明质酸作为生物油墨,与小鼠的心肌细胞混合后成功打印出双层结构[72]。 除了混合墨水,对于承载细胞的支架也做了许多的改良。使用丝素蛋白和聚吡啶组成一种具有导电性的支架,将心肌细胞接种到支架上后,通过电刺激可促进心肌细胞的成熟,使心肌补片具有良好的收缩性和导电性。移植到体内后,可改善梗死心肌的重构和射血功能,并且能有效降低发生心律失常的次数[73]。在聚乙二醇水凝胶上打印导电碳化钛(Ti3C2Tx)MXene形成一个复合物支架,在此支架上接种心肌细胞可以形成一个改善心肌同步跳动和传导速度的心肌补片[74]。还有研究人员3D打印出了一种明胶水凝胶微通道,天然的心肌细胞可在此通道内生长分化,并出现同步跳动;通过此水凝胶通道,还可控制心肌细胞的生长方向[75]。 2.2.3 提高治疗效果 细胞片的治疗作用不仅是细胞片提高了移植细胞的数量,还有另一个重要的原因是细胞片保留了细胞外基质,而细胞外基质中含有多种细胞因子。将肝细胞生长因子通过基因工程修饰到骨髓源性的间充质细胞内,再将工程化后的细胞与正常的间充质干细胞一起装载到心肌补片中。基因工程化以后的细胞可以持续分泌干细胞生长因子激活间充质干细胞的心肌修复作用,并且未发现致癌作用[76]。 心肌的组织对氧气的需求量很高,因此心肌补片能否形成充足的血管网络来运输氧气和代谢废物对心肌补片在体内的存活非常重要[77]。由于坏死的心肌本身血流量就少,不利于心肌补片在体内的存活,因此寻找一个可以在心肌补片内提前构建血管网络的方法非常重要。将内皮细胞和成纤维细胞共同培养或者将单培养的细胞片按照不同的排列顺序制成多层细胞片,发现2种方法均可在细胞片内提前形成血管网络[78]。对于复杂的三维心脏组织,也开发了几种生物反应器在体外构建血管:一种是以股肌为基础的血管床;还有一种是以胶原凝胶为基础的血管床。在大鼠的股肌上放置与内皮细胞共培养的心肌细胞片,然后与股肌上的动静脉形成动静脉环,再经过3-5 d的体外循环灌注培养后,细胞片内形成了血管网络并且与体外血管床相连[61]。后来有人使用猪小肠组织的血管组织代替小鼠股肌的血管组织,也取得了不错的效果[47]。 除此之外,对细胞片进行缺氧处理、加入抗坏血酸、单向拉伸细胞片或者改变细胞片的培养环境或培养基内容物,都是提高细胞片治疗效果的潜在研究方向。 2.2.4 抑制免疫反应 无论是哪一种心肌补片,移植后的免疫排斥反应一直是干扰心肌补片广泛应用的限制条件之一。通过基因工程技术将诱导免疫应答的基因敲除,是一种抑制免疫反应的可行方案。组织相容性抗原的缺乏使得异种间的移植成为可能,在缺乏组织相容性抗原的情况下,人的脂肪源性间充质干细胞已经在小鼠、大鼠、兔、斑马鱼等不同动物中进行了实验,并且无不良事件发生[79]。 间充质干细胞可以在不应用免疫抑制治疗的情况下,移植到同种异体中,因此间充质干细胞被认为是抑制排斥反应的有效细胞[80]。骨髓来源的间充质干细胞可以改变免疫系统中的免疫细胞数量和功能从而诱导免疫耐受作用,把具有免疫耐受的间充质细胞应用到心肌补片的移植过程中,可以减少受体的免疫排斥反应。由多能干细胞诱导的心肌细胞和同种的间充质干细胞一起移植到体内,共同移植后,间充质干细胞增加了移植部位的调节性T细胞群的数量并且降低了CD8/CD4比值、th1阳性细胞在CD4阳性细胞中的比例以及几种炎症相关细胞因子的分泌,从而诱导免疫耐受[81],并且有可能是通过前列腺素E2来诱导的。因为增加前列腺素E2以后,梗死区的移植细胞存活数量增多,并且心功能得到改善[82]。脐带基质间充质干细胞分化而来的内皮细胞不仅缺乏组织相容性抗原,还一定程度上抑制了免疫细胞的活化,因而具有免疫特权[83]。并且脐带基质间充质干细胞已经开始应用于临床[84]。也有报道称,子宫来源的间充质干细胞也具有免疫特权,注射到心肌梗死小鼠中,可以促进梗死区的血管再生,并且没有观察到明显的免疫排斥反应[85]。 2.2.5 纯化多能干细胞 多能干细胞作为当今制作人工心肌补片最有潜力的细胞来源,如何诱导足够数量的心肌细胞和如何解决干细胞的致瘤问题是阻碍多功能干细胞取得临床应用的主要原因[86]。 传统的2D培养系统由于空间有限,无法大规模诱导多功能干细胞。好在3D悬浮培养系统已经开始用于干细胞的大规模诱导[87]。3D悬浮培养诱导出来的血管内皮细胞也与心肌细胞和成纤维细胞共同培养成功制成心肌补片,并且在培养系统中加入细胞因子可以进一步扩增细胞数量。常规的培养基体系中由于乳酸堆积,导致pH值改变,从而致使细胞扩增和分化不足。由此提出了一种连续灌注系统,此系统可以维持乳酸和pH值的浓度。 任何未分化的多功能干细胞自身都具有致瘤性。因此,分化后的心肌细胞纯化方案在发展干细胞来源的心肌补片中至关重要。可以使用Wnt信号分子诱导人多能干细胞分化为心肌细胞[88],虽然在体内没有发生畸胎瘤,但是也没有100%分化为心肌细胞。TRPV-1是非选择性阳离子通道(non-selective cation channel)中的一个亚型,可以在≥42 ℃或者在化学刺激下被激活,TRPV-1的诱导激活可以介导细胞死亡或者抑制肿瘤发生。在42 ℃的条件下培养多能干细胞48 h后,多能干细胞几乎全部消失,但是由多能干细胞诱导而来的心肌细胞和成纤维细胞却继续存活,不受影响。因此可以利用诱导性多能干细胞及其衍生的心肌细胞对TRPV-1的耐受性差异用来消除生物工程细胞片组织中剩余的诱导性多能干细胞[89]。之后使用无蛋氨酸培养基在42 ℃的情况下培养2 d后,消除了大部分的诱导性多能干细胞,并且没有影响心肌细胞和成纤维细胞的存活率,也没有发现有畸胎瘤产生[90]。此外,他们还发现,细胞周期蛋白依赖性激酶抑制剂(Dinaciclib)能有效抑制抗凋亡蛋白MCL-1,也能在不影响心脏组织活力的情况下选择性诱导人诱导性多能干细胞死亡[91]。 当干细胞分化完成之后,鉴定是否成功分化为心肌细胞或心肌细胞的纯度是多少非常重要,这直接影响了心肌补片在体内的安全性和治疗效果,因为未分化的多能干细胞在体内具有致瘤性。但是对于纯化后心肌细胞的鉴定并没有一套完整的鉴定方案,目前常用的方案是使用荧光定量PCR和免疫荧光两种检测方式对分化后对心肌细胞标志物和干细胞标志物进行检测,验证分化后心肌细胞的纯度[92]。"

| [1] TSAO CW, ADAY AW, ALMARZOOQ ZI, et al. Heart disease and stroke statistics-2023 update: a report from the american heart association. Circulation. 2023;147(8):e93-e621. [2] KITSUKA T, SHIRAKI A, OYAMA J, et al. A novel soluble epoxide hydrolase vaccine protects murine cardiac muscle against myocardial infarction. Sci Rep. 2022;12(1):6923. [3] YU H, LU K, ZHU J, et al. Stem cell therapy for ischemic heart diseases. Br Med Bull. 2017; 121(1):135-154. [4] KITSUKA T, TAKAHASHI F, REINHARDT J, et al. Advances in cardiac tissue engineering. Bioengineering. 2022;9(11):696. [5] 王颖薇,秦子夕,武征.心肌补片的制备与结构功能特点[J].中国组织工程研究,2015, 19(38):6211-6216. [6] THUMMARATI P, LAIWATTANAPAISAL W, NITTA R, et al. Recent advances in cell sheet engineering: from fabrication to clinical translation. Bioengineering (Basel). 2023;10(2):211. [7] 周术奎,张楷乐,王营,等.细胞膜片技术在组织工程中的应用与研究进展[J].中国组织工程研究,2016,20(11):1630-1636. [8] WANG Z, WANG L, LI T, et al. 3D bioprinting in cardiac tissue engineering. Theranostics. 2021;11(16):7948-7969. [9] WEI Q, ZHOU J, AN Y, et al. Modification, 3D printing process and application of sodium alginate based hydrogels in soft tissue engineering: a review. Int J Biol Macromol. 2023; 232:123450. [10] SEKINE H, SHIMIZU T, DOBASHI I, et al. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Engineering Part A. 2011;17(23-24):2973-2980. [11] MASUDA N, SEKINE H, NIINAMI H, et al. Engineering of functional cardiac tubes by stepwise transplantation of cardiac cell sheets onto intestinal mesentery. Heart Vessels. 2020;35(6):859-867. [12] KAYNAK BAYRAK G, GÜMÜŞDERELIOĞLU M. Construction of cardiomyoblast sheets for cardiac tissue repair: comparison of three different approaches. Cytotechnology. 2019; 71(4):819-833. [13] QI YX, HAN Y, JIANG ZL. Mechanobiology and vascular remodeling: from membrane to nucleus. Adv Exp Med Biol. 2018;1097:69-82. [14] SHUDO Y, COHEN JE, MACARTHUR JW, et al. A tissue-engineered chondrocyte cell sheet induces extracellular matrix modification to enhance ventricular biomechanics and attenuate myocardial stiffness in ischemic cardiomyopathy. Tissue Engineering Part A. 2015;21(19-20):2515-2525. [15] VENUGOPAL H, HANNA A, HUMERES C, et al. Properties and functions of fibroblasts and myofibroblasts in myocardial infarction. Cells. 2022;11(9):1386. [16] CHAUDHURI R, RAMACHANDRAN M, MOHARIL P, et al. Biomaterials and cells for cardiac tissue engineering: current choices. Mater Sci Eng C. 2017;79:950-957. [17] IWAMIYA T, MATSUURA K, MASUDA S, et al. Cardiac fibroblast-derived VCAM-1 enhances cardiomyocyte proliferation for fabrication of bioengineered cardiac tissue. Regen Ther. 2016;4:92-102. [18] ZAKHAROVA L, MASTROENI D, MUTLU N, et al. Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function. Cardiovasc Res. 2010;87(1):40-49. [19] THUMMARATI P, KINO-OKA M. Effect of co-culturing fibroblasts in human skeletal muscle cell sheet on angiogenic cytokine balance and angiogenesis. Front Bioeng Biotechnol. 2020,8:578140. [20] GUO R, MORIMATSU M, FENG T, et al. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res Ther. 2020;11(1):19. [21] OLIVA J, FLORENTINO A, BARDAG-GORCE F, et al. Engineering, differentiation and harvesting of human adipose-derived stem cell multilayer cell sheets. Regen Med. 2019; 14(3):151-163. [22] XIAO Y, CHEN Y, SHAO C, et al. Strategies to improve the therapeutic effect of pluripotent stem cell-derived cardiomyocytes on myocardial infarction. Front Bioeng Biotechnol. 2022;10:973496. [23] LEOR J, ABOULAFIA-ETZION S, DAR A, et al. Bioengineered cardiac grafts: a new approach to repair the infarcted myocardium? Circulation. 2000;102(19 Suppl 3):I56-I61. [24] KEHAT I, KENYAGIN-KARSENTI D, SNIR M, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108(3):407-414. [25] TAKAHASHI K, TANABE K, OHNUKI M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861-872. [26] STEVENS KR, PABON L, MUSKHELI V, et al. Scaffold-free human cardiac tissue patch created from embryonic stem cells. Tissue Eng Part A. 2009;15(6):1211-1222. [27] MENASCHÉ P, VANNEAUX V, HAGÈGE A, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report: Figure 1. Eur Heart J. 2015;36(30):2011-2017. [28] BUDHARAJU H, SUNDARAMURTHI D, SETHURAMAN S. Efficient dual crosslinking of protein–in–polysaccharide bioink for biofabrication of cardiac tissue constructs. Biomat Adv. 2023;152:213486. [29] SHIN HS, THAKORE A, TADA Y, et al. Angiogenic stem cell delivery platform to augment post-infarction neovasculature and reverse ventricular remodeling. Sci Rep. 2022;12(1): 17605. [30] ZHANG L, GUO J, ZHANG P, et al. Derivation and high engraftment of patient-specific cardiomyocyte sheet using induced pluripotent stem cells generated from adult cardiac fibroblast. Circ Heart Fail. 2015;8(1):156-166. [31] TRIESCHMANN J, BETTIN D, HAUSTEIN M, et al. The interaction between adult cardiac fibroblasts and embryonic stem cell-derived cardiomyocytes leads to proarrhythmic changes inin vitro cocultures. Stem Cells Int. 2016;2016:1-12. [32] MEHANNA RA, ESSAWY MM, BARKAT MA, et al. Cardiac stem cells: current knowledge and future prospects. World J Stem Cells. 2022;14(1):1-40. [33] DERGILEV K, TSOKOLAEVA Z, MAKAREVICH P, et al. C-kit cardiac progenitor cell based cell sheet improves vascularization and attenuates cardiac remodeling following myocardial infarction in rats. BioMed Res Int. 2018;2018:1-13. [34] GAETANI R, FEYEN DAM, VERHAGE V, et al. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. 2015;61:339-348. [35] KAMATA S, MIYAGAWA S, FUKUSHIMA S, et al. Improvement of cardiac stem cell sheet therapy for chronic ischemic injury by adding endothelial progenitor cell transplantation: analysis of layer-specific regional cardiac function. Cell Transplantation. 2014,23(10): 1305-1319. [36] HATA H, MATSUMIYA G, MIYAGAWA S, et al. Grafted skeletal myoblast sheets attenuate myocardial remodeling in pacing-induced canine heart failure model. J Thorac Cardiovasc Surg. 2006;132(4):918-924. [37] CHANG D, FAN T, GAO S, et al. Application of mesenchymal stem cell sheet to treatment of ischemic heart disease. Stem Cell Res Ther. 2021;12(1):384. [38] NAKAO M, INANAGA D, NAGASE K, et al. Characteristic differences of cell sheets composed of mesenchymal stem cells with different tissue origins. Regen Ther. 2019;11:34-40. [39] KIM J, JOO HJ, KIM M, et al. Transplantation of adipose-derived stem cell sheet attenuates adverse cardiac remodeling in acute myocardial infarction. Tissue Engineering Part A. 2017;23(1-2):1-11. [40] ABBASGHOLIZADEH R, ISLAS JF, NAVRAN S, et al. A highly conductive 3d cardiac patch fabricated using cardiac myocytes reprogrammed from human adipogenic mesenchymal stem cells. Cardiovasc Eng Technol. 2020;11(2):205-218. [41] HIGUCHI A, HIRAD AH, KUMAR SS, et al. Thermoresponsive surfaces designed for the proliferation and differentiation of human pluripotent stem cells. Acta Biomaterialia. 2020;116:162-173. [42] MIKI K, UENAKA H, SAITO A, et al. Bioengineered myocardium derived from induced pluripotent stem cells improves cardiac function and attenuates cardiac remodeling following chronic myocardial infarction in rats. Stem Cells Transl Med. 2012;1(5):430-437. [43] MASUMOTO H, IKUNO T, TAKEDA M, et al. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci Rep. 2014;4(1):6716. [44] ISHIGAMI M, MASUMOTO H, IKUNO T, et al. Human iPS cell-derived cardiac tissue sheets for functional restoration of infarcted porcine hearts. PLoS One. 2018;13(8):e201650. [45] WANG M, DENG Y, XIE P, et al. Optimal design and biomechanical analysis of a biomimetic lightweight design plate for distal tibial fractures: a finite element analysis. Front Bioeng Biotechnol. 2022;10:820921. [46] YOSHIDA S, MIYAGAWA S, FUKUSHIMA S, et al. Maturation of human induced pluripotent stem cell-derived cardiomyocytes by soluble factors from human mesenchymal stem cells. Mol Ther. 2018;26(11):2681-2695. [47] INUI A, SEKINE H, SANO K, et al. Generation of a large-scale vascular bed for the in vitro creation of three-dimensional cardiac tissue. Regen Ther. 2019;11:316-323. [48] JIA Z, GUO H, XIE H, et al. Harvesting prevascularized smooth muscle cell sheets from common polystyrene culture dishes. PLOS ONE. 2018;13(9):e204677. [49] MASUDA S, MATSUURA K, ANAZAWA M, et al. Formation of vascular network structures within cardiac cell sheets from mouse embryonic stem cells. Regen Ther. 2015;2:6-16. [50] GAO L, GREGORICH ZR, ZHU W, et al. Large cardiac muscle patches engineered from human induced-pluripotent stem cell–derived cardiac cells improve recovery from myocardial infarction in swine. Circulation. 2018;137(16):1712-1730. [51] GAO L, KUPFER ME, JUNG JP, et al. Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold. Circ Res. 2017;120(8):1318-1325. [52] KIM KS, JOO HJ, CHOI SC, et al. Transplantation of 3D bio-printed cardiac mesh improves cardiac function and vessel formation via ANGPT1/Tie2 pathway in rats with acute myocardial infarction. Biofabrication. 2021. doi: 10.1088/1758-5090/ac1e78. [53] YEUNG E, FUKUNISHI T, BAI Y, et al. Cardiac regeneration using human‐induced pluripotent stem cell‐derived biomaterial‐free 3D‐bioprinted cardiac patch in vivo. J Tissue Eng Regen Med. 2019;13(11):2031-2039. [54] ARAI K, MURATA D, TAKAO S, et al. Drug response analysis for scaffold-free cardiac constructs fabricated using bio-3D printer. Sci Rep. 2020;10(1):8972. [55] KAWAI Y, TOHYAMA S, ARAI K, et al. Scaffold-free tubular engineered heart tissue from human induced pluripotent stem cells using bio-3D printing technology in vivo. Front Cardiovasc Med. 2022;8:806215. [56] LANCASTER JJ, SANCHEZ P, REPETTI GG, et al. Human induced pluripotent stem cell–derived cardiomyocyte patch in rats with heart failure. Ann Thoracic Surg. 2019;108(4): 1169-1177. [57] ROCHE CD, SHARMA P, ASHTON AW, et al. Printability, durability, contractility and vascular network formation in 3D bioprinted cardiac endothelial cells using alginate–gelatin hydrogels. Front Bioeng Biotechnol. 2021;9:636257. [58] ALONZO M, EL KHOURY R, NAGIAH N, et al. 3D biofabrication of a cardiac tissue construct for sustained longevity and function. ACS App Mater Interfaces. 2022;14(19):21800-21813. [59] ANIL KUMAR S, ALONZO M, ALLEN SC, et al. A visible light-cross-linkable, fibrin–gelatin-based bioprinted construct with human cardiomyocytes and fibroblasts. ACS Biomater Sci Eng. 2019;5(9):4551-4563. [60] GAEBEL R, MA N, LIU J, et al. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials. 2011;32(35):9218-9230. [61] SEKINE H, SHIMIZU T, SAKAGUCHI K, et al. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4(1):1399. [62] SEKINE H, SHIMIZU T, HOBO K, et al. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118(14_suppl_1):S145-S152. [63] TSURUYAMA S, MATSUURA K, SAKAGUCHI K, et al. Pulsatile tubular cardiac tissues fabricated by wrapping human iPS cells-derived cardiomyocyte sheets. Regen Ther. 2019; 11:297-305. [64] KOBAYASHI H, SHIMIZU T, YAMATO M, et al. Fibroblast sheets co-cultured with endothelial progenitor cells improve cardiac function of infarcted hearts. J Artific Organs. 2008;11(3):141-147. [65] KAWAMURA M, PAULSEN MJ, GOLDSTONE AB, et al. Tissue-engineered smooth muscle cell and endothelial progenitor cell bi-level cell sheets prevent progression of cardiac dysfunction, microvascular dysfunction, and interstitial fibrosis in a rodent model of type 1 diabetes-induced cardiomyopathy. Cardiovasc Diabetol. 2017;16(1):142. [66] SHUDO Y, COHEN JE, MACARTHUR JW, et al. Spatially oriented, temporally sequential smooth muscle cell-endothelial progenitor cell bi-level cell sheet neovascularizes ischemic myocardium. Circulation. 2013;128(11_suppl_1):S59-S68. [67] NOOR N, SHAPIRA A, EDRI R, et al. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci. 2019;6(11):1900344. [68] SCHAEFER JA, GUZMAN PA, RIEMENSCHNEIDER S B, et al. A cardiac patch from aligned microvessel and cardiomyocyte patches. J Tissue Eng Regen Med. 2018;12(2):546-556. [69] ZHANG H, WANG Y, ZHENG Z, et al. Strategies for improving the 3D printability of decellularized extracellular matrix bioink. Theranostics. 2023;13(8):2562-2587. [70] SU T, HUANG K, MATHEWS KG, et al. Cardiac stromal cell patch integrated with engineered microvessels improves recovery from myocardial infarction in rats and pigs. ACS Biomate Sci Eng. 2020;6(11):6309-6320. [71] BEJLERI D, STREETER BW, NACHLAS ALY, et al. A bioprinted cardiac patch composed of cardiac‐specific extracellular matrix and progenitor cells for heart repair. Adv Healthc Mater. 2018;7(23):1800672. [72] ANILKUMAR S, ALLEN SC, TASNIM N, et al. The applicability of furfuryl-gelatin as a novel bioink for tissue engineering applications. J Biomed Mater Res Part B Appl Biomater. 2019;107(2):314-323. [73] YIN Q, ZHU P, LIU W, et al. A conductive bioengineered cardiac patch for myocardial infarction treatment by improving tissue electrical integrity. Adv Healthc Mater. 2023; 12(1):2201856. [74] BASARA G, SAEIDI-JAVASH M, REN X, et al. Electrically conductive 3D printed Ti(3)C(2)T(x) MXene-PEG composite constructs for cardiac tissue engineering. Acta Biomater. 2022;139:179-189. [75] TIJORE A, IRVINE SA, SARIG U, et al. Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication. 2018;10(2):25003. [76] PARK BW, JUNG SH, DAS S, et al. In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Sci Adv. 2020;6(13):y6994. [77] KOMAE H, ONO M, SHIMIZU T. Cell sheet-based vascularized myocardial tissue fabrication. Eur Surg Res. 2018,59(3-4):276-285. [78] ASAKAWA N, SHIMIZU T, TSUDA Y, et al. Pre-vascularization of in vitro three-dimensional tissues created by cell sheet engineering. Biomaterials. 2010;31(14):3903-3909. [79] LIN C, LIN G, LUE TF. Allogeneic and xenogeneic transplantation of adipose-derived stem cells in immunocompetent recipients without immunosuppressants. Stem Cells Dev. 2012;21(15):2770-2778. [80] DENG Y, OUYANG H, XIE P, et al. Biomechanical assessment of screw safety between far cortical locking and locked plating constructs. Comput Methods Biomech Biomed Eng. 2021;24(6):663-672. [81] YOSHIDA S, MIYAGAWA S, TOYOFUKU T, et al. Syngeneic mesenchymal stem cells reduce immune rejection after induced pluripotent stem cell-derived allogeneic cardiomyocyte transplantation. Sci Rep. 2020;10(1):4593. [82] DHINGRA S, LI P, HUANG X, et al. Preserving prostaglandin E2 level prevents rejection of implanted allogeneic mesenchymal stem cells and restores postinfarction ventricular function. Circulation. 2013;128(11_suppl_1):S69-S78. [83] EL OMAR R, XIONG Y, DOSTERT G, et al. Immunomodulation of endothelial differentiated mesenchymal stromal cells:impact on T and NK cells. Immunol Cell Biol. 2016;94(4):342-356. [84] MUSIALEK P, MAZUREK A, JAROCHA D, et al. Myocardial regeneration strategy using wharton’s jelly mesenchymal stem cells as an off-the-shelf ‘unlimited’ therapeutic agent:results from the acute myocardial infarction first-in-man study. Postepy Kardiol Interwencyjnej. 2015;11(2):100-107. [85] LUDKE A, WU J, NAZARI M, et al. Uterine-derived progenitor cells are immunoprivileged and effectively improve cardiac regeneration when used for cell therapy. J Mol Cell Cardiol. 2015;84:116-128. [86] GAO B, MATSUURA K, SHIMIZU T. Recent progress in induced pluripotent stem cell-derived cardiac cell sheets for tissue engineering. BioSci Trends. 2019;13(4):292-298. [87] KONG E, BIDDLE J, KALAIPANDIAN S, et al. Coconut callus initiation for cell suspension culture. Plants (Basel). 2023;12(4):968. [88] BUIKEMA JW, LEE S, GOODYER WR, et al. Wnt activation and reduced cell-cell contact synergistically induce massive expansion of functional human iPSC-derived cardiomyocytes. Cell Stem Cell. 2020;27(1):50-63. [89] MATSUURA K, SETA H, HARAGUCHI Y, et al. TRPV-1-mediated elimination of residual iPS cells in bioengineered cardiac cell sheet tissues. Sci Rep. 2016,6(1):21747. [90] MATSUURA K, ITO K, SHIRAKI N, et al. Induced pluripotent stem cell elimination in a cell sheet by methionine-free and 42°C condition for tumor prevention. Tissue Engineering Part C Methods. 2018;24(10):605-615. [91] ALSAYEGH K, MATSUURA K, SEKINE H, et al. Dinaciclib potently suppresses MCL-1 and selectively induces the cell death in human iPS cells without affecting the viability of cardiac tissue. Sci Rep. 2017;7(1):45577. [92] 陈欣欣,刘珠媛,顾寰宇,等.人类胚胎干细胞来源的心肌细胞模型的建立及系统鉴定方案[J].上海大学学报(自然科学版),2017,23(3):378-386. [93] 赵东良,任明明,欧阳昆富,等.心血管跨尺度机制研究与生物力学模型[J].中国科学基金,2022,36(2):235-244. [94] ZHAO D, NIU P, SUN X, et al. Mechanical difference of left ventricle between rabbits of myocardial infarction and hypertrophy. J Biomech. 2020;111:110021. |
| [1] | Yu Jingbang, Wu Yayun. Regulatory effect of non-coding RNA in pulmonary fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1659-1666. |
| [2] | Wang Qiuyue, Jin Pan, Pu Rui . Exercise intervention and the role of pyroptosis in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1667-1675. |
| [3] | Yuan Weibo, Liu Chan, Yu Limei. Potential application of liver organoids in liver disease models and transplantation therapy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1684-1692. |
| [4] | Yang Zhihang, Sun Zuyan, Huang Wenliang, Wan Yu, Chen Shida, Deng Jiang. Nerve growth factor promotes chondrogenic differentiation and inhibits hypertrophic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1336-1342. |
| [5] | Hu Taotao, Liu Bing, Chen Cheng, Yin Zongyin, Kan Daohong, Ni Jie, Ye Lingxiao, Zheng Xiangbing, Yan Min, Zou Yong. Human amniotic mesenchymal stem cells overexpressing neuregulin-1 promote skin wound healing in mice [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1343-1349. |
| [6] | Jin Kai, Tang Ting, Li Meile, Xie Yuan. Effects of conditioned medium and exosomes of human umbilical cord mesenchymal stem cells on proliferation, migration, invasion, and apoptosis of hepatocellular carcinoma cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1350-1355. |
| [7] | Li Dijun, Jiu Jingwei, Liu Haifeng, Yan Lei, Li Songyan, Wang Bin. Three-dimensional gelatin microspheres loaded human umbilical cord mesenchymal stem cells for chronic tendinopathy repair [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1356-1362. |
| [8] | Liu Qi, Li Linzhen, Li Yusheng, Jiao Hongzhuo, Yang Cheng, Zhang Juntao. Icariin-containing serum promotes chondrocyte proliferation and chondrogenic differentiation of stem cells in the co-culture system of three kinds of cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1371-1379. |
| [9] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [10] | Peng Hongcheng, Peng Guoxuan, Lei Anyi, Lin Yuan, Sun Hong, Ning Xu, Shang Xianwen, Deng Jin, Huang Mingzhi . Role and mechanism of platelet-derived growth factor BB in repair of growth plate injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1497-1503. |
| [11] | Li Jialin, Zhang Yaodong, Lou Yanru, Yu Yang, Yang Rui. Molecular mechanisms underlying role of mesenchymal stem cell secretome [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1512-1522. |
| [12] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| [13] | Sun Xianjuan, Wang Qiuhua, Zhang Jinyi, Yang Yangyang, Wang Wenshuang, Zhang Xiaoqing. Adhesion, proliferation, and vascular smooth muscle differentiation of bone marrow mesenchymal stem cells on different electrospinning membranes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 661-669. |
| [14] | Liu Haoyang, Xie Qiang, Shen Mengran, Ren Yansong, Ma Jinhui, Wang Bailiang, Yue Debo, Wang Weiguo . Application, research hotspots, and shortcomings of degradable zinc-based alloys in bone defect repair and reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 839-845. |
| [15] | Zhang Yu, Xu Ruian, Fang Lei, Li Longfei, Liu Shuyan, Ding Lingxue, Wang Yuexi, Guo Ziyan, Tian Feng, Xue Jiajia. Gradient artificial bone repair scaffold regulates skeletal system tissue repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 846-855. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||