Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (11): 1780-1788.doi: 10.12307/2024.206
Previous Articles Next Articles
Role and mechanism of reactive oxygen species in tendinopathy
Liu Ke1, Xu Weidong2, Zhou Hengyu3, Bai Shuo4, Zhang Zhen5, Ge Ruidong6, 7
- 1School of Exercise and Health, Shanghai University of Sport, Shanghai 200438, China; 2Department of Orthopedics, Changhai Hospital, Navy Medical University, Shanghai 200433, China; 3School of Life Science, Beijing University of Chinese Medicine, Beijing 100029, China; 4Beijing Chaoyang District Taiyanggong Community Health Service Center, Beijing 100028, China; 5Department of Rehabilitation Medicine, Beijing Da Wang Lu Emergency Hospital, Beijing 100122, China;6Department of Rehabilitation Medicine, China-Japan Friendship Hospital, Beijing 100029, China; 7School of Sports Medicine and Rehabilitation, Beijing Sport University, Beijing 100084, China
-
Received:2023-01-30Accepted:2023-03-14Online:2024-04-18Published:2023-07-27 -
Contact:Ge Ruidong, PhD, Associate chief therapist, Department of Rehabilitation Medicine, China-Japan Friendship Hospital, Beijing 100029, China; School of Sports Medicine and Rehabilitation, Beijing Sport University, Beijing 100084, China -
About author:Liu Ke, Master candidate, School of Exercise and Health, Shanghai University of Sport, Shanghai 200438, China -
Supported by:Fundamental Research Funds for the Central Universities, No. 2020064 (to GRD)
CLC Number:
Cite this article
Liu Ke, Xu Weidong, Zhou Hengyu, Bai Shuo, Zhang Zhen, Ge Ruidong. Role and mechanism of reactive oxygen species in tendinopathy[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(11): 1780-1788.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
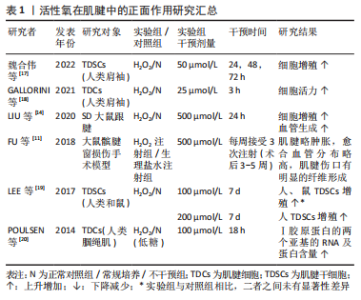
2.1 活性氧在肌腱病中的作用 肌腱损伤后会出现一系列的自我修复过程,但由于其愈合能力较差,若不利刺激持续存在,肌腱则会偏离理想愈合路径,出现愈合不足或愈合失败等现象,引起肌腱组成成分、细胞数量和微观结构的异常改变,最终造成不可逆的肌腱损伤,反而阻止肌腱正常机械性能的恢复[5]。因此,根据活性氧对肌腱细胞外基质和细胞表型的影响效果,可将其作用大致分为正面作用和负面作用两类。 2.1.1 活性氧在肌腱病中的正面作用 Ⅰ型胶原蛋白为细胞外基质的主要成分,该胶原蛋白的合成增加,可促进肌腱正常细胞外基质的维持或补充。如表1所示,活性氧可增强肌腱中Ⅰ型胶原蛋白mRNA和蛋白质的合成,活性氧刺激还可以增强肌腱干细胞和成纤维细胞的活力和增殖能力[11,14,17-20]。"

肌腱干细胞是目前发现的肌腱中惟一具有再生修复能力的细胞[21],而成纤维细胞作为肌腱内的主要细胞类型,负责监测细胞外基质稳态,调节适应负荷,维持肌腱胶原蛋白和非胶原成分[4]。因此肌腱细胞的活力与数量的增加,对愈合过程中细胞的再生分化和细胞外基质中损伤和再生胶原蛋白的补充代谢有重要意义。同时,活性氧还可以刺激新生血管的形成[11,14],这可能对后期在损伤肌腱组织中完善愈合血管网,提供必要损伤修复因子和细胞具有重要意义。因此,活性氧通过促进胶原蛋白的合成、肌腱细胞的增殖以及新生血管的合成等,对损伤后肌腱的愈合具有一定的正面促进作用。 2.1.2 活性氧在肌腱中的负面作用 表2介绍了活性氧在肌腱中负面作用的研究成果[22-48],与正面作用相反,活性氧可以造成肌腱中胶原蛋白含量的大量下降[13-14,24-25,44],并增加Ⅲ/Ⅰ型胶原的比例[30]。虽然Ⅲ型胶原蛋白为肌腱损伤修复的原始补丁蛋白,但长期累积过量,不能及时向Ⅰ型胶原蛋白转化时,肌腱胶原结构将会更加紊乱,出现排列恶化等愈合失败现象[35,42]。同时,活性氧也可以在细胞层面上导致大量肌腱干细胞和成纤维细胞的活力和增殖能力的下降[14,17-19,24-26,31-32,34,36-37,40-41,43,45],细胞凋亡数量的显著上升[14,17,19,23-25,27,29,33,39-40,43-45],部分细胞出现自噬性死亡现 象[13,16,29,31,37,41],整体肌腱中活细胞数目减少[13,22,24,30,32,38,43,45]。肌腱细胞代谢活性的下降和凋亡可能会再次导致细胞外基质中胶原的调节能力和代谢转化平衡的失衡,因此活性氧可能通过同时作用于肌腱细胞外基质和细胞,导致肌腱出现早期发病特征。并且活性氧也可显著降低肌腱干细胞的迁移[19]、集落形成以及干性分化能力[19,23,25,31,37],导致其无法在肌腱修复过程中向必要腱细胞、软骨细胞以及骨细胞的生成和转化,从而可能会影响腱-骨软骨细胞生成和排列,降低肌腱机械强度[35,42]。 而抗活性氧剂可以明显改善肌腱病模型中肌腱细胞的凋亡与毒性,并增强其肌腱组织中胶原蛋白的转录和合成[27-29,33,39]。因此,活性氧可通过造成肌腱细胞外基质和细胞的损害,并抑制肌腱干细胞的修复能力来对肌腱的愈合过程造成负面影响。以上结果显示,活性氧作用于肌腱时,可能会同时影响肌腱细胞外基质和细胞的改变,而两者之间具有密切相互作用[49]。细胞外基质不仅是肌腱的主要组成部分,同时还是肌腱细胞的生存环境,具有支持、保护和传递细胞信息的功能[49]。因此在活性氧干预后,细胞表型的改变可能是细胞外基质作用的结果,而细胞信号的变化又可以反过来引起细胞微环境的改变[50-51],两者之间的相互作用可能会放大活性氧在肌腱病中的作用,从而导致其向某一方面的持续发展[52]。"


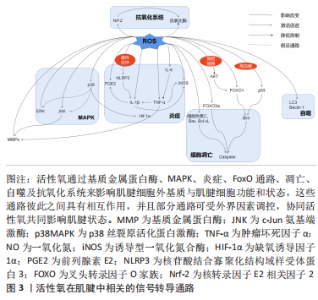
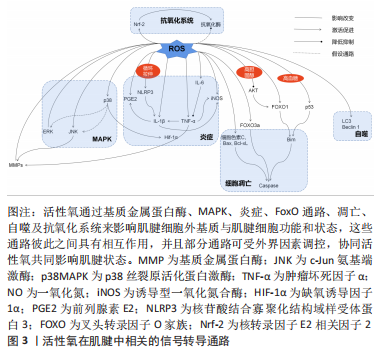
而在探究活性氧双重作用特点原因方面,浓度可能是决定其发挥何种作用效果的关键,即仅在低浓度活性氧刺激下,才会诱导肌腱发生正确愈合反应。究其原因,一方面可能是在正常肌腱代谢活动中,活性氧可不断产生消耗,参与细胞增殖及胶原代谢等肌腱正常修复生理过程[47];另一方面也可能是适量低浓度的活性氧刺激会引发肌腱组织和细胞自适变化,帮助肌腱微小损伤的修复和愈合[48]。有研究虽使用的H2O2浓度较高但仍出现正面作用结果,或许与其实验对象为动物体内模型有关[11, 14]。相比于体外模型,体内模型含有自身抗氧化系统,因此可能相对需要更高的H2O2浓度才会达到活性氧有效作用阈值。 因此,活性氧虽为氧化应激的主要效应分子,但在肌腱病中却存在肌腱保护作用[53]。然而,目前研究仍大多集中在对活性氧负面作用的研究上,如探究肌腱病中过量活性氧引起的发病机制以及各类抗氧化剂的治疗作用,未对活性氧的保护作用进行详细探究。这可能与早期肌腱病患者症状隐匿,难以监测其活性氧的积累及控制愈合方向的发展有关。但此类活性氧正面作用的研究,可以为未来开发活性氧治疗肌腱病的新方向,以及之后的再生组织支架微环境的构造奠定研究基础。 2.2 活性氧在肌腱中的相关信号转导 活性氧在肌腱中的作用涉及多方面的信号转导通路,见表3。这些通路之间存在相互作用,可对肌腱不同方面的功能造成影响,具体可见图3。"
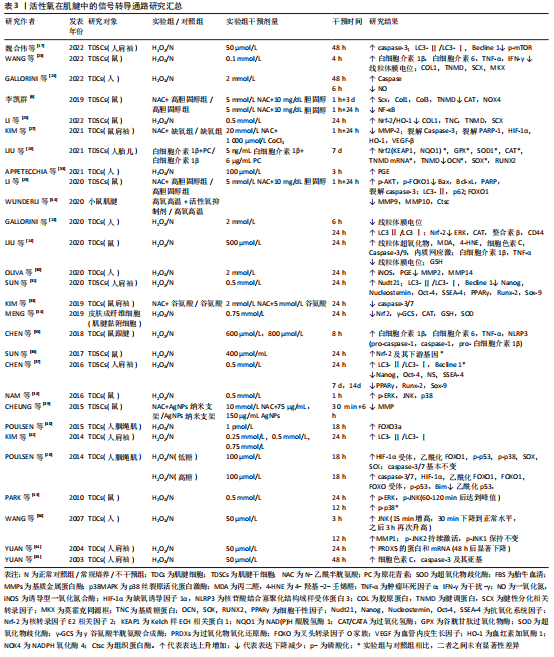

2.2.1 基质金属蛋白酶影响细胞外基质平衡转化 在活性氧刺激下,人肌腱细胞中的基质金属蛋白酶含量会发生不同变化[27,30,54-56];而抗活性氧剂干预后,则会明显下调腱病模型中的基质金属蛋白酶水平[39]。基质金属蛋白酶是一类维持细胞外基质正常结构所必需的酶家族,几乎所有家族成员均可分解肌腱细胞外基质中的胶原蛋白,诱发肌腱断裂或疼痛;但部分基质金属蛋白酶还可以积极促进组织重塑及损伤后的愈合反应[57]。因此活性氧刺激可能会打破肌腱中基质金属蛋白酶的合成与降解平衡,改变肌腱中胶原比例[57],从而影响肌腱生物学特性。 2.2.2 MAPK激活细胞的存亡通路 有研究发现,1.0-2.0 h的H2O2模拟刺激可增强肌腱细胞中的胞外信号调节激酶(extracellular regulated kinase,ERK)通路的磷酸化水平[38,43]。但在干预24 h后,磷酸化的ERK蛋白水平反而显著降低,甚至低于干预前水平[13,43]。大量研究认为,ERK1/2的激活在肌腱细胞的存活中起核心作用[58-60],ERK1/2通路的活化减少会对肌腱细胞有负面影响[61]。但也有研究认为ERK激活有助于过量活性氧刺激后诱导的细胞凋亡[62]。因此,活性氧在肌腱细胞中对ERK通路的不同调节作用可能与干预时间相关,长时间的活性氧干预使ERK通路处于抑制状态,这可能是慢性肌腱病中导致细胞凋亡原因之一。但早期ERK通路的短暂激活是否为细胞的存活反应,与细胞凋亡有无关系仍需进一步探究[63]。 同样,c-Jun氨基端激酶(c-Jun N-terminal kinase,JNK)通路也可在肌腱中被活性氧刺激激活[38,43]。但1.0-2.0 h后,激活的JNK蛋白水平会出现较大差距的回落[43]。有研究发现,肌腱中的JNK通路在活性氧的刺激下呈双相激活特点,分别在干预后15 min和3 h达到高峰[56]。这种现象的出现可能是由于JNK的瞬时和持续激活阶段都有助于细胞存亡基因的表达:即早期瞬时的JNK活化可促进细胞存活,而持续的JNK活化可介导细胞凋亡[64]。同时,活性氧诱导的肌腱JNK通路激活还可能导致基质金属蛋白酶1的表达含量增加[56]。因此以上研究结果提示,在活性氧的作用下,JNK的激活可能不仅对肌腱细胞表型有影响,还对细胞外基质的合成降解有重要介导作用。 在肌腱细胞中,活性氧激活的p38MAPK因子可能为炎症因子表达的关键调控因子,但其后续作用效果未有统一定论。NAM等[38]认为,可能与细胞凋亡通路的激活有关。PARK等[43]认为,p38MAPK的弱磷酸化,可能是激活ERK和JNK通路磷酸化的前提。由于POULSEN等[20]发现p38MAPK可以更多地在低糖过氧化物处理的肌腱细胞中被激活,并增强缺氧诱导因子1α受体活性,从而促进腱细胞分化蛋白的表达,对肌腱具有保护作用。因此他们认为,p38MAPK激活缺氧诱导因子1α通路是细胞正常氧化通路,是细胞的保护机制[20]。但JIAO等[65]的研究结果证明,抑制缺氧诱导因子1α因子的表达反而可缓解肌腱病的严重程度,促进肌腱修复。同样抗活性氧剂干预后,发现缺氧诱导因子1α的降低与细胞活力增加、凋亡减少有关[27]。因此JIAO等[65]认为,抑制与炎症发展密切相关的缺氧诱导因子1α因子,可以减轻炎症和肌腱的异常修复,从而促进正确愈合进程。根据以上众多实验结果,或许可以推测弱磷酸化的p38MAPK通路才是肌腱遇到有害刺激之后的正保护机制,而长时间大量激活的p38MAPK则会导致缺氧诱导因子1α等炎症因子长期存在,甚至可能引起细胞凋亡,影响肌腱正常修复。 MAPK是将信号从细胞表面传到到核内的重要传递者,与细胞的基因调节、细胞对环境的反应以及细胞生长和凋亡活动密切相关[66],而活性氧是其主要刺激因子之一。因此在肌腱中,活性氧刺激MAPK通路所产生的不同变化及其对肌腱细胞和细胞外基质产生保护或是有害作用,可能与不同的刺激时间和强度有关。 2.2.3 细胞色素C与Caspases激活线粒体凋亡路径 在大部分活性氧刺激导致细胞凋亡的实验中,都可以检测到细胞色素C、凋亡诱导蛋白含量的增加以及Caspases酶活性的大量激活[14,17,24,45],而抗活性氧剂干预后又可以显著降低这些蛋白在肌腱中的表达[27,29,33]。其中,Caspase酶家族是细胞程序性死亡和炎症作用的关键执行因子,通常当细胞接收到凋亡诱导信号时,该类酶将以凋亡或促炎方式直接切割细胞关键存活蛋白或底物,导致细胞死亡[67]。因此,活性氧可能是通过诱导线粒体中的细胞色素C或其他凋亡诱导蛋白(如Bax和Bcl-xL等)释放,与胞质中的凋亡酶激活因子1(apoptosis protease activating factor-1,Apaf-1)结合,激活Caspase级联,从而最终导致线粒体依赖的细胞凋亡发生[68]。 同时,一些研究者也发现,活性氧刺激会导致肌腱细胞中线粒体膜电位的显著下降,出现去极化表现[13,23]。线粒体为细胞的主要能量代谢场所,是胞内活性氧的重要来源。线粒体功能失调可能会导致胞内出现活性氧水平和脂质过氧化物的进一步上调,并诱导氧化损伤和内质网应激,从而引发凋亡级联反应[37,69-71]。LIU等[14]利用阻止线粒体去极化的药物对H2O2处理后的小鼠肌腱细胞进行干预发现,药物预处理可以减少活性氧诱导的有毒细胞代谢产物,保护线粒体,同时也可以降低线粒体凋亡路径中细胞色素C和Caspase酶的表达。 因此,在肌腱损伤后,活性氧可能会攻击线粒体,促进线粒体膜去极化,导致线粒体内容物释放,如细胞色素C等,从而诱导线粒体凋亡级联反应,造成肌腱细胞数目进一步减少,细胞外基质持续恶化。 2.2.4 叉头转录因子O家族通路受多种影响因素调控 叉头转录因子O家族(forkhead transcription factors of the Oclass,FoxO)家族是一类具有复杂功能的转录因子,是多种下游通路的作用基础[72]。在肌腱中,活性氧可能会协同多种细胞外环境激活不同分子通路作用于FoxO,从而对细胞造成不同方面的影响。例如在高血糖情况下,活性氧可激活p53通路,从而增强FOXO1通过Bim(Bcl-2-Like Protein 11,Bcl-2样蛋白11)蛋白介导的细胞凋亡作用[20]。而高胆固醇状态下,抗活性氧剂可激活上游蛋白激酶B(protein kinase B,AKT),从而抑制FOXO1在细胞自噬和凋亡中的作用[29]。并且,即使是细胞培养生长补充剂胎牛血清浓度也会对活性氧在肌腱中的FoxO通路产生不同影响[40]。如POULSEN等[40]发现,在细胞增殖率较小的5%胎牛血清组,FOXO3a会通过抑制细胞增殖,减少活性氧暴露后DNA受损细胞的增生复制,来发挥肌腱保护作用;而在无法抑制细胞大量增殖的10%胎牛血清组中,FOXO3a则会表现出诱导肌腱细胞出现大量凋亡的负面作用。因此,在活性氧环境中,肌腱中的FoxO通路可能是放大或改变活性氧作用的一个枢纽,各类代谢性疾病或细胞外环境可通过影响FoxO的作用,来改善或加重活性氧在肌腱中的作用。 2.2.5 自噬激活细胞保护/死亡作用 多项实验发现,活性氧暴露会增加微管相关蛋白1轻链3(microtubule-associated protein 1A/1B-Light chain3,LC3)和Beclin 1蛋白等自噬标志物在肌腱中的表达,诱导肌腱细胞自噬率和死亡率的上升[13,17,31,37,41]。KIM等[41]分别利用凋亡抑制剂和自噬抑制剂证明,活性氧可诱导人肌腱成纤维细胞的自噬死亡。有研究发现,肌腱细胞自噬死亡率会随细胞外基质的破裂程度的增加而增加,因此提出自噬诱导的细胞死亡会导致基质胶原合成减少、肌腱退变的假说机制[48,73]。但细胞的自噬活动可能对于肌腱的修复愈合并不是一件完全负面事件。Beclin1蛋白为自噬通路因子,该因子在持续凋亡应激下会被裂解;而在自噬情况下可以反过来抑制凋亡因子的表达。因此适量活性氧刺激下,Beclin1会促进细胞自噬,回收胞内因活性氧攻击受损的蛋白质或细胞器,从而减少细胞凋亡,维持肌腱细胞数量[17]。CHEN等[37]同样发现自噬抑制剂会使N-乙酰半胱氨酸在实验中的保护作用失效,间接证明自噬有助于维护保持活性氧刺激下肌腱干细胞的干性、分化以及自我更新能力。同时,自噬也可在一定程度上减少活性氧引起的炎症负面反应[74]。 因此在肌腱组织中,活性氧介导的自噬反应对细胞有致死或保护作用,而自噬的激活程度是决定细胞反应的关键。适当的活性氧激活可以促进自噬向利于细胞存活,促进肌腱损伤愈合方向发展,但自噬程度过大则会导致细胞死亡。 2.2.6 炎症因子的产生及相互平衡作用 当肌腱细胞暴露于活性氧后,前列腺素E2的分泌显著增加[30,53]。前列腺素E2是一种常见的慢性炎症参与因子[75],对于Ⅰ型胶原蛋白合成具有抑制作用,可减少肌腱成纤维细胞的增殖[32,76]。因此,前列腺素E2对肌腱结构具有分解作用,同时在其他研究发现前列腺素E2可呈剂量依赖性减少肌腱干细胞的增殖,并影响其干性与分化能力[77]。 多项研究发现,活性氧刺激可显著提高小鼠肌腱细胞中白细胞介素1β和肿瘤坏死因子α的表达[14,23,55]。白细胞介素1β是典型炎症因子,可直接下调Ⅰ型胶原蛋白mRNA和蛋白的表达,降解细胞外基质,导致细胞外基质重塑。同时,白细胞介素1β可导致多种促炎分子的产生,如环氧合酶2和前列腺素E2等,这些因子可进一步降解肌腱细胞外基质并损害其机械性能[78]。肿瘤坏死因子α同样可能通过上调人肌腱细胞中基质金属蛋白酶1或白细胞介素1β的表达来直接或间接影响细胞外基质[79-80]。同时肿瘤坏死因子α也可通过上调caspase-3的mRNA水平来诱导人肌腱细胞凋亡[81]。而CHEN等[55]还发现,活性氧刺激后,白细胞介素1β将会在循环拉伸的刺激下再次大量释放,而肿瘤坏死因子α却未出现类似改变,其原因可能是循环拉伸可以增加活性氧对核苷酸结合寡聚化结构域样受体蛋白3(nod-like receptor protein3,NLRP3)炎症小体的激活作用,从而放大炎性细胞因子,尤其是白细胞介素1β的成熟释放[55]。 白细胞介素6的表达也可以在活性氧刺激下显著提升[23,55]。在肌腱细胞中,白细胞介素6具有增加总胶原蛋白合成、调节STAT3转录因子、增强早期炎症反应的作用[23,78]。其他研究发现,白细胞介素6可以抑制肿瘤坏死因子α表达,被认为可能是肌腱修复中的重要抗炎因子[82]。LIN等[83]的实验证明了这一结论,即白细胞介素6缺乏小鼠的肌腱恢复强度明显弱于正常对照组。但仍不确定该结果的出现是因为白细胞介素6作为炎症因子分泌较少,引起的炎症反应较弱导致肌腱愈合出现迟缓;还是白细胞介素6确实在肌腱愈合过程中发挥抗炎的作用。因此,未来对于白细胞介素6在肌腱病中的角色仍需继续探讨。 最后,实验发现,肌腱在活性氧干预暴露6 h后,肌腱中的一氧化氮(nitric oxide,NO)生成量减少[24];而在暴露后24 h,诱导型一氧化氮合酶(inducible nitric oxide synthase,iNOS)的水平反而显著升高[30]。一氧化氮是一种炎症发病机制中的关键信号分子[84],而诱导型一氧化氮合酶是生成一氧化氮效率最高的一种化合酶[30],可由白细胞介素1β或肿瘤坏死因子α诱导释放[85]。大量动物实验、体外实验及临床试验证明,一氧化氮会诱导胶原蛋白产生,增强肌腱细胞外基质修复,从而促进肌腱愈合,使受伤的肌腱具有更好的机械强度和性能[85-86]。因此,一氧化氮可能为肌腱受伤后的重要恢复因子。但一氧化氮可能在肌腱损伤的不同时期含量不同,目前已有研究证明诱导型一氧化氮合酶的活动可在肌腱损伤后7 d达到峰值,并在14 d时再度恢复到基线水平[85],但目前短时间之内的诱导型一氧化氮合酶和一氧化氮变化规律仍处于未知状态,因此未来探究一氧化氮在肌腱中的水平变化规律一定程度上有助于肌腱病的风险预测和最佳治疗时机的寻找。 综合以上观点,活性氧可导致各种炎症因子在肌腱中的释放,这些因子之间并不是独立运行,而是一个相互协调、互相平衡的整体。当炎症反应失衡或不能及时解决时,就会引发长期持续性的炎症,导致肌腱的进一步退变和过度纤维化[87],因此促炎因子与抗炎因子之间的平衡是保证肌腱避免异常修复的重要前提条件[78]。同时,文章还发现循环拉伸干预会促进或加强活性氧刺激下肌腱炎症因子的释放,如白细胞介素1β。由此或许可以说明,过度运动导致的循环拉伸与肌腱中活性氧通路具有协同作用,可直接或间接引发、加重炎症,从而加速肌腱退化。 2.2.7 抗氧化系统的应激保护与失效 活性氧干预后,启动细胞内源性抗氧化反应的关键分子——核转录因子E2相关因子2(nuclear factor erythroid 2-like 2,Nrf-2)表达显著升高[13,25,88],其介导的下游抗氧化基因和蛋白也同样出现上调,虽然在SUN等[36]的实验中未出现显著结果。然而大部分研究显示,Nrf-2终端产物,各类抗氧化酶的水平却并未升高,反而部分呈现下降趋势[13,25,34]。只有YUAN等[44]发现,在H2O2的干预下,一种亚细胞中广泛分布的抗氧化酶含量的显著上升。因此,活性氧可以激活肌腱细胞中的Nrf-2内源性抗氧化通路,保护细胞,维持氧化还原稳态。而过量活性氧导致抗氧化酶底物浓度过饱和,可能是出现中心因子Nrf-2显著上调,但仍无法激活末端抗氧化酶现象的原因[13]。 但在MENG等[34]的实验中发现,H2O2干预会使Nrf-2下降。探究其原因,该综述作者认为可能与其使用的特殊实验对象有关。相比于其他实验均利用肌腱组织或细胞探究初次活性氧刺激后肌腱的损伤情况,MENG等[34]利用皮肤成纤维细胞探究活性氧对于肌腱粘连的影响。因此可能在肌腱粘连状态下,Nrf-2通路不再响应过量活性氧刺激,细胞内源性抗氧化系统能力再次下降,应激加重,形成恶性循环,导致肌腱条件继续恶化。所以未来通过促抗氧化酶信号通路保护肌腱细胞,可能是治疗肌腱病的重要前景之一。 此外,活性氧还可通过其他多种信号转导通路影响肌腱的损伤或愈合过程,如肌腱干细胞中的核转录因子κB通路[6]、干性基因表达[31,37],肌腱细胞的整合素或CD44类胞膜表面信号受体蛋白以及血管调节因子等[13, 25, 27]。但在此类方向上的研究数目较少,作用仍不明确,有待未来进一步的探究。 综上所述,基质金属蛋白酶、MAPK、线粒体凋亡通路、FoxO、自噬通路、炎症反应以及胞内的抗氧化系统可能为目前活性氧在肌腱中的统一下游作用通路。这些通路既可以接受外界环境的影响,也可以彼此之间相互调节,共同增强或减弱活性氧在肌腱中的作用,影响肌腱细胞外基质的组成结构和肌腱细胞生存、修复、响应外界刺激的能力。这可为未来控制肌腱病中危险因素和探究活性氧在肌腱中的整体分子作用机制打下研究基础。但目前大部分分子机制尚未完全探清,仅发现活性氧对部分关键因子的上下调作用,其具体作用效果与机制仅参考其他骨骼肌肉疾病的活性氧分子机制做出假设讨论,未进行独立证明实验。所以未来探究活性氧在肌腱中的分子作用与机制仍需向特殊化、具体化全面发展。"

| [1] NOURISSAT G, BERENBAUM F, DUPREZ D. Tendon injury: from biology to tendon repair. Nat Rev Rheumatol. 2015;11(4):223-233. [2] TEMPLEHOF S, RUPP S, SEIL R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg. 1999;8(4):296-299. [3] LUI PP. Stem cell technology for tendon regeneration: current status, challenges, and future research directions. Stem Cells Cloning. 2015;8: 163-174. [4] LIM WL, LIAU LL, NG MH, et al. Current progress in tendon and ligament tissue engineering. Tissue Eng Regen Med. 2019;16(6):549-571. [5] FU SC, ROLF C, CHEUK YC, et al. Deciphering the pathogenesis of tendinopathy: a three-stages process. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:30. [6] 李凯群.高胆固酵通过激活活性氧介导的NF-кB通路抑制肌腱干细胞的腱系分化[D].广州:南方医科大学,2019. [7] ABATE M, DI CARLO L, COCCO G, et al. Oxidative stress and abnormal tendon sonographic features in elite soccer players (a pilot study). Rev Bras Ortop. 2021;56(4):432-437. [8] NOH KC, PARK SH, YANG CJ, et al. Involvement of synovial matrix degradation and angiogenesis in oxidative stress-exposed degenerative rotator cuff tears with osteoarthritis. J Shoulder Elbow Surg. 2018;27(1):141-150. [9] YOSHIDA K, ITOIGAWA Y, WADA T, et al. Association of superoxide-induced oxidative stress with rotator cuff tears in juman patients. J Orthop Res. 2020;38(1):212-218. [10] ITOIGAWA Y, YOSHIDA K, NOJIRI H, et al. Association of recurrent tear after arthroscopic rotator cuff repair and Superoxide-Induced oxidative stress. Am J Sports Med. 2021;49(8):2048-2055. [11] FU SC, YEUNG MY, ROLF CG, et al. Hydrogen peroxide induced tendinopathic changes in a rat model of patellar tendon injury. J Orthop Res. 2018;36(12): 3268-3274. [12] RAY PD, HUANG BW, TSUJI Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981-990. [13] GALLORINI M, BERARDI AC, GISSI C, et al. Nrf2-mediated cytoprotective effect of four different hyaluronic acids by molecular weight in human tenocytes. J Drug Target. 2020;28(2):212-224. [14] LIU YC, WANG HL, HUANG YZ, et al. Alda-1, an activator of ALDH2, ameliorates Achilles tendinopathy in cellular and mouse models. Biochem Pharmacol. 2020;175:113919. [15] BESTWICK CS, MAFFULLI N. Reactive oxygen species and tendinopathy: do they matter? Br J Sports Med. 2004;38(6):672-674. [16] LUI PPY, ZHANG X, YAO S, et al. Roles of oxidative stress in acute tendon injury and degenerative tendinopathy-a target for intervention. Int J Mol Sci. 2022;23(7):3571. [17] 魏合伟,郑维蓬,刘治军,等.化应激诱导肩袖肌腱干细胞自噬的表达[J].中国组织工程研究,2022,26(31):4954-4961. [18] GALLORINI M, BERARDI AC, RICCI A, et al. Dual acting carbon monoxide releasing molecules and carbonic anhydrase inhibitors differentially modulate inflammation in human tenocytes. Biomedicines. 2021;9(2):141. [19] LEE YW, FU SC, YEUNG MY, et al. Effects of redox modulation on cell proliferation, viability, and migration in cultured rat and human tendon progenitor cells. Oxid Med Cell Longev. 2017;2017:8785042. [20] POULSEN RC, KNOWLES HJ, CARR AJ, et al. Cell differentiation versus cell death: Extracellular glucose is a key determinant of cell fate following oxidative stress exposure. Cell Death Dis. 2014;5(2):e1074. [21] ZHANG C, ZHANG E, YANG L, et al. Histone deacetylase inhibitor treated cell sheet from mouse tendon stem/progenitor cells promotes tendon repair. Biomaterials. 2018;172:66-82. [22] LU K, ZHOU M, WANG L, et al. N-Acetyl-L-cysteine facilitates tendon repair and promotes the tenogenic differentiation of tendon stem/progenitor cells by enhancing the integrin α5/β1/PI3K/AKT signaling. BMC Mol Cell Biol. 2023;24(1):1. [23] WANG S, YAO Z, ZHANG X, et al. Energy-supporting enzyme-mimic nanoscaffold facilitates tendon regeneration based on a mitochondrial protection and microenvironment remodeling strategy. Adv Sci (Weinh). 2022;9(31):e2202542. [24] GALLORINI M, ANTONETTI LAMORGESE PASSERI C, CATALDI A, et al. Hyaluronic acid alleviates oxidative stress and apoptosis in human tenocytes via Caspase 3 and 7. Int J Mol Sci. 2022;23(15):8817. [25] LI X, SU Z, SHEN K, et al. Eugenol-Preconditioned mesenchymal stem Cell-Derived extracellular vesicles promote antioxidant capacity of tendon stem cells in vitro and in vivo. Oxid Med Cell Longev. 2022;2022:3945195. [26] SONG K, JIANG T, PAN P, et al. Exosomes from tendon derived stem cells promote tendon repair through miR-144-3p-regulated tenocyte proliferation and migration. Stem Cell Res Ther. 2022;13(1):80. [27] KIM RJ, AN SH, GWARK JY, et al. Antioxidant effects on hypoxia-induced oxidative stress and apoptosis in rat rotator cuff fibroblasts. Eur Cell Mater. 2021;41:680-693. [28] LIU R, ZHOU B, ZHANG H, et al. Inhibition of ROS activity by controlled release of proanthocyanidins from mesoporous silica nanocomposites effectively ameliorates heterotopic ossification in tendon. Chem Eng J. 2021;420(1):129415. [29] LI K, DENG Y, DENG G, et al. High cholesterol induces apoptosis and autophagy through the ROS-activated AKT/FOXO1 pathway in tendon-derived stem cells. Stem Cell Res Ther. 2020;11(1):131. [30] OLIVA F, GALLORINI M, PASSERI CAL, et al. Conjugation with methylsulfonylmethane improves hyaluronic acid anti-inflammatory activity in a hydrogen peroxide-exposed tenocyte culture in vitro model. Int J Mol Sci. 2020;21(21):1-15. [31] SUN Y, CHEN H, YE H, et al. Nudt21-mediated alternative polyadenylation of HMGA2 3’-UTR impairs stemness of human tendon stem cell. Aging (Albany NY). 2020;12(18):18436-18452. [32] SHEHZADI S, JAVED M, AWAN SJ, et al. Developing a novel, efficient and cost effective tissue injury model (in-vitro) on equine tendon fibroblasts. Pak Vet J. 2020;40(3):360-364. [33] KIM RJ, HAH YS, GWARK JY, et al. N-acetylcysteine reduces glutamate-induced cytotoxicity to fibroblasts of rat supraspinatus tendons. Connect Tissue Res. 2019;60(5):431-443. [34] MENG J, YU P, TONG J, et al. Hydrogen treatment reduces tendon adhesion and inflammatory response. J Cell Biochem. 2019;120(2):1610-1619. [35] MORIKAWA D, NOJIRI H, ITOIGAWA Y, et al. Antioxidant treatment with vitamin C attenuated rotator cuff degeneration caused by oxidative stress in Sod1-deficient mice. JSES Open Access. 2018;2(1):91-96. [36] SUN W, MENG J, WANG Z, et al. Proanthocyanidins attenuation of H2O2-Induced oxidative damage in Tendon-Derived stem cells via upregulating nrf-2 signaling pathway. Biomed Res Int. 2017;2017:7529104. [37] CHEN H, GE HA, WU GB, et al. Autophagy prevents oxidative Stress-Induced loss of Self-Renewal capacity and stemness in human tendon stem cells by reducing ROS accumulation. Cell Physiol Biochem. 2016;39(6):2227-2238. [38] NAM DC, HAH YS, NAM JB, et al. Cytoprotective mechanism of cyanidin and delphinidin against oxidative Stress-Induced tenofibroblast death. Biomol Ther. 2016;24(4):426-432. [39] CHEUNG TS, LAU PM, LU H, et al. Cytotoxic and sublethal effects of silver nanoparticles on tendon-derived stem cells - implications for tendon engineering. Toxicol Res. 2015;5(1):318-330. [40] POULSEN RC, CARR AJ, HULLEY PA. Cell proliferation is a key determinant of the outcome of FOXO3a activation. Biochem Biophys Res Commun. 2015;462(1):78-84. [41] KIM RJ, HAH YS, SUNG CM, et al. Do antioxidants inhibit oxidative-stress-induced autophagy of tenofibroblasts? J Orthop Res. 2014;32(7):937-943. [42] MORIKAWA D, ITOIGAWA Y, NOJIRI H, et al. Contribution of oxidative stress to the degeneration of rotator cuff entheses. J Shoulder Elbow Surg. 2014;23(5):628-635. [43] PARK HB, HAH YS, YANG JW, et al. Antiapoptotic effects of anthocyanins on rotator cuff tenofibroblasts. J Orthop Res. 2010;28(9):1162-1169. [44] YUAN J, MURRELL GA, TRICKETT A, et al. Overexpression of antioxidant enzyme peroxiredoxin 5 protects human tendon cells against apoptosis and loss of cellular function during oxidative stress. Biochim Biophys Acta. 2004;1693(1):37-45. [45] YUAN J, MURRELL GA, TRICKETT A, et al. Involvement of cytochrome c release and caspase-3 activation in the oxidative stress-induced apoptosis in human tendon fibroblasts. Biochim Biophys Acta. 2003;1641(1):35-41. [46] JAMES S, SCHUIJERS J, DAFFY J, et al. Ciprofloxacin reduces tenocyte viability and proteoglycan synthesis in short-term explant cultures of equine tendon. PeerJ. 2021;9:e12003. [47] PIZZINO G, IRRERA N, CUCINOTTA M, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017;2017:8416763. [48] CHEN J, WANG A, XU J, et al. In chronic lateral epicondylitis, apoptosis and autophagic cell death occur in the extensor carpi radialis brevis tendon. J Shoulder Elbow Surg. 2010;19(3):355-362. [49] SCREEN HRC, BERK DE, KADLER KE, et al. Tendon functional extracellular matrix. Journal of Orthopaedic Research. 2015;33(6):793-799. [50] BONNANS C, CHOU J, WERB Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786-801. [51] ROZARIO T, DESIMONE DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341(1):126-140. [52] BHABRA G, WANG A, EBERT JR, et al. Lateral elbow tendinopathy: development of a pathophysiology-based treatment algorithm. Orthop J Sports Med. 2016;4(11):2325967116670635. [53] APPETECCHIA F, CONSALVI S, BERRINO E, et al. A Novel class of dual-acting DCH-CORMs counteracts oxidative stress-induced inflammation in human primary tenocytes. Antioxidants (Basel). 2021;10(11):1828. [54] WUNDERLI SL, BLACHE U, BERETTA PICCOLI A, et al. Tendon response to matrix unloading is determined by the patho-physiological niche. Matrix Biol. 2020;89:11-26. [55] CHEN Q, ZHOU J, ZHANG B, et al. Cyclic stretching exacerbates tendinitis by enhancing NLRP3 inflammasome activity via F-actin depolymerization. Inflammation. 2018;41(5):1731-1743. [56] WANG F, MURRELL GA, WANG MX. Oxidative stress-induced c-Jun N-terminal kinase (JNK) activation in tendon cells upregulates MMP1 mRNA and protein expression. J Orthop Res. 2007;25(3):378-389. [57] DEL BUONO A, OLIVA F, OSTI L, et al. Metalloproteases and tendinopathy. Muscles Ligaments Tendons J. 2013;3(1):51-57. [58] DONG SQ, XU HZ, XIA XB, et al. Activation of the ERK 1/2 and STAT3 signaling pathways is required for 661W cell survival following oxidant injury. Int J Ophthalmol. 2012;5(2):138-142. [59] PAXTON JZ, HAGERTY P, ANDRICK JJ, et al. Optimizing an intermittent stretch paradigm using ERK1/2 phosphorylation results in increased collagen synthesis in engineered ligaments. Tissue Eng Part A. 2012;18(3-4):277-284. [60] JOHNSON G L, LAPADAT R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600): 1911-1912. [61] POULSEN RC, CARR AJ, HULLEY PA. Protection against glucocorticoid-induced damage in human tenocytes by modulation of ERK, Akt, and forkhead signaling. Endocrinology. 2011;152(2):503-514. [62] MARTIN P, POGNONEC P. ERK and cell death: cadmium toxicity, sustained ERK activation and cell death. FEBS J. 2010;277(1):39-46. [63] SUGIURA R, SATOH R, TAKASAKI T. ERK: A double-edged sword in cancer. ERK-dependent apoptosis as a potential therapeutic strategy for cancer. Cells. 2021;10(10):2509. [64] VENTURA JJ, HÜBNER A, ZHANG C, et al. Chemical genetic analysis of the time course of signal transduction by JNK. Mol Cell. 2006;21(5):701-710. [65] JIAO X, ZHANG Y, LI W, et al. HIF-1αinhibition attenuates severity of Achilles tendinopathy by blocking NF-κB and MAPK pathways. Int Immunopharmacol. 2022;106:108543. [66] KIM EK, CHOI EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802(4):396-405. [67] KESAVARDHANA S, MALIREDDI RKS, KANNEGANTI TD. Caspases in cell death, inflammation, and gasdermin-induced pyroptosis. Ann Rev Immunol. 2020;38:567-595. [68] GREEN DR. Apoptotic pathways: the roads to ruin. Cell. 1998;94(6):695-698. [69] YUAN J, WANG MX, MURRELL GA. Cell death and tendinopathy. Clin Sports Med. 2003;22(4):693-701. [70] KUDRYAVTSEVA AV, KRASNOV GS, DMITRIEV AA, et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget. 2016; 7(29):44879-44905. [71] XIAO M, ZHONG H, XIA L, et al. Pathophysiology of mitochondrial lipid oxidation: role of 4-hydroxynonenal (4-HNE) and other bioactive lipids in mitochondria. Free Radic Biol Med. 2017;111:316-327. [72] XING YQ, LI A, YANG Y, et al. The regulation of FOXO1 and its role in disease progression. Life Sci. 2018;193:124-131. [73] WU B, CHEN J, DELA ROSA T, et al. Cellular response and extracellular matrix breakdown in rotator cuff tendon rupture. Arch Orthop Trauma Surg. 2011;131(3):405-411. [74] BIASIZZO M, KOPITAR-JERALA N. Interplay between NLRP3 inflammasome and autophagy. Front Immunol. 2020;11:591803. [75] LOIACONO C, PALERMI S, MASSA B, et al. Tendinopathy: pathophysiology, therapeutic options, and role of nutraceutics. a narrative literature review. Medicina (Kaunas). 2019;55(8):447. [76] CILLI F, KHAN M, FU F, et al. Prostaglandin E2 affects proliferation and collagen synthesis by human patellar tendon fibroblasts. Clin J Sport Med. 2004;14(4):232-236. [77] ZHANG J, WANG JH. Production of PGE(2) increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J Orthop Res. 2010;28(2):198-203. [78] MILLAR NL, MURRELL GA, MCINNES IB. Inflammatory mechanisms in tendinopathy - towards translation. Nat Rev Rheumatol. 2017;13(2):110-122. [79] SCHULZE-TANZIL G, AL-SADI O, WIEGAND E, et al. The role of pro-inflammatory and immunoregulatory cytokines in tendon healing and rupture: new insights. Scand J Med Sci Sports. 2011;21(3):337-351. [80] JOHN T, LODKA D, KOHL B, et al. Effect of pro-inflammatory and immunoregulatory cytokines on human tenocytes. J Orthop Res. 2010; 28(8):1071-1077. [81] BACKMAN LJ, ERIKSSON DE, DANIELSON P. Substance P reduces TNF-α-induced apoptosis in human tenocytes through NK-1 receptor stimulation. Br J Sports Med. 2014;48(19):1414-1420. [82] ACKERMANN PW, DOMEIJ-ARVERUD E, LECLERC P, et al. Anti-inflammatory cytokine profile in early human tendon repair. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1801-1806. [83] LIN TW, CARDENAS L, GLASER DL, et al. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J Biomech. 2006;39(1):61-69. [84] PALMER RM, ASHTON DS, MONCADA S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333(6174):664-666. [85] BOKHARI AR, MURRELL GA. The role of nitric oxide in tendon healing. J Shoulder Elbow Surg. 2012;21(2):238-244. [86] MURRELL GA. Using nitric oxide to treat tendinopathy. Br J Sports Med. 2007;41(4):227-231. [87] GRZANNA MW, AU RY, AU AY, et al. Avocado/Soybean unsaponifiables, glucosamine and chondroitin sulfate combination inhibits proinflammatory COX-2 expression and prostaglandin E2 production in tendon-derived cells. J Med Food. 2020;23(2):139-146. [88] HYBERTSON BM, GAO B, BOSE SK, et al. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32(4-6):234-246. [89] LIGUORI I, RUSSO G, CURCIO F, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757-772. [90] YUAN T, QIAN H, YU X, et al. Proteomic analysis reveals rotator cuff injury caused by oxidative stress. Ther Adv Chronic Dis. 2021;12: 2040622320987057. |
| [1] | Yu Weijie, Liu Aifeng, Chen Jixin, Guo Tianci, Jia Yizhen, Feng Huichuan, Yang Jialin. Advantages and application strategies of machine learning in diagnosis and treatment of lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1426-1435. |
| [2] | Lin Zeyu, Xu Lin. Research progress in gout-induced bone destruction mechanism [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1295-1300. |
| [3] | Ma Shuwei, He Sheng, Han Bing, Zhang Liaoyun. Exosomes derived from mesenchymal stem cells in treatment of animals with acute liver failure: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1137-1142. |
| [4] | Zhang Kefan, Shi Hui. Research status and application prospect of cytokine therapy for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 961-967. |
| [5] | Li Jiaqi, Huang Yuanli, Li Yan, Wang Chunren, Han Qianqian. Mechanism and influencing factors in molecular weight degradation of non-cross-linked hyaluronic acid [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 747-752. |
| [6] | Xu Rong, Wang Haojie, Geng Mengxiang, Meng Kai, Wang Hui, Zhang Keqin, Zhao Huijing. Research advance in preparation and functional modification of porous polytetrafluoroethylene artificial blood vessels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 759-765. |
| [7] | Chen Xiaofang, Zheng Guoshuang, Li Maoyuan, Yu Weiting. Preparation and application of injectable sodium alginate hydrogels [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 789-794. |
| [8] | Liu Chuang, Shan Shuo, Yu Tengbo, Zhou Huan, Yang Lei. Advantages, discomfort and challenges of clinical application of orthopedic hemostatic materials [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 795-803. |
| [9] | Jiang Zihao, Wang Guanglan, Chen Peng, Sun Xianghong, Wang Ting, Jia Shaohui, Zheng Cheng. Effect of eccentric training combined with different frequency whole body vibration training on patellar tendinopathy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 493-498. |
| [10] | Zhang Ming, Wang Bin, Jia Fan, Chen Jie, Tang Wei. Application of brain-computer interface technology based on electroencephalogram in upper limb motor function rehabilitation of stroke patients [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 581-586. |
| [11] | He Yuanjie, Chen Yuheng, Zhao Yongchao, Wang Zhenglong. Progress in epigenetic regulation of vascular smooth muscle cell remodeling in the occurrence and development of aortic aneurysms [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 602-608. |
| [12] | Ma Sicong, Chen Jing, Li Yunqing. Functions and roles of connective tissue growth factor in nervous systems [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 615-620. |
| [13] | Yan Binghan, Li Zhichao, Su Hui, Xue Haipeng, Xu Zhanwang, Tan Guoqing. Mechanisms of traditional Chinese medicine monomers in the treatment of osteoarthritis by targeting autophagy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 627-632. |
| [14] | Wang Xinyi, Xie Xianrui, Chen Yujie, Wang Xiaoyu, Xu Xiaoqing, Shen Yihong, Mo Xiumei. Electrospun nanofiber scaffolds for soft and hard tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 426-432. |
| [15] | Long Jundong, Shi Yehong, Wang Cheng, Chen Shijiu. Effects of different freezing techniques on the rejection of allogeneic vascular transplantation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(3): 433-438. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||