Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (19): 3076-3083.doi: 10.12307/2024.185
Previous Articles Next Articles
Notch signaling pathway regulates proliferation and differentiation of mesenchymal stem cells
Wang Xuesong1, 2, Zhou Lin1, 3, 4, 5, Li Lincai1, 3, 4, 5, Zou Zhengwei1, 3, 4, 5, Tang Xingkun1, 2, Lu Wenming1, 2, Chen Wenjie2, Wang Yue6, Ye Junsong1, 3, 4, 5
- 1 Subcenter for Stem Cell Clinical Translation, First Affiliated Hospital of Gannan Medical University, Ganzhou 341000, Jiangxi Province, China; 2 School of Rehabilitation Medicine, Gannan Medical University, Ganzhou 341000, Jiangxi Province, China; 3 Ganzhou Key Laboratory of Stem Cell and Regenerative Medicine, Ganzhou 341000, Jiangxi Province, China; 4 Key Laboratory of Medical Tissue Engineering Materials and Biological Manufacturing of Jiangxi Province,Gannan Medical University, Ganzhou 341000, Jiangxi Province, China; 5 Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases, Ministry of Education, Gannan Medical University, Ganzhou 341000, Jiangxi Province, China; 6 College of Nursing, Gannan Medical University, Ganzhou 341000, Jiangxi Province, China
-
Received:2023-06-17Accepted:2023-07-29Online:2024-07-08Published:2023-09-26 -
Contact:Ye Junsong, PhD, Associate Professor, Subcenter for Stem Cell Clinical Translation, First Affiliated Hospital of Gannan Medical University, Ganzhou 341000, Jiangxi Province, China; Ganzhou Key Laboratory of Stem Cell and Regenerative Medicine, Ganzhou 341000, Jiangxi Province, China; Key Laboratory of Medical Tissue Engineering Materials and Biological Manufacturing of Jiangxi Province, Gannan Medical University, Ganzhou 341000, Jiangxi Province, China; Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases, Ministry of Education, Gannan Medical University, Ganzhou 341000, Jiangxi Province, China -
About author:Wang Xuesong, Master candidate, Subcenter for Stem Cell Clinical Translation, First Affiliated Hospital of Gannan Medical University, Ganzhou 341000, Jiangxi Province, China; School of Rehabilitation Medicine, Gannan Medical University, Ganzhou 341000, Jiangxi Province, China -
Supported by:National Natural Science Foundation of China, No. 32060232 (to YJS); Natural Science Foundation of Jiangxi Province, No. 20212BAB206057 (to YJS); Science and Technology Project of Jiangxi Provincial Health Commission, No. 20191079 (to YJS)
CLC Number:
Cite this article
Wang Xuesong, Zhou Lin, Li Lincai, Zou Zhengwei, Tang Xingkun, Lu Wenming, Chen Wenjie, Wang Yue, Ye Junsong. Notch signaling pathway regulates proliferation and differentiation of mesenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 3076-3083.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
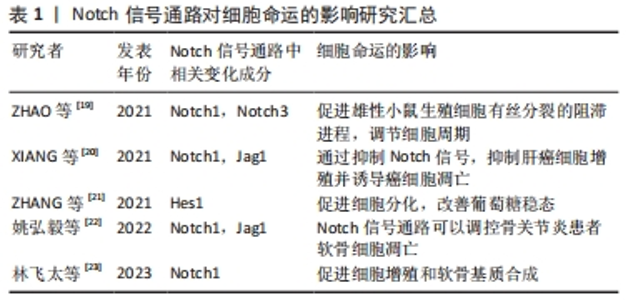
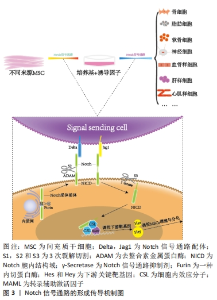
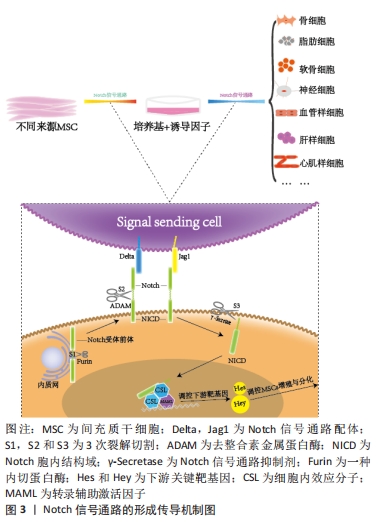
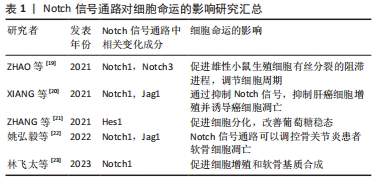
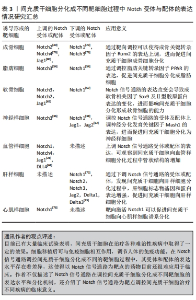
2.1 Notch信号通路的构成与激活 Notch信号通路是进化保守的信号通路。由G蛋白偶联受体和酶联型受体介导的经典信号通路[8-9],在膜受体和效应因子之间有多个中间体,与经典信号通路不同的是Notch信号通路在传导的过程中没有中间体介导,而是受体在3次裂解切割后直接传递到细胞核中[10]。完整的Notch信号通路由Notch受体、Notch配体、相关酶、转录因子(CBF-1/suppressor of hairless/Lag1,CSL)、调节因子和Notch信号下游分子组成[11]。在哺乳动物中由不同基因编码的Notch的4个受体分别为(Notch1-4)[12],5个经典配体分别是DLL1(Delta-like1,DLL1),DLL3,DLL4,Jag1(jagged1,Jag1)和Jag2[13]。 Notch受体是一种单次跨膜异源二聚体,由Notch细胞外结构域、跨膜区和Notch细胞内结构域(Notch intracellular domain,NICD)3部分组成。细胞外结构域(N端)包含29-36个表皮生长因子样重复序列(epidermal growth factor-like repeats,EGF-R)和一个负性调控区(negative control region,NRR)[14]。NRR结构域由3个富含半胱氨酸的Lin12-Notch重复序列(Lin12-Notch repeats,LNR)和对切割位点至关重要的异源二聚体构成,在没有配体的情况下NRR可以阻止受体激活[15]。经典Notch信号通路中Notch受体在内质网中合成后,在高尔基体中被furin样蛋白酶切割成异源二聚体(S1切割)并运送到细胞膜[16],Notch信号受体与相邻细胞的Notch配体结合。在Neur或Mib泛素化后NRR结构域被扩展,Notch受体细胞外结构域中的去整合素金属蛋白酶(A disintegrin and metalloproteinase,ADAM)酶切已经暴露的S2位点。S2位点被切割后Notch受体剩余部分称为Notch胞外截短体(Notch extracellular truncation,NEXT),NEXT可以进一步被细胞膜上的γ-分泌酶在跨膜区域的(S3)位点处剪切成NICD,随后NICD进入细胞质或转移到细胞核,与其他信号通路相互作用。进入细胞核的NICD与CSL结合进而调控基因的转录[17]。NICD与CSL结合后可以改变CSL的构象,使其由转录抑制因子转变为转录激活因子并募集转录辅助激活因子(mastermind-like,MAML),形成NICD/MAML/CSL三元复合物,进而启动下游相关基因Hes,Hey的表达[18]。Notch信号通路可以决定细胞命运[19-23],相关研究见表1。"
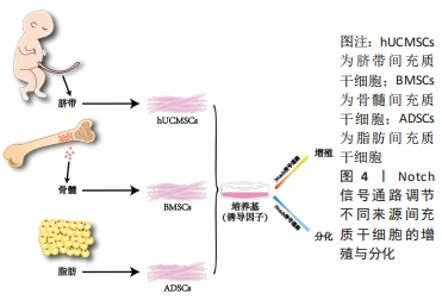
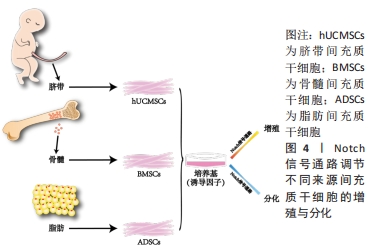
2.2 Notch信号通路调节不同来源间充质干细胞的增殖与分化 间充质干细胞的来源种类众多,在人体内广泛分布,可以从成人的骨髓、内脏和脂肪等处获得,研究表明骨髓来源的间充质干细胞(bone mesenchymal stem cell,BMSC)临床使用最普遍,目前诸多文献报道骨髓间充质干细胞被更好的记录用于临床试验中且数据相对完善;脂肪来源的间充质干细胞(adipose mesenchymal stem cell,ADSC)的获得方式对比于骨髓间充质干细胞的获得方式对人的侵入性更小,安全性更高并可以大量获得[25],而被广泛运用;新生儿的脐带也是间充质干细胞来源的重要途径[26],婴儿脐带来源的间充质干细胞(human umbilical cord mesenchymal stem cell,hUCMSC)向脂肪定向分化能力较弱[27],但是对比于骨髓间充质干细胞其形成成纤维细胞集落的能力明显更高且增殖能力更强。不同来源的间充质干细胞在增殖和分化能力上因所处不同微环境而有其独有的特性,但同样都受到Notch信号通路的调节。Notch信号通路通过受体与配体的结合后,经过-分泌酶剪切,释放NICD进入细胞质并移位至细胞核内。在细胞核内,NICD与其他转录因子结合形成转录激活复合物,进而激活下游基因Hes及Hey的表达,发挥调控骨髓、脂肪和脐带间充质干细胞增殖与分化的作用,见图4。"
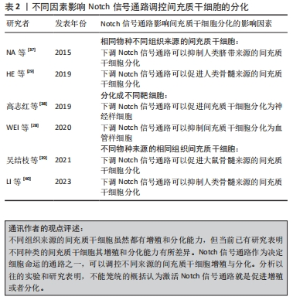
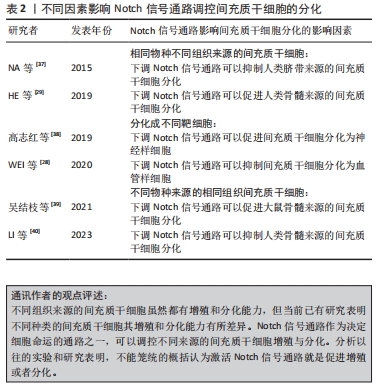
骨髓间充质干细胞的增殖与分化受到Notch信号通路的调节,临床上,骨髓间充质干细胞同时也被用作种子细胞定向分化为不同的靶细胞进行多种疾病治疗。研究发现通过非典型趋化因子CXCR7上调Notch1受体的同源配体Jag1使Notch信号通路激活传导,可以在体内或体外显著促进骨髓间充质干细胞向血管样细胞分化[28]。另一项研究表明通过siRNA干扰Notch1受体或者γ-分泌酶抑制剂(DAPT)抑制Notch信号可以降低骨髓间充质干细胞的增殖[29]。同样Notch信号通路也可以调节脂肪间充质干细胞的生物活性,中药杜仲籽总苷通过抑制Notch信号通路的Hes1和Hey1表达进而促进了脂肪间充质干细胞的成骨分化[30]。脂肪间充质干细胞的增殖能力比骨髓间充质干细胞的增殖能力更强。分别激活或抑制Notch信号通路并对脂肪间充质干细胞增殖进行评估发现,可以利用内源性Notch信号调节脂肪间充质干细胞的增殖,通过慢病毒转染Notch1细胞内结构域可以部分恢复通过DAPT所抑制脂肪间充质干细胞增殖的活性,脂肪间充质干细胞的增殖可能部分依赖于丝裂原活化蛋白激酶和下游Notch信号元件之间的串扰[31]。同脂肪间充质干细胞一样,Notch1基因的表达上调能显著促进脐带间充质干细胞的增殖[32]。实验发现人参皂苷Rg1通过抑制Notch1的表达能增强脐带间充质干细胞向神经干细胞诱导定向分化的能力[33]。 通过调节间充质干细胞的增殖,保证提供具有足够数量的间充质干细胞移植是其治疗各种疾病的基础。调控Notch信号通路的表达水平是促使间充质干细胞增殖的有效手段之一。Notch信号通路的表达在不同来源的间充质干细胞增殖过程中的调控有所差异。在未添加诱导剂的情况下,骨髓间充质干细胞和脐带间充质干细胞的情况大致相同,即激活Notch信号可以促进增殖[34],反之则抑制。DENG等[35]采用Notch信号通路上游的血管内皮生长因子(vascular endothelial growth factor,VEGF)对骨髓间充质干细胞进行干预发现,Notch信号通路的激活能改善血管再生障碍性贫血的间充质干细胞增殖降低现象,而Notch信号通路的抑制则会加剧血管再生障碍性贫血患者的间充质干细胞增殖抑制。但也有研究发现在脂肪间充质干细胞增殖过程中激活Notch信号通路会抑制脂肪间充质干细胞的增殖,反之则促进增殖[36]。 Notch信号通路如何调控间充质干细胞的增殖与分化不是简单地用其高表达促进干细胞增殖或低表达促进干细胞分化就能概括,在间充质干细胞定向分化的过程中,有诸多原因影响Notch信号受体或者配体的表达,甚至导致实验结论截然相反[37-40],见表2。造成这种情况的原因可能与间充质干细胞来源于不同组织及不同物种有关,也有可能与间充质干细胞被诱导成不同的靶细胞有关。"
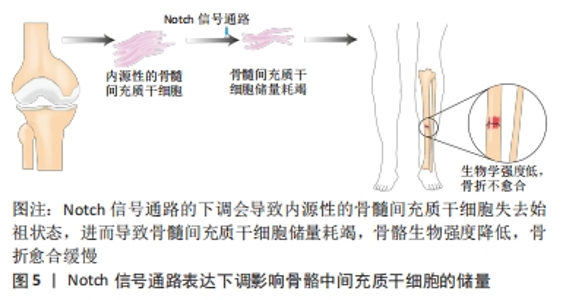
2.3 Notch信号通路在调控间充质干细胞分化为不同靶细胞中的表达及临床意义 间充质干细胞除了基本的三系分化外,还能定向分化成许多特定的靶细胞,比如间充质干细胞可以分化为肝样细胞、心肌细胞或神经细胞等,其分化潜能的差异主要依赖于培养基中诱导因子的种类。由于目前临床上对于间充质干细胞治疗各种难治性疾病的需要,检测和调控间充质干细胞增殖和分化至关重要。Notch信号通路通过相邻细胞之间的作用传递信息,Notch受体与配体Jag或DLL结合后被蛋白酶裂解,然后经γ-分泌酶在跨膜结构域裂解,生成NICD并易位至细胞核,与转录因子结合后通过下游靶基因Hes,Hey在分化成不同的靶细胞过程中调控分化的具体进程。但目前大部分研究都集中在Notch信号通路调控间充质干细胞分化成骨、软骨和脂肪中的影响,却少有在其分化成其他靶细胞时对其自身信号通路表达的探究与总结。文章总结后发现,Notch受体及其配体的表达水平在间充质干细胞分化成不同的靶细胞中是不同的,甚至结论完全相悖,如抑制Notch信号通路可以促进间充质干细胞向骨细胞分化,但是在间充质干细胞向血管样细胞分化的过程中抑制Notch信号通路的表达反而会抑制间充质干细胞的分化。 2.3.1 Notch信号通路调控间充质干细胞向骨细胞分化 间充质干细胞成骨分化是目前研究间充质干细胞定向分化最多的方向之一。从表观遗传学角度讲,骨髓间充质干细胞分化为成骨细胞的潜能比其他来源的间充质干细胞更高[41]。分析骨髓间充质干细胞分化为骨细胞过程中Notch受体及其配体的表达情况发现,Notch信号通路中受体与配体的表达水平对骨髓间充质干细胞分化的影响很大。通过siRNA干预Notch1受体,以及用Notch信号通路抑制剂DAPT对Notch信号通路进行干预发现Notch1的表达降低可以促进骨髓间充质干细胞的分化[29],表明Notch1可以抑制骨髓间充质干细胞向骨细胞分化。Notch1的激活可能导致成骨关键转录因子Runx2的表达以及转录活性受抑制,但是调控间充质干细胞成骨分化不仅仅只有Notch1受体,还可通过激活Notch2、Notch4、Jag1以及抑制Notch3增强间充质干细胞成骨分化。总之,在骨髓间充质干细胞向骨细胞分化的过程中,Notch信号通路其受体与配体发挥了重要的调节作用。 事实上,一直以来对于Notch调控间充质干细胞向骨细胞分化众说纷纭,有研究者认为Notch信号通路的激活抑制了间充质干细胞成骨细胞的分化,因为NICD或Hey1的物理相互作用抑制Runx2活性,且在缺氧环境下通过激活Notch信号通路的确可以抑制骨髓间充质干细胞向成骨细胞分化[42]。但是也有研究者发现固定化的Jag1模拟配体通过NICD的上调激活Notch信号转导,可以导致间充质干细胞成骨分化增强[43],这些差异的结果可能与间充质干细胞的供体来源不同物种有关[44-45]。 临床上有10%-20%的骨折患者会出现骨折不愈合,最终导致长期甚至终身残疾。数据表明在骨折修复期间骨骼愈合除通过骨周围和软组织内骨祖细胞的增殖和分化外,骨髓间充质干细胞也同样发挥着关键作用,临床上患者骨髓间充质干细胞的减少与骨折不愈合息息相关[46]。有研究证实,在骨祖细胞中特异性下调Notch信号会导致骨量早期增加,骨髓间充质干细胞细胞群失去祖始状态,最终骨髓间充质干细胞储量耗尽[47],生物力学强度降低,骨折不易愈合,见图5。在骨骼愈合期间Notch1的下调会导致软骨的面积增大,骨组织减少[48],而配体Jag1的下调则会导致骨量减少增加骨折风险[49],探究Notch信号通路调控间充质干细胞增殖与分化的具体机制,进而帮助骨折患者在骨折愈合期间促进骨组织的形成有至关重要的意义。"
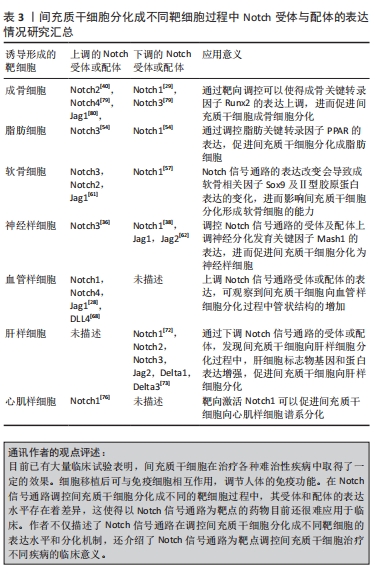
2.3.2 Notch信号通路调控间充质干细胞向脂肪细胞分化 Notch信号通路的表达水平同样影响间充质干细胞成脂分化,但是Notch信号通路在调控间充质干细胞向脂肪定向分化过程中的作用一直都争议不断,原因在于单个Notch受体的作用会因为实验条件而出现较大的差异。尽管研究发现通过许多方法抑制Notch信号通路可以增强间充质干细胞的成脂分化[50-53],但是Notch单个受体对成脂的作用却少有分析。通过比较Notch1和Notch3在体外诱导成脂期间的表达发现,在脂肪生成的早期阶段Notch3被上调,Notch1下降,而成脂诱导3 d后Notch1的表达上调,Notch3下降[54]。因此,在间充质干细胞成脂分化的早期Notch3的表达是必要的,在此之后抑制Notch1的表达可以促进脂肪的形成。 目前,由于脂肪细胞在人体内占有一定的比例,且体脂率的变化在一定程度上对人体健康的影响并不明显,故调控间充质干细胞向脂肪细胞分化的临床应用较少。但有些疾病同样需要调控间充质干细胞向脂肪细胞转换。过量或不足的脂肪细胞是某些病理状况的一种特征,比如再生障碍性贫血症患者骨和骨髓脂肪之间的稳态紊乱。有研究者提出,在骨髓中间充质干细胞被默认编程分化为脂肪细胞,并且人们随着衰老成骨细胞会受到损害,导致脂肪生成过多[55],寻找调控间充质干细胞脂肪分化的策略成为该领域的一个重要问题。研究表明,可以通过下调Notch信号通路的表达促进由PTEN-P13K/Akt/mTOR通路介导的人骨髓间充质干细胞向脂肪分化[50],Notch信号传导可能作为未来临床上调节间充质干细胞向脂肪细胞分化进而治疗相关疾病的新靶点。 2.3.3 Notch信号通路调控间充质干细胞向软骨细胞分化 关节软骨由软骨细胞和细胞外基质组成,成软骨细胞分化是间充质干细胞三系分化中的重要能力之一,Notch信号通路的表达可以影响间充质干细胞的软骨分化[56]。王勇超等[57]通过过表达一种长链非编码RNA显著降低Notch1和Hes1的蛋白表达,进而促进间充质干细胞向软骨细胞分化。可溶性Jag1短暂下调Notch信号也可以用于提高间充质干细胞成软骨分化能力[58],这可能是通过下调Notch信号可以促进软骨转录因子Sox9和胶原蛋白Ⅱ型启动子的表达[59-60]。但另一种观点认为,Notch信号通路的表达上调促进了间充质干细胞的软骨分化,JEONG等[61]评估了间充质干细胞向软骨分化前期Notch信号通路各受体以及配体表达,发现只有Notch3在分化的前4 d高表达,其他受体并无变化,5个配体中只有Jag1在软骨分化过程中被逐渐诱导,通过DAPT与原代间充质干细胞共培养发现DAPT可以抑制间充质干细胞向软骨分化,造成以上差异的可能是因为Notch信号表达的不同时期有关,在分化开始后需关闭Notch信号,但是在软骨分化的初始期Notch信号的临时激活也很重要,而持续激活的Notch信号通路反而会通过中断Sox9和胶原蛋白Ⅱ型启动子的表达,进而抑制间充质干细胞成软骨分化[60-61]。 关节损伤以及关节退行性变一直以来困扰了许多的患者,由于人体自身的软骨细胞有限以及软骨修复的局限性,亟需找到一种大量高效修复的方法。目前基于组织工程的再生策略,在临床上采用以Notch信号通路为靶点调控间充质干细胞修复受损关节软骨是非常有前景的策略。 2.3.4 Notch信号通路调控间充质干细胞向神经样细胞分化 神经细胞属于终末期细胞,这是一种连续不断死亡且不再增殖的过程,解决神经损伤相关疾病一直以来都备受现代医学的重视。间充质干细胞可以分化成神经样细胞,Notch信号通路的表达水平可以调控此过程。实验发现,在间充质干细胞向神经元样细胞诱导的7 d后,Jag1的表达显著降低,在诱导的第14天时Jag1,Jag2和Hes1的表达都有显著的降低[62],下调Notch1的表达可以促进间充质干细胞向神经样细胞分化[38]。但是王志敏[36]通过研究Notch信号通路对间充质干细胞向神经样细胞分化的影响,发现激活Notch信号通路反而会促进间充质干细胞向神经样细胞分化,而抑制Notch信号通路则会起到反作用。以上实验分别采用了不同物种及不同组织来源的间充质干细胞,造成以上差异的原因可能是间充质干细胞的来源不同导致。 近年来,运用间充质干细胞治疗神经相关疾病取得了一定的成果。动物实验与临床治疗均证明间充质干细胞治疗神经系统疾病的有效性和巨大的应用前景[63],通过调控Notch信号通路的表达,不仅可以减轻神经元的损伤还可以增强间充质干细胞向神经细胞样细胞的分化能力[64-65]。 2.3.5 Notch信号通路调控间充质干细胞向血管样细胞分化 近年来间充质干细胞向血管样细胞分化的研究逐渐成为干细胞领域的焦点之一,经证实间充质干细胞不但有助于推动血管成熟和调节血管功能[66],而且能够在诱导血管生成因子的刺激下分化为血管内皮谱系细胞,并进一步形成毛细血管样网络结构[67]。通过血管内皮诱导培养基对间充质干细胞向血管内皮细胞进行诱导,发现Notch1,Notch4,DLL4,Hes1和Hes5等相关因子的表达随着时间的增加而增加,加入DAPT进行干预后发现管状结构对比正常诱导组显著减少[68]。Notch信号通路在间充质干细胞向血管进行分化过程中起促进作用,其通过与血管内皮生长因子信号互相作用一起调节血管的生成[28]。不但如此,通过过表达Notch1还可以提高间充质干细胞分泌的细胞外囊泡,并进一步促进血管的生成[69]。 间充质干细胞的促进血管新生作用在治疗缺血性脑卒中效果良好,但是实际上单纯间充质干细胞的移植治疗效果与预期效果相比有差距,主要是因为干细胞向梗死区迁移能力有限。Notch信号通路可以促进干细胞向梗死灶区域迁移,进而显著提高促进血管的生成效率[70]。以Notch信号通路为靶点,未来在临床上治疗血管相关疾病可能会产生一种新的治疗思路。 2.3.6 Notch信号通路调控间充质干细胞向肝样细胞分化 间充质干细胞还可以分化为肝样细胞,这对目前以肝移植做为终末期肝病唯一治疗手段的患者来讲是一大福音。以Notch信号通路的受体或配体作为靶点,调控间充质干细胞分化是目前研究的热点。Numb是一种进化高度保守的基因,其对Notch信号起着负调节的作用,通过调控Numb水平进而影响Notch的表达,可以决定间充质干细胞分化为肝样细胞或胆管样细胞,Notch信号的下调可以促进间充质干细胞向肝样细胞分化[71]。将含有肝细胞生长因子(hepatocyte grow factor,HGF)和成纤维生长因子4(fibroblast growth factor,FGF-4)的培养基诱导间充质干细胞向肝样细胞分化,并检测Notch信号通路受体及配体的表达情况发现,随着间充质干细胞的分化,对比于未加HGF和FGF-4的培养基的对照组,Notch1蛋白的表达逐渐降低,且若抑制Notch1的表达会进一步提高诱导的分化率[72]。通过诱导间充质干细胞向肝样细胞分化,第21天检测到细胞中的Notch1,Notch2,Notch3,Jag2,Delta1和Delta3明显低于第0,7,11天[73]。上调Jag1激活的Notch信号后间充质干细胞分化被阻滞,这是因为Notch信号的激活导致Hes1和Hey1的表达水平增高,进而抑制了间充质干细胞的白蛋白表达[73]。 目前已有许多国家开始在临床上尝试用间充质干细胞治疗肝脏相关疾病,并获得了不错的效果。在2012年,有研究对脐带来源的间充质干细胞治疗乙型肝炎患者的效果进行了评估,结果显示间充质干细胞注射后降低了终末期肝病模型的评分[74]。不但如此,间充质干细胞治疗慢性肝衰竭、丙型肝炎病毒相关性肝硬化和酒精性肝硬化等肝脏相关疾病都有较好的疗效。而以Notch通路作为靶点,提高间充质干细胞在体内分化为肝样细胞的效率,可以提高间充质干细胞介导的治疗效果。 2.3.7 Notch信号通路调控间充质干细胞向心肌样细胞分化 近年来,间充质干细胞在临床上治疗心血管相关疾病的研究一直在进行,且取得了不错的结果[75]。临床研究表明,间充质干细胞有助于心肌梗死和扩张型心肌病等疾病后的心脏修复,间充质干细胞中的Notch1信号的表达水平在心肌细胞的形成过程中起着重要作用。Notch1是间充质干细胞心肌分化中主要表达的Notch受体,当间充质干细胞过量表达NICD具有活性的Notch1信号时,可以促进间充质干细胞分化成心肌样细胞和内皮细胞谱系[76-77],而且Notch1信号的激活还可以减轻缺血性损伤,减少心肌纤维化并改善心脏功能[78]。 不过值得注意的是,虽然临床试验均已证明骨髓、脂肪和脐带来源的间充质干细胞在治疗心血管疾病方面是可行的,但间充质干细胞的来源与其治疗潜力之间的差异性仍然不确定。因此,通过以Notch信号的受体或配体为靶点调控间充质干细胞[79-80],用于治疗心脏疾病在未来还需要大规模和精心设计的随机临床试验,进而去分析具体的治疗效果和作用机制。 文章总结了充质干细胞分化成不同靶细胞过程中Notch受体与配体的表达情况研究成果,见表3。"

2.4 Notch信号通路在不同种属间充质干细胞分化过程中的表达差异 尽管人来源的间充质干细胞和其他动物来源的间充质干细胞在表面标志物和三系分化能力等基本特征方面有着相似之处,但是已经有研究证明不同物种间间充质干细胞的增殖与分化能力有着明显的差异性[81]。 调控Notch信号通路作为一种影响间充质干细胞分化的手段,需要厘清Notch信号通路在不同物种之间的间充质干细胞分化过程中的表达情况。经典Notch激活通过2个相邻细胞的细胞表面Notch受体与配体的接触而激活,激活后被蛋白酶裂解,在此基础上γ-分泌酶在跨膜结构域进一步切割,生成NICD并易位至细胞核,与转录因子CSL结合后使其共阻遏物被去除,随后募集MAML形成三元转录复合物,激活下游碱性-螺旋-环-螺旋(basic-helix-loop-helix,bHLH)转录抑制因子家族成员转录,进而调控不同种属相同组织来源的间充质干细胞分化。以骨髓间充质干细胞成骨分化的实验举例,任锟等[82]研究证明中高浓度的水蛭素可以通过上调VEGF/Notch1信号通路抑制人骨髓间充质干细胞的凋亡,并上调Runx2等成骨早期分化标志物的表达,进而促进人骨髓间充质干细胞成骨分化。然而在小鼠来源的骨髓间充质干细胞分化成骨过程中,抑制剂DAPT导致Notch信号的瞬时抑制会暂时促进小鼠骨髓间充质干细胞分化并增加成骨细胞生成[83]。吴结枝等[39]通过药物调控Notch信号通路,上调Notch信号通路中的Notch1,Jag1和Hes的表达,发现可以促进大鼠来源的骨髓间充质干细胞成骨分化,这一点与人类骨髓间充质干细胞类似。 不同来源的间充质干细胞的差异性是研究干细胞靶向药物所必须考虑的问题,以同种异体的间充质干细胞和异种异体的间充质干细胞作为干细胞靶向治疗手段差别甚大,并且Notch信号通路在调控不同物种的间充质干细胞分化过程中受体和配体的表达有所不同[80]。"

| [1] SACHAN N, SHARMA V, MUTSUDDI M, et al. Notch signalling: multifaceted role in development and disease. FEBS J. 2023. doi: 10.1111/febs.16815. [2] HOANG DM, PHAM PT, BACH TQ, et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7(1):272. [3] 何龙, 云蔓, 吴薇薇,等. lncRNA GAS5调控Notch通路对炎症环境下牙髓干细胞成骨分化的影响[J]. 实用口腔医学杂志,2022,38(6):730-736. [4] YUAN M, HU X, YAO L, et al. Mesenchymal stem cell homing to improve therapeutic efficacy in liver disease. Stem Cell Res Ther. 2022; 13(1):179. [5] LEONG DJ, SUN HB. Mesenchymal stem cells in tendon repair and regeneration: basic understanding and translational challenges. Ann N Y Acad Sci. 2016;1383(1):88-96. [6] SOLTANI L, MAHDAVI AH. Role of signaling pathways during cardiomyocyte differentiation of mesenchymal stem cells. Cardiology. 2022;147(2):216-224. [7] WANG Y, ZHANG F, YAO B, et al. Notch4 participates in mesenchymal stem cell-induced differentiation in 3D-printed matrix and is implicated in eccrine sweat gland morphogenesis. Burns Trauma. 2023;11:tkad032. [8] CHEN Q, TESMER JJG. G protein-coupled receptor interactions with arrestins and GPCR kinases: the unresolved issue of signal bias. J Biol Chem. 2022;298(9):102279. [9] MAJUMDER S, CRABTREE JS, GOLDE TE, et al. Targeting Notch in oncology: the path forward. Nat Rev Drug Discov. 2021;20(2):125-144. [10] SIEBEL C, LENDAHL U. Notch signaling in development, tissue homeostasis, and disease. Physiol Rev. 2017;97(4):1235-1294. [11] ZHANG Z, WU W, SHAO Z. NOTCH signaling in osteosarcoma. Curr Issues Mol Biol. 2023;45(3):2266-83. [12] ZHOU B, LIN W, LONG Y, et al. Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct Target Ther. 2022;7(1):95. [13] HUANG Q, LI J, ZHENG J, et al. The carcinogenic role of the notch signaling pathway in the development of hepatocellular carcinoma. J Cancer. 2019; 10(6):1570-1579. [14] KOVALL RA, GEBELEIN B, SPRINZAK D, et al. The canonical notch signaling pathway: structural and biochemical insights into shape, sugar, and force. Dev Cell. 2017;41(3):228-241. [15] NOWELL CS, RADTKE F. Notch as a tumour suppressor. Nat Rev Cancer. 2017;17(3):145-159. [16] HU B, PHAN SH. Notch in fibrosis and as a target of anti-fibrotic therapy. Pharmacol Res. 2016;108:57-64. [17] SHIM YS, LEE HS, HWANG JS. Aberrant notch signaling pathway as a potential mechanism of central precocious puberty. Int J Mol Sci. 2022; 23(6):3332. [18] GUO M, NIU Y, XIE M, et al. Notch signaling, hypoxia, and cancer. Front Oncol. 2023;13:1078768. [19] ZHAO J, LU P, WAN C, et al. Cell-fate transition and determination analysis of mouse male germ cells throughout development. Nat Commun. 2021; 12(1):6839. [20] XIANG Z, MIAO Q, ZHANG J, et al. AB4 inhibits Notch signaling and promotes cancer cell apoptosis in liver cancer. Oncol Rep. 2021;45(6):112. [21] ZHANG X, TAO J, YU J, et al. Inhibition of Notch activity promotes pancreatic cytokeratin 5-positive cell differentiation to beta cells and improves glucose homeostasis following acute pancreatitis. Cell Death Dis. 2021;12(10):867. [22] 姚弘毅,李燕霞,梅其杰,等.O3抑制Notch信号通路减少OA大鼠软骨细胞凋亡的实验研究[J].生物骨科材料与临床研究,2022,19(5):55-60. [23] 林飞太,林煜,林钡,等.Notch信号通路对骨关节炎软骨细胞增殖和软骨基质合成的作用研究[J].中国合理用药探索,2023,20(2):110-118. [24] 韦沅汛,陈锋,林宗汉,等.Notch信号通路与骨质疏松症及中医药防治[J].中国组织工程研究,2024,28(4):587-593. [25] POMATTO M, GAI C, NEGRO F, et al. Differential therapeutic effect of extracellular vesicles derived by bone marrow and adipose mesenchymal stem cells on wound healing of diabetic ulcers and correlation to their cargoes. Int J Mol Sci. 2021;22(8):3851. [26] NAJI A, EITOKU M, FAVIER B, et al. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76(17):3323-3348. [27] JING Y, ZHOU J, GUO F, et al. Betaine regulates adipogenic and osteogenic differentiation of hAD-MSCs. Mol Biol Rep. 2023;50(6):5081-5089. [28] WEI ST, HUANG YC, HSIEH ML, et al. Atypical chemokine receptor ACKR3/CXCR7 controls postnatal vasculogenesis and arterial specification by mesenchymal stem cells via Notch signaling. Cell Death Dis. 2020;11(5):307. [29] HE Y, ZOU L. Notch-1 inhibition reduces proliferation and promotes osteogenic differentiation of bone marrow mesenchymal stem cells. Exp Ther Med. 2019;18(3):1884-1890. [30] ZHOU Y H, XIE Q. Total glycosides from Eucommia ulmoides seed promoted osteogenic differentiation of adipose-derived mesenchymal stem cells and bone formation in ovariectomized rats through regulating Notch signaling pathway. J Orthop Surg Res. 2021;16(1):660. [31] LOUGH DM, CHAMBERS C, GERMANN G, et al. Regulation of ADSC osteoinductive potential using notch pathway inhibition and gene rescue: a potential on/off switch for clinical applications in bone formation and reconstructive efforts. Plast Reconstr Surg. 2016;138(4):642e-652e. [32] 常运光.Notch1转染人脐带间充质干细胞治疗SD大鼠缺血性脑血管病的实验研究 [D].新乡:新乡医学院,2017. [33] XIAO L, WANG M, ZOU K, et al. Effects of ginsenoside Rg1 on proliferation and directed differentiation of human umbilical cord mesenchymal stem cells into neural stem cells. Neuroreport. 2022;33(10):413-421. [34] 梁学奇,周慧,武杰,等.细粒棘球蚴促进骨髓间充质干细胞的增殖[J].中国组织工程研究,2022,26(19):3004-3010. [35] DENG S, XIANG JJ, SHEN YY, et al. Effects of VEGF-notch signaling pathway on proliferation and apoptosis of bone marrow MSC in patients with aplastic anemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2019;27(6):1925-1932. [36] 王志敏.miR-204-5p过表达调控脂肪间充质干细胞生长特性和分化潜能的分子机制研究 [D].呼和浩特:内蒙古大学,2021. [37] NA T, LIU J, ZHANG K, et al. The notch signaling regulates CD105 expression, osteogenic differentiation and immunomodulation of human umbilical cord mesenchymal stem cells. PLoS One. 2015;10(2):e0118168. [38] 高志红,左亚奇,李鹏涛,等.黄芪甲苷Ⅳ诱导小鼠骨髓间充质干细胞向神经细胞分化机制的研究[J].中药材,2019,42(7):1640-1645. [39] 吴结枝,鄢然,刘湘琳,等.壮骨止痛胶囊调控Notch通路对去卵巢大鼠Notch1、Jagged1、HES蛋白的影响[J].辽宁中医杂志,2021,48(12): 183-186, 227-228. [40] LI SD, XING W, WANG SC, et al. Fibulin2: a negative regulator of BMSC osteogenic differentiation in infected bone fracture healing. Exp Mol Med. 2023;55(2):443-456. [41] XU L, LIU Y, SUN Y, et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res Ther. 2017;8(1):275. [42] PFEIFFENBERGER M, DAMERAU A, LANG A, et al. Fracture healing research-shift towards in vitro modeling? Biomedicines. 2021;9(7):748. [43] DENG Y, LI R, WANG H, et al. Biomaterial-mediated presentation of jagged-1 mimetic ligand enhances cellular activation of notch signaling and bone regeneration. ACS Nano. 2022;16(1):1051-1062. [44] ZHAO Y, YANG R, BOUSRAOU Z, et al. Probing Notch1-Dll4 signaling in regulating osteogenic differentiation of human mesenchymal stem cells using single cell nanobiosensor. Sci Rep. 2022;12(1):10315. [45] KORNSUTHISOPON C, CHANSAENROJ A, MANOKAWINCHOKE J, et al. Non-canonical Wnt signaling participates in Jagged1-induced osteo/odontogenic differentiation in human dental pulp stem cells. Sci Rep. 2022;12(1):7583. [46] SEEBACH C, HENRICH D, TEWKSBURY R, et al. Number and proliferative capacity of human mesenchymal stem cells are modulated positively in multiple trauma patients and negatively in atrophic nonunions. Calcif Tissue Int. 2007;80(4):294-300. [47] WANG C, INZANA J A, MIRANDO A J, et al. NOTCH signaling in skeletal progenitors is critical for fracture repair. J Clin Invest. 2016;126(4):1471-1481. [48] NOVAK S, ROEDER E, SINDER BP, et al. Modulation of Notch1 signaling regulates bone fracture healing. J Orthop Res. 2020;38(11):2350-2361. [49] DISHOWITZ MI, ZHU F, SUNDARARAGHAVAN HG, et al. Jagged1 immobilization to an osteoconductive polymer activates the Notch signaling pathway and induces osteogenesis. J Biomed Mater Res A. 2014;102(5):1558-1567. [50] SONG BQ, CHI Y, LI X, et al. Inhibition of Notch signaling promotes the adipogenic differentiation of mesenchymal stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR pathway. Cell Physiol Biochem. 2015;36(5):1991-2002. [51] CHEUNG YM, CHOOK CY, YEUNG HW, et al. A wrong fate decision in adipose stem cells upon obesity. Cells. 2023;12(4):662. [52] WAN X, ZHU L, ZHAO L, et al. hPER3 promotes adipogenesis via hHSP90AA1-mediated inhibition of Notch1 pathway. Cell Death Dis. 2021;12(4):301. [53] ZHOU S, CHEN S, JIANG Q, et al. Determinants of stem cell lineage differentiation toward chondrogenesis versus adipogenesis. Cell Mol Life Sci. 2019;76(9):1653-1680. [54] LIU MC, LOGAN H, NEWMAN JJ. Distinct roles for Notch1 and Notch3 in human adipose-derived stem/stromal cell adipogenesis. Mol Biol Rep. 2020;47(11):8439-8450. [55] RIPPO MR, BABINI L, PRATTICHIZZO F, et al. Low FasL levels promote proliferation of human bone marrow-derived mesenchymal stem cells, higher levels inhibit their differentiation into adipocytes. Cell Death Dis. 2013;4(4):e594. [56] OLDERSHAW RA, HARDINGHAM TE. Notch signaling during chondrogenesis of human bone marrow stem cells. Bone. 2010;46(2):286-293. [57] 王勇超,粟钦,林昕,等.lncRNA GAS5调控Notch通路对骨髓间充质干细胞软骨分化的影响[J].山东医药,2021,61(35):26-30. [58] SUN J, LUO Z, WANG G, et al. Notch ligand Jagged1 promotes mesenchymal stromal cell-based cartilage repair. Exp Mol Med. 2018;50(9):1-10. [59] PAKVASA M, HARAVU P, BOACHIE-MENSAH M, et al. Notch signaling: Its essential roles in bone and craniofacial development. Genes Dis. 2021; 8(1):8-24. [60] ZIEBA JT, CHEN YT, LEE BH, et al. Notch signaling in skeletal development, homeostasis and pathogenesis. Biomolecules. 2020;10(2):332. [61] JEONG SY, HA J, LEE M, et al. Autocrine action of thrombospondin-2 determines the chondrogenic differentiation potential and suppresses hypertrophic maturation of human umbilical cord blood-derived mesenchymal stem cells. Stem Cells. 2015;33(11):3291-3303. [62] ZHANG J, YANG B, LUO L, et al. Effect of NTN and Lmx1α on the Notch signaling pathway during the differentiation of human bone marrow mesenchymal stem cells into dopaminergic neuron-like cells. Parkinsons Dis. 2021;2021:6676709. [63] 何正义,邹征伟,罗耀玲,等.脂肪间充质干细胞治疗脑瘫大鼠的作用机理研究[J]. 江西医药,2022,57(7):681-686, 689. [64] LONG Q, LUO Q, WANG K, et al. Mash1-dependent notch signaling pathway regulates GABAergic neuron-like differentiation from bone marrow-derived mesenchymal stem cells. Aging Dis. 2017;8(3):301-313. [65] 刘宗秀,张紫微,成媛,等.Notch信号通路对脑缺血大鼠神经干细胞移植后神经再生的影响[J].解剖学报,2020,51(1):15-20. [66] BELOGLAZOVA I, STEPANOVA V, ZUBKOVA E, et al. Mesenchymal stromal cells enhance self-assembly of a HUVEC tubular network through uPA-uPAR/VEGFR2/integrin/NOTCH crosstalk. Biochim Biophys Acta Mol Cell Res. 2022;1869(1):119157. [67] SHAO F, LIU R, TAN X, et al. MSC transplantation attenuates inflammation, prevents endothelial damage and enhances the angiogenic potency of endogenous MSCs in a model of pulmonary arterial hypertension. J Inflamm Res. 2022;15:2087-2101. [68] 秦浩添.镁离子通过Notch通路促进骨髓间充质干细胞成血管分化的实验研究 [D].北京:北京大学医学部,2021. [69] XUAN W, KHAN M, ASHRAF M. Extracellular vesicles from notch activated cardiac mesenchymal stem cells promote myocyte proliferation and neovasculogenesis. Front Cell Dev Biol. 2020;8:11. [70] SHI W L, ZHAO J, YUAN R, et al. Combination of ligusticum chuanxiong and radix paeonia promotes angiogenesis in ischemic myocardium through notch signalling and mobilization of stem cells. Evid Based Complement Alternat Med. 2019;2019:7912402. [71] XU Y N, XU W, ZHANG X, et al. BM-MSCs overexpressing the Numb enhance the therapeutic effect on cholestatic liver fibrosis by inhibiting the ductular reaction. Stem Cell Res Ther. 2023;14(1):45. [72] 徐洪雨,王瑞峰,李宝杰.Notch-1蛋白在诱导骨髓间充质干细胞向肝细胞分化中的表达[J].哈尔滨医科大学学报,2009,43(2):122-125. [73] KE Z, MAO X, LI S, et al. Dynamic expression characteristics of Notch signal in bone marrow-derived mesenchymal stem cells during the process of differentiation into hepatocytes. Tissue Cell. 2013;45(2):95-100. [74] SHI M, ZHANG Z, XU R, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1(10):725-731. [75] MATHIASEN AB, QAYYUM AA, JØRGENSEN E, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: final 4-year follow-up of the MSC-HF trial. Eur J Heart Fail. 2020;22(5):884-892. [76] DING R, JIANG X, HA Y, et al. Activation of Notch1 signalling promotes multi-lineage differentiation of c-Kit (POS)/NKX2.5 (POS) bone marrow stem cells: implication in stem cell translational medicine. Stem Cell Res Ther. 2015;6(1):91. [77] NIU P, ZHAO YQ, YU SQ, et al. Notch1 signaling enhances the differentiation of mesenchymal stem cells to cardiomyogenic cells. Nanosci Nanotechnol Lett. 2018;10(10):1380-1386. [78] KORE RA, JENKINS SV, JAMSHIDI-PARSIAN A, et al. Proteomic analysis of transcription factors involved in the alteration of ischemic mouse heart as modulated by MSC exosomes. Biochem Biophys Rep. 2023;34:101463. [79] BALLHAUSE TM, JIANG S, BARANOWSKY A, et al. Relevance of Notch signaling for bone metabolism and regeneration. Int J Mol Sci. 2021; 22(3):1325. [80] ZHU F, SWEETWYNE MT, HANKENSON KD. PKCδ is required for Jagged-1 induction of human mesenchymal stem cell osteogenic differentiation. Stem Cells. 2013;31(6):1181-1192. [81] VOGA M, ADAMIC N, VENGUST M, et al. Stem cells in veterinary medicine-current state and treatment options. Front Vet Sci. 2020;7:278. [82] 任锟,吉鸿涛,韩磊,等.水蛭素上调VEGF/Notch1信号通路促进人骨髓间充质干细胞成骨分化[J].中国骨质疏松杂志,2022,28(8):1154-1158. [83] WANG C, SHEN J, YUKATA K, et al. Transient gamma-secretase inhibition accelerates and enhances fracture repair likely via Notch signaling modulation. Bone. 2015;73:77-89. [84] LEE S, REMARK LH, JOSEPHSON AM, et al. Notch-Wnt signal crosstalk regulates proliferation and differentiation of osteoprogenitor cells during intramembranous bone healing. NPJ Regen Med. 2021;6(1):29. [85] PORCELLI L, MAZZOTTA A, GAROFOLI M, et al. Active notch protects MAPK activated melanoma cell lines from MEK inhibitor cobimetinib. Biomed Pharmacother. 2021;133:111006. [86] LUO K. Signaling cross talk between TGF-β/Smad and other signaling pathways. Cold Spring Harb Perspect Biol. 2017;9(1):a022137. [87] JACOBS CT, HUANG P. Complex crosstalk of Notch and Hedgehog signalling during the development of the central nervous system. Cell Mol Life Sci. 2021;78(2):635-644. |
| [1] | Yu Weijie, Liu Aifeng, Chen Jixin, Guo Tianci, Jia Yizhen, Feng Huichuan, Yang Jialin. Advantages and application strategies of machine learning in diagnosis and treatment of lumbar disc herniation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1426-1435. |
| [2] | Chen Kaijia, Liu Jingyun, Cao Ning, Sun Jianbo, Zhou Yan, Mei Jianguo, Ren Qiang. Application and prospect of tissue engineering in treatment of osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1450-1456. |
| [3] | Bai Chen, Yang Wenqian, Meng Zhichao, Wang Yuze. Strategies for repairing injured anterior cruciate ligament and promoting graft healing [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1457-1463. |
| [4] | Yang Yifeng, Ye Nan, Wang Lin, Guo Shuaicheng, Huang Jian. Signaling pathway of dexmedetomidine against ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1464-1469. |
| [5] | Lin Zeyu, Xu Lin. Research progress in gout-induced bone destruction mechanism [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1295-1300. |
| [6] | Yue Yun, Wang Peipei, Yuan Zhaohe, He Shengcun, Jia Xusheng, Liu Qian, Li Zhantao, Fu Huiling, Song Fei, Jia Menghui. Effects of croton cream on JNK/p38 MAPK signaling pathway and neuronal apoptosis in cerebral ischemia-reperfusion injury rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1186-1192. |
| [7] | Liu Xin, Hu Man, Zhao Wenjie, Zhang Yu, Meng Bo, Yang Sheng, Peng Qing, Zhang Liang, Wang Jingcheng. Cadmium promotes senescence of annulus fibrosus cells via activation of PI3K/Akt signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1217-1222. |
| [8] | Wei Juan, Li Ting, Huan Mengting, Xie Ying, Xie Zhouyu, Wei Qingbo, Wu Yunchuan. Mechanism by which static exercise improves insulin resistance in skeletal muscle of type 2 diabetes [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1271-1276. |
| [9] | Xu Canli, He Wenxing, Wang Lei, Wu Fangting, Wang Jiahui, Duan Xuelin, Zhao Tiejian, Zhao Bin, Zheng Yang. Bibliometric analysis of researches on liver organoids [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1099-1104. |
| [10] | Liu Hanfeng, Wang Jingjing, Yu Yunsheng. Artificial exosomes in treatment of myocardial infarction: current status and prospects [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1118-1123. |
| [11] | Ma Shuwei, He Sheng, Han Bing, Zhang Liaoyun. Exosomes derived from mesenchymal stem cells in treatment of animals with acute liver failure: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1137-1142. |
| [12] | Sun Yukang, Song Lijuan, Wen Chunli, Ding Zhibin, Tian Hao, Ma Dong, Ma Cungen, Zhai Xiaoyan. Visualization analysis of stem cell therapy for myocardial infarction based on Web of Science in recent ten years [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1143-1148. |
| [13] | Feng Ruiqin, Han Na, Zhang Meng, Gu Xinyi, Zhang Fengshi. Combination of 1% platelet-rich plasma and bone marrow mesenchymal stem cells improves the recovery of peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 985-992. |
| [14] | Wang Wen, Zheng Pengpeng, Meng Haohao, Liu Hao, Yuan Changyong. Overexpression of Sema3A promotes osteogenic differentiation of dental pulp stem cells and MC3T3-E1 [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 993-999. |
| [15] | Qiu Xiaoyan, Li Bixin, Li Jingdi, Fan Chuiqin, Ma Lian, Wang Hongwu. Differentiation of insulin-producing cells from human umbilical cord mesenchymal stem cells infected by MAFA-PDX1 overexpressed lentivirus [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1000-1006. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||