Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (28): 4566-4570.doi: 10.12307/2023.431
Previous Articles Next Articles
Roles of N6-methyladenosine methyltransferase-like 3 in regulating bone metabolism and related diseases
Xiong Bo1, Zeng Ping2, Liu Jinfu1, Lu Guanyu1, Chen Cai1, Huang Yue1, Chen Lihua1
- 1Graduate School of Guangxi University of Chinese Medicine, Nanning 530299, Guangxi Zhuang Autonomous Region, China; 2The First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning 530023, Guangxi Zhuang Autonomous Region, China
-
Received:2022-04-06Accepted:2022-07-23Online:2023-10-08Published:2023-01-29 -
Contact:Zeng Ping, MD, Professor, The First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning 530023, Guangxi Zhuang Autonomous Region, China -
About author:Xiong Bo, Master candidate, Graduate School of Guangxi University of Chinese Medicine, Nanning 530299, Guangxi Zhuang Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, Nos. 81960876 and 82160913 (both to ZP); Guangxi Zhuang Autonomous Region Administration of Traditional Chinese Medicine Appropriate Technology Development and Promotion Project, No. 2017ZD002 (to ZP); School-level Project of Guangxi University of Chinese Medicine, No. YCXJ2021070 (to XB)
CLC Number:
Cite this article
Xiong Bo, Zeng Ping, Liu Jinfu, Lu Guanyu, Chen Cai, Huang Yue, Chen Lihua. Roles of N6-methyladenosine methyltransferase-like 3 in regulating bone metabolism and related diseases[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(28): 4566-4570.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
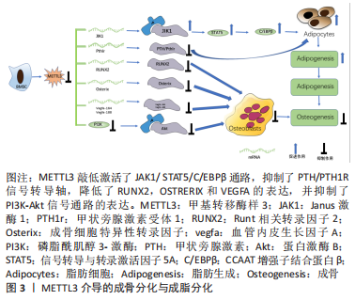
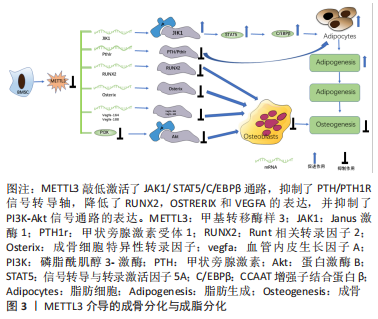
2.1 METTL3调控成骨细胞 2.1.1 METTL3调控成骨细胞分化 有报道称METTL3敲除可抑制成骨细胞相关因子[包括Runt相关转录因子2(Runt-related transcription factor 2,Runx2),OSTERIX,ALPL和COL1AL]的转录水平和蛋白质水平,同时抑制SMAD1/5/9复合物的磷酸化,导致细胞核中的Runt相关转录因子2含量减少,以致不能激活成骨基因表达,从而导致成骨分化的负调控[15-16]。此外,METTL3表达异常可能对成骨细胞的分化造成不良影响;另一方面,METTL3在成骨方面表现出不同功能,METTL3与去甲基酶ALKBH5协同作用,通过促进髓样分化因子88(MyD88)的m6A甲基化修饰来正向调节髓样分化因子88的表达,促进成骨细胞的分化[17]。因此关于METTL3在成骨方面的作用存在一定争议,还有待进一步的深入研究。 2.1.2 METTL3调控骨髓间充质干细胞成骨分化 作为表观遗传调控的必不可少的部分,m6A修饰被认为是参与骨骼成骨过程的关键调控因子。例如,研究人员发现多条信号通路参与调控骨髓间充质干细胞成骨向分化,如PI3K-Akt、Notch、BMPs/SMAD、MAPK、Wnt/β-catenin等,而 m6A甲基化修饰则通过调控通路中关键分子的表达进而介导对骨髓间充质干细胞成骨分化的调节[18]。TIAN等[19]研究发现骨髓间充质干细胞成骨分化过程中METTL3的表达上调,通过进一步研究,他们发现敲低METTL3可以抑制PI3K-Akt通路从而抑遏骨髓间充质干细胞成骨分化的能力。METTL3通过调控血管内皮生长因子mRNA的剪接影响成骨细胞的成熟,在敲低骨髓间充质干细胞中METTL3的表达后,剪接体血管内皮生长因子188的mRNA表达量下调,而血管内皮生长因子120的 mRNA表达量却没有明显变化,有研究显示前者参与调控骨髓间充质干细胞的成骨分化。 甲状旁腺激素其主要功能之一是调控脊椎动物体内钙及磷的代谢,促使血浆中钙离子浓度升高,骨与肾脏是其发挥作用的主要靶器官[18]。甲状旁腺激素通过介导Notch信号通路影响骨髓间充质干细胞成骨分化间接调控骨代谢与骨稳态[18]。甲状旁腺激素受体1是钙稳态的中心,其对甲状旁腺激素功能的介导至关重要,阿巴洛肽是一种已经批准上市用于治疗绝经后骨质疏松症的新药,其机制即是通过激活甲状旁腺激素/甲状旁腺激素受体1信号通路发挥作用,而甲状旁腺激素/甲状旁腺激素受体1信号通路是METTL3介导骨髓间充质干细胞成骨分化的下游通路[20]。该团队通过进一步研究发现敲除METTL3可抑制甲状旁腺激素受体1的翻译影响骨髓间充质干细胞向成骨分化[20]。YAN 等[21]通过 MeRIP 高通量测序发现在敲低METTL3的骨髓间充质干细胞中pre-miRNA 与pri-miRNA出现明显变化,其中m6A 甲基化修饰水平最高的是pre-miR-320。因此,研究人员重点关注这个miRNA,通过深入研究发现敲低METTL3 可使miR-320及pre-miR-320的表达明显上调,miR-320可调控骨髓间充质干细胞成骨分化的关键转录因子Runt相关转录因子2,从而抑制骨髓间充质干细胞向成骨分化,而敲除METTL3 本身也可使Runt相关转录因子2的mRNA 和蛋白水平明显下调[21]。但是,据报道pri-miRNA上的m6A 被“reader”异质核核糖核蛋白A2B1(heterogeneous nuclear ribonucleoprotein A2B1,HNRNPA2B1)识别后可促使其加工成pre-miRNA和成熟的miRNA,METTL3实际上参与了调控miRNA的成熟过程,然而这无法解释上述研究中敲低METTL3后pre-miR-320及miR-320的mRNA水平上调的现象[22]。综上所述,m6A/METTL3能够通过多种机制调控节骨髓间充质干细胞的成骨分化。 2.1.3 METTL3调控骨髓间充质干细胞成脂与成骨分化之间的转换介导成骨分化 骨髓间充质干细胞具备多向分化的潜能,可以促进骨、软骨、肌腱等间充质组织的再生。WU等[23]通过CRISPR/Cas9 技术敲除小鼠中的METTL3基因,发现敲除小鼠的成骨分化能力明显降低、骨髓脂肪组织却显著增加。然后,通过分离小鼠的骨髓间充质干细胞进行体外成骨及成脂分化的研究,结果显示成骨标志物如骨钙素、Runt相关转录因子2、碱性磷酸酶和锌指转录因子SP7/OSTERIX的表达明显下调,而过氧化物酶体增殖物激活受体γ、人CCAAT增强子结合蛋白α、脂联素、人围脂滴蛋白1和脂肪酸转位酶CD36的表达明显增加,研究结果表明敲除METTL3后,小鼠的成骨分化能力降低,然而成脂分化能力却明显提高。随后,为了进一步阐明实验结果的可靠性,该团队在绝经后骨质疏松症小鼠模型中过表达 METTL3,发现较之于对照组其骨量增加、骨小梁密度和骨皮质厚度升高,且发现过表达METTL3的小鼠成骨细胞的增殖及分化能力明显提高;最后,通过MeRIP-Seq鉴定,证实METTL3/m6A 通过甲状旁腺激素/甲状旁腺激素受体1 信号通路影响骨髓间充质干细胞的成骨与成脂分化介导骨重塑。最近,YAO 等[24]研究发现METTL3在骨髓间充质干细胞分化和脂肪形成中起重要作用,METTL3表达与骨髓间充质干细胞脂肪形成之间存在负相关[24]。具体而言,METTL3通过m6A甲基化介导YTHDF2调控JAK1/STAT5/C/ EBPβ通路抑制骨髓间充质干细胞成脂分化促进成骨分化[24]。结果表明,METTL3的敲除通过m6A甲基化途径显著促进了骨髓间充质干细胞的脂肪形成过程和酪胺酸蛋白激酶JAK1的表达[24]。综上所述,METTL3在介导成骨分化与成脂分化之间的转换中起了关键作用。然而,由于研究的广度和深度有限,目前的认识仍然受到限制有待进一步探索。METTL3介导的成骨分化与成脂分化见图3。"
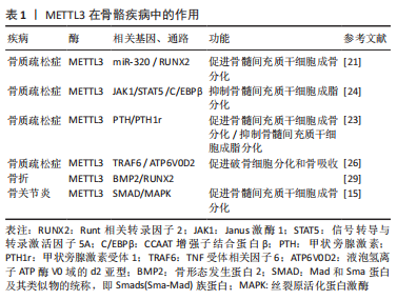
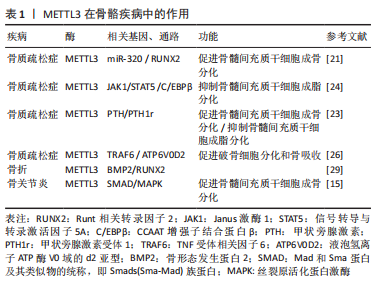
2.2 METTL3调控破骨细胞分化 破骨细胞的分化功能对于维持骨稳态以及保持骨骼完整性至关重要,METTL3被认为是调控破骨细胞分化的关键调控因子。例如,TIAN等[19]发现骨髓间充质干细胞经成骨诱导后,m6A甲基转移酶METTL3的表达显著升高,敲低METTL3会降低与骨髓间充质干细胞增殖和分化相关的基因的mRNA水平,如血管内皮生长因子A164和血管内皮生长因子A188。破骨细胞形成过程中METTL3的水平升高,而METTL3的敲除抑制了破骨细胞的分化和骨吸收能力,从机制上讲,破骨细胞前体细胞融合的主要调控因子ATP6V0D2 mRNA的稳定与METT3的甲基化密切相关[25] 。同样,LI等[26]在破骨细胞诱导分化后,均检测到m6A甲基化及METTL3过表达。此外,研究人员用巨噬细胞集落刺激因子和核因子κB受体活化因子配体处理si-METTL3 的骨髓单核巨噬细胞,发现其分化的破骨细胞活性也会降低,调控破骨细胞分化相关基因DCSTAMP、NFATC1和c-FOS及骨吸收相关基因ACP5、CTSK的mRNA和蛋白表达水平均下调[26]。METTL3 沉默的细胞中AKT、IKKα/β、IκBα、P65、ERK、P38和JNK的磷酸化水平均下调,这表明METTL3可能通过激活核因子kB、AKT和MAPK信号通路来调控核因子κb受体活化因子配体诱导的破骨细胞分化。METTL3通过影响YTHDF2及TRAF6 mRNA核输出介导的ATP6V0D2 mRNA降解的不同机制进而调控破骨细胞的分化功能[26]。RNA甲基化酶METTL3作用于circ-0008542-1956-bp的m6A功能位点,通过circ-0008542促进miRNA-185-5p的竞争性结合,促进破骨细胞诱导的骨吸收[27]。这些研究表明METTL3为调控破骨细胞分化的潜在靶点,并提示抑制 METTL3作为治疗骨代谢疾病的潜在治疗靶点。 2.3 METTL3调节骨代谢相关疾病 2.3.1 METTL3调节骨代谢影响骨质疏松症的发生发展 骨质疏松症的发生与骨代谢异常密切相关。多项研究增强了对m6A修饰在骨质疏松症中作用的认识。例如,MO等[28]通过全基因组鉴定发现多个m6A相关的单核苷酸多态性对骨矿物质密度产生影响,而骨矿物质密度与骨质疏松症密切相关。此外,YAN 等[21]通过Northern Blot和ELISA法检测发现骨质疏松症患者的骨组织以及骨质疏松症小鼠中总体RNA的m6A和METTL3 mRNA 含量较对照组明显下调,且其成骨细胞分化标志物骨钙素、Runt相关转录因子2、碱性磷酸酶和锌指转录因子SP7/OSTERIX的表达均低于对照组。最近,研究人员还发现METTL3/m6A通过影响pre-miR-320 的甲基化水平下调miR-320的表达,从而促进成骨转录因子Runt相关转录因子2的表达调控骨质疏松症中成骨细胞分化[18,21]。另外,该团队还通过沉默METTL3来探索m6A甲基化水平是否影响骨质疏松症中骨髓间充质干细胞的成骨分化能力,研究结果显示转染si-METTL3的骨质疏松症中骨髓间充质干细胞中成骨相关标志物骨钙素、Runt相关转录因子2、碱性磷酸酶和锌指转录因子SP7/OSTERIX的表达明显下调,表明骨质疏松症中骨髓间充质干细胞的成骨分化能力减弱[21]。此次研究为进一步了解METTL3/m6A与骨质疏松症之间关联的功能机制提供了新的线索。国内干细胞再生医学领域正处在规范、快速发展过程中,推动干细胞治疗相关领域的深入研究将可能为骨质疏松症患者治疗带来新希望。METTL3调节骨代谢影响骨质疏松症的发生发展,见表1。"

2.3.2 METTL3调控骨关节炎发生发展 骨关节炎是一种常见难治的骨关节疾病,其主要以关节软骨退变、关节基质崩解和继发性骨质增生为特征[30]。正常情况下,破骨细胞介导的骨吸收与成骨细胞调节的骨重建构成协同偶联效应,使软骨下骨重塑保持在稳定状态从而保证骨关节适应平常的机械负荷[31]。METTL3 通过调控SMAD和 MAPK 信号通路介导成骨细胞分化以及炎症反应影响软骨下骨重塑。具体来说,METTL3通过介导SMAD信号通路中的关键调节因子YTHDF2调控炎症微环境进而抑制成骨分化,并通过MAPK信号通路促进成骨细胞中促炎因子的表达,最终致使骨关节炎的进展[15]。既往研究认为靶向炎症相关通路是治疗骨关节炎方法之一,已有研究发现表观遗传调控与炎症因子和反应有关[32]。 作为表观遗传调控的必不可少的修饰,m6A在骨关节炎中研究逐渐成为热点。LIU等[33]研究发现METTL3通过增强炎症反应和细胞外基质合成来调节骨关节炎过程。从机制上讲,沉默METTL3抑制骨关节炎细胞中的炎性细胞因子水平和核因子kB信号传导,从而延缓骨关节炎的进展。同时,抑制METTL3通过下调基质金属蛋白酶13和X型胶原酶促进软骨细胞细胞外基质的降解,从而促进骨关节炎的发展。上述研究表明METTL3在骨关节炎的发生发展过程中发挥了重要作用。 2.3.3 METTL3调节骨代谢影响骨折愈合 骨折愈合是由多种因素调控的复杂生物学过程,影响骨折愈合的具体机制至今尚未探索清楚,骨代谢异常会增加骨折的风险[26]。MI等[29]研究发现骨折后的前7 d内METTL3、KIAA1429和 Wilms肿瘤1相关蛋白的水平明显下调,随后研究人员通过在小鼠股骨骨折部位注入质粒METTL3,发现成骨相关基因Runt相关转录因子2 在质粒 METTL3 组中的表达明显下降,此外,该团队又在细胞水平验证了这一结果。表明 METTL3 在骨折愈合过程中起负向调节作用。 2.3.4 METTL3治疗骨代谢相关疾病的临床应用 随着METTL3对骨代谢调控相关研究的开展,越来越多的METTL3相关疗法被应用于临床。谭素明[20]通过微量RNA甲基化(m6A)免疫共沉淀高通量测序技术发现对照组、成骨诱导剂组及不同浓度的墨旱莲提取物处理组骨髓间充质干细胞成骨分化过程中mRNA的m6A甲基化水平以及甲基转移酶METTL3的表达水平存在明显差异,随后,通过研究墨旱莲调控骨髓间充质干细胞成骨分化过程中m6A甲基化水平的动态变化规律,联合成骨分化的特征指标评价,认为墨旱莲通过METTL3介导m6A动态修饰调控骨髓间充质干细胞成骨分化。另有研究发现,适当的机械牵张应力刺激可以使METTL3的表达上调以及介导细胞内m6A甲基化水平调控成骨分化相关因子的表达促进骨髓间充质干细胞向成骨分化,进而起到减缓骨质丢失、提高机体骨量、骨密度,防治骨质疏松症作用[34]。清热活血方可能通过调控RNA m6A甲基化修饰酶METTL3延缓踝关节骨质破坏[35]。综上所述,METTL3在骨代谢相关疾病的发生发展中发挥着重要作用,因此,使METTL3过表达可能会成为治疗人类骨代谢相关疾病如骨质疏松症的一种新的替代疗法。"

| [1] CHEN X, WANG Z, DUAN N, et al. Osteoblast-osteoclast interactions. Connect Tissue Res. 2018;59(2):99-107. [2] OZAKI D, KUBOTA R, MAENO T, et al. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos Int. 2021;32(1):145-156. [3] CIPRIANI C, COLANGELO L, SANTORI R, et al. The Interplay Between Bone and Glucose Metabolism. Front Endocrinol (Lausanne). 2020;11:122. [4] ZHAO X, CUI P, HU G, et al. PIP5k1beta controls bone homeostasis through modulating both osteoclast and osteoblast differentiation. J Mol Cell Biol. 2020; 12(1):55-70. [5] REPPE S, LIEN TG, HSU YH, et al. Distinct DNA methylation profiles in bone and blood of osteoporotic and healthy postmenopausal women. Epigenetics. 2017; 12(8):674-687. [6] CHEN X, HUA W, HUANG X, et al. Regulatory Role of RNA N(6)-Methyladenosine Modification in Bone Biology and Osteoporosis. Front Endocrinol (Lausanne). 2019;10:911. [7] TRAUBE FR, CARELL T. The chemistries and consequences of DNA and RNA methylation and demethylation. RNA Biol. 2017;14(9):1099-1107. [8] GUTBROD MJ, MARTIENSSEN RA. Conserved chromosomal functions of RNA interference. Nat Rev Genet. 2020;21(5):311-331. [9] ZHAO BS, ROUNDTREE IA, HE C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18(1):31-42. [10] YU S, LI X, LIU S, et al. N(6)-Methyladenosine: A Novel RNA Imprint in Human Cancer. Front Oncol. 2019;9:1407. [11] DESROSIERS R, FRIDERICI K, ROTTMAN F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974; 71(10):3971-3975. [12] Fu Y, Dominissini D, Rechavi G, et al. Gene expression regulation mediated through reversible m(6)A RNA methylation. Nat Rev Genet. 2014;15(5):293-306. [13] YANG Y, HSU PJ, CHEN YS, et al. Dynamic transcriptomic m(6)A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res.2018;28(6):616-624. [14] DENG X, SU R, WENG H, et al. RNA N(6)-methyladenosine modification in cancers: current status and perspectives. Cell Res. 2018;28(5):507-517. [15] ZHANG Y, GU X, LI D, et al. METTL3 Regulates Osteoblast Differentiation and Inflammatory Response via Smad Signaling and MAPK Signaling. Int J Mol Sci. 2019;21(1):199. [16] 黎琮南, 李毅成, 杨渊. m6A RNA甲基化在骨骼疾病中的作用[J]. 中国生物化学与分子生物学报,2020,36(11):1303-1311. [17] YU J, SHEN L, LIU Y, et al. The m6A methyltransferase METTL3 cooperates with demethylase ALKBH5 to regulate osteogenic differentiation through NF-kappaB signaling. Mol Cell Biochem. 2020;463(1-2):203-210. [18] 陈伟坚, 张罡瑜, 林天烨, 等. RNA m~6A甲基化修饰参与及调控骨科相关疾病[J]. 中国组织工程研究,2021,25(26):4236-4242. [19] TIAN C, HUANG Y, LI Q, et al. Mettl3 Regulates Osteogenic Differentiation and Alternative Splicing of Vegfa in Bone Marrow Mesenchymal Stem Cells. Int J Mol Sci. 2019;20(3):551. [20] 谭素明. 墨旱莲通过Mettl3/14介导m6A动态修饰调控骨髓间充质干细胞成骨分化的作用机制研究[D]. 大连:大连医科大学,2021. [21] YAN G, YUAN Y, HE M, et al. m(6)A Methylation of Precursor-miR-320/RUNX2 Controls Osteogenic Potential of Bone Marrow-Derived Mesenchymal Stem Cells. Mol Ther Nucleic Acids. 2020;19:421-436. [22] ALARCON C R, GOODARZI H, LEE H, et al. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell. 2015;162(6):1299-1308. [23] WU Y, XIE L, WANG M, et al. Mettl3-mediated m(6)A RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis. Nat Commun. 2018;9(1):4772. [24] YAO Y, BI Z, WU R, et al. METTL3 inhibits BMSC adipogenic differentiation by targeting the JAK1/STAT5/C/EBPbeta pathway via an m(6)A-YTHDF2-dependent manner. FASEB J. 2019;33(6):7529-7544. [25] KIM T, HA H I, KIM N, et al. Adrm1 interacts with Atp6v0d2 and regulates osteoclast differentiation. Biochem Biophys Res Commun. 2009;390(3):585-590. [26] LI D, CAI L, MENG R, et al. METTL3 Modulates Osteoclast Differentiation and Function by Controlling RNA Stability and Nuclear Export.Int J Mol Sci. 2020;21(5):1660. [27] WANG W, QIAO S C, WU XB, et al. Circ_0008542 in osteoblast exosomes promotes osteoclast-induced bone resorption through m6A methylation. Cell Death Dis. 2021;12(7):628. [28] MO XB, ZHANG Y H, LEI SF. Genome-wide identification of m(6)A-associated SNPs as potential functional variants for bone mineral density. Osteoporos Int. 2018;29(9):2029-2039. [29] MI B, XIONG Y, YAN C, et al. Methyltransferase-like 3-mediated N6-methyladenosine modification of miR-7212-5p drives osteoblast differentiation and fracture healing. J Cell Mol Med. 2020;24(11):6385-6396. [30] 骆帝, 许波, 李刚, 等. 骨碎补治疗骨关节炎的网络药理学研究[J]. 中草药, 2018,49(15):3516-3522. [31] Cui Z, Crane J, Xie H, et al. Halofuginone attenuates osteoarthritis by inhibition of TGF-beta activity and H-type vessel formation in subchondral bone. Ann Rheum Dis. 2016;75(9):1714-1721. [32] Shen J, Abu-Amer Y, O’Keefe R J, et al. Inflammation and epigenetic regulation in osteoarthritis. Connect Tissue Res. 2017;58(1):49-63. [33] Liu Q, Li M, Jiang L, et al. METTL3 promotes experimental osteoarthritis development by regulating inflammatory response and apoptosis in chondrocyte. Biochem Biophys Res Commun. 2019;516(1):22-27. [34] 黄媚. METTL3在机械牵张促进BMSC成骨分化中的作用研究[D]. 上海:上海体育学院,2021. [35] 李露. 基于m6A甲基化探讨清热活血方治疗活动期RA的作用机制[D]. 北京:中国中医科学院,2021. |
| [1] | Guo Shuhui, Yang Ye, Jiang Yangyang, Xu Jianwen. Screening and validation of neurogenic bladder miRNA-mRNA regulatory network [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-8. |
| [2] | Zheng Bo, Zhang Xiuli, Zhou Hao, He Zebi, Zhou Jin, Zhou Weiyun, Li Peng. Arthroscopy-assisted locking hollow screw fixation and open reduction plate internal fixation in the treatment of Schatzker II-III tibial plateau fractures: early CT evaluation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1410-1416. |
| [3] | Pan Zhongjie, Qin Zhihong, Zheng Tiejun, Ding Xiaofei, Liao Shijie. Targeting of non-coding RNAs in the pathogenesis of the osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1441-1447. |
| [4] | Dang Yi, Du Chengyan, Yao Honglin, Yuan Nenghua, Cao Jin, Xiong Shan, Zhang Dingmei, Wang Xin. Hormonal osteonecrosis and oxidative stress [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1469-1476. |
| [5] | Nie Chenchen, Su Kaiqi, Gao Jing, Fan Yongfu, Ruan Xiaodi, Yuan Jie, Duan Zhaoyuan, Feng Xiaodong. The regulatory role of circular RNAs in cerebral ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1286-1291. |
| [6] | Sun Jiajia, Zhu Haidi, Lu Yun, Zhang Kai. Comparison of bone metabolism markers between type 2 diabetes mellitus and non-type 2 diabetes mellitus patients with hip fracture [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1156-1160. |
| [7] | Zhao Lu, Zhao Yifei, Gao Da, Liu Yanfang, Fu Tingting, Xu Jiangyan. Expression of suppressor of Zeste 12 in kidney tissues of rats with diabetic nephropathy [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1179-1186. |
| [8] | Yin Shuoxin, Zhang Tao, Wang Shuping, Lu Xin, Huang Xuping, Yin Mengying, Yang Yuwei, Yuan Bingmao, Mao Zhihua, Chen Yuanneng. Bibliometric and visualized analysis of researches on gastric cancer stem cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 941-947. |
| [9] | Qin Yuxing, Ren Qiangui, Li Zilong, Quan Jiaxing, Shen Peifeng, Sun Tao, Wang Haoyu. Action mechanism and prospect of bone microvascular endothelial cells for treating femoral head necrosis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 955-961. |
| [10] | Chen Guanting, Zhang Linqi, Li Qingru. Meta-analysis of the value of exosomal miRNA for the diagnosis of chronic kidney disease [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 970-976. |
| [11] | Liu Hongwen, Li Jiao, Xu Wenhao, Nie Hua, Liu Shaojiang, Xu Jie, Yin Li. Differential expression profiles of microRNAs in muscle tissue of denervated skeletal muscle atrophy rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 732-737. |
| [12] | Hu Xinming, Qiao Yanhua, Wang Xiaofan, Li Linyu, Zhao Bing. Mechanism of long non-coding RNA plasmacytoma variant translocation 1 involved in pelvic organ prolapse [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 669-675. |
| [13] | Zhang Qing, Gao Chunlan, Yu Feifei, Zhang Zhenghao, Ma Fang, Gao Yuan, Li Guizhong, Jiang Yideng, Ma Shengchao. Ephrin A receptor 2 DNA methylation increases in pancreatic beta cell apoptosis induced by homocysteine [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 714-719. |
| [14] | Li Long, Li Guangdi, Shi Hao, Deng Keqi. Circular RNA as a competing endogenous RNA is involved in the regulation of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 751-757. |
| [15] | Li Zhichao, Tan Guoqing, Su Hui, Xu Zhanwang, Xue Haipeng. Regulatory role of non-coding RNAs as potential therapeutic targets in spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 758-764. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||