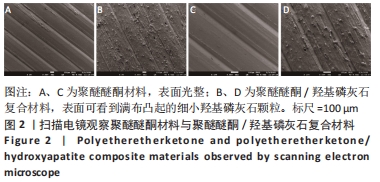
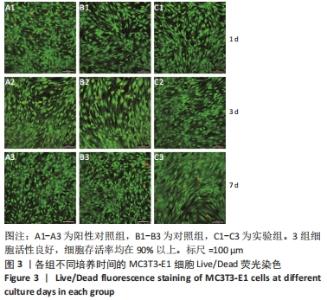
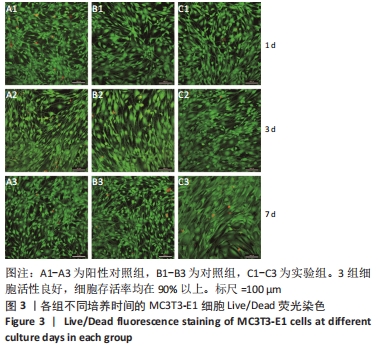
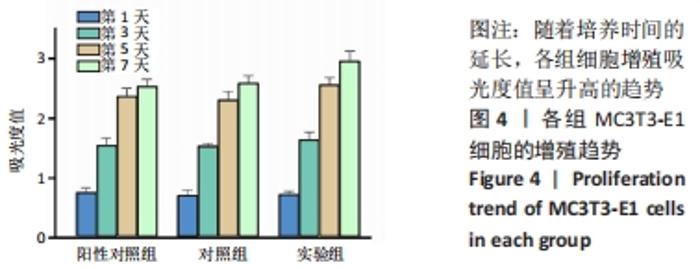
Chinese Journal of Tissue Engineering Research ›› 2023, Vol. 27 ›› Issue (12): 1932-1937.doi: 10.12307/2023.079
Previous Articles Next Articles
Biocompatibility of 3D printed polyetheretherketone/hydroxyapatite composites
Wu Boyu1, 2, Ye Kai1, 2, Chen Jiahan1, 2, Wang Jianghua1, Wurikaixi·Aiyiti3, Jiang Houfeng3, Teng Yong2
- 1Graduate School of Xinjiang Medical University, Urumqi 830054, Xinjiang Uygur Autonomous Region, China; 2Department of Spinal Surgery, General Hospital of Xinjiang Military Region PLA, Urumqi 830000, Xinjiang Uygur Autonomous Region, China; 3School of Mechanical Engineering, Xinjiang University, Urumqi 830017, Xinjiang Uygur Autonomous Region, China
-
Received:2022-01-06Accepted:2022-03-07Online:2023-04-28Published:2022-07-30 -
Contact:Teng Yong, Chief physician, Department of Spinal Surgery, General Hospital of Xinjiang Military Region PLA, Urumqi 830000, Xinjiang Uygur Autonomous Region, China -
About author:Wu Boyu, Master candidate, Graduate School of Xinjiang Medical University, Urumqi 830054, Xinjiang Uygur Autonomous Region, China; Department of Spinal Surgery, General Hospital of Xinjiang Military Region PLA, Urumqi 830000, Xinjiang Uygur Autonomous Region, China -
Supported by:Regional Collaborative Innovation Special Program of Xinjiang Uygur Autonomous Region (Science and Technology Assistance to Xinjiang), No. 2019E0277 (to TY)
CLC Number:
Cite this article
Wu Boyu, Ye Kai, Chen Jiahan, Wang Jianghua, Wurikaixi·Aiyiti, Jiang Houfeng, Teng Yong. Biocompatibility of 3D printed polyetheretherketone/hydroxyapatite composites[J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1932-1937.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

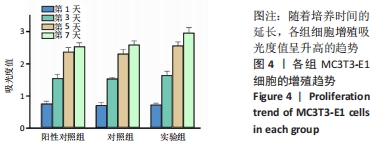
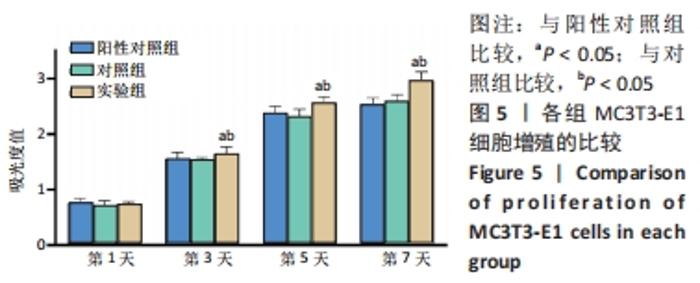
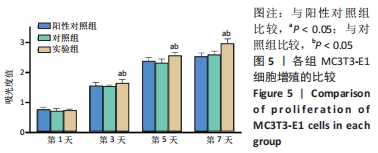
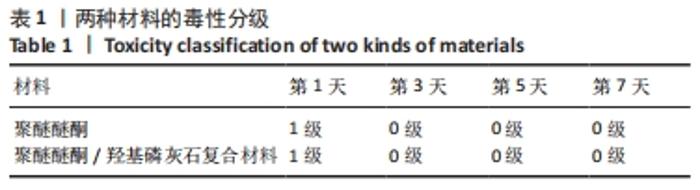
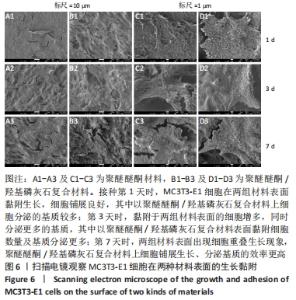
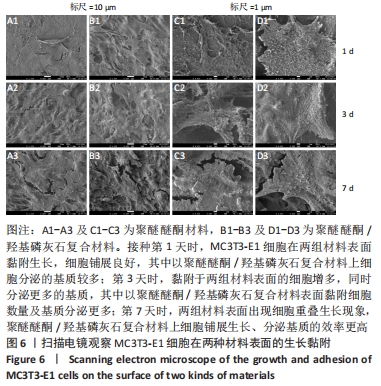
培养第1天时,低倍电镜下可观察到MC3T3-E1在两种材料表面黏附生长,细胞铺展良好,聚醚醚酮/羟基磷灰石复合材料表面细胞分泌出的基质多于聚醚醚酮材料;高倍电镜下可观察到细胞伸出伪足和表面分泌的钙化颗粒,细胞生长良好。培养第3天时,低倍电镜下观察到两组材料表面黏附的细胞增多,同时分泌出较多的基质,高倍电镜下同样观测到细胞伪足和钙化颗粒,细胞在材料表面生长增殖良好,其中聚醚醚酮/羟基磷灰石复合材料上的细胞生长黏附、基质分泌更多。培养第7天时,低倍电镜下观察到了细胞已经在两种材料表面出现细胞重叠生长的现象,其中聚醚醚酮/羟基磷灰石复合材料上的细胞铺展生长、分泌基质效率更高。 2.5 材料的生物相容性 由以上结果可知,聚醚醚酮/羟基磷灰石复合材料具有良好的生物相容性。 "

| [1] MA R, TANG T. Current strategies to improve the bioactivity of PEEK. Int J Mol Sci. 2014;15(4):5426-5445. [2] MOO IH, KAM CJW, LAI MWS, et al. A comparison of contiguous two-level anterior cervical discectomy and fusion using a structural allograft versus a Polyetheretherketone (PEEK) cage: the results of a three-year follow-up. BMC Musculoskelet Disord. 2020;21(1):331. [3] HU B, YANG X, HU Y, et al. The n-HA/PA66 Cage Versus the PEEK Cage in Anterior Cervical Fusion with Single-Level Discectomy During 7 Years of Follow-Up. World Neurosurg. 2019;123:e678-e684. [4] WANG H, XU M, ZHANG W, et al. Mechanical and biological characteristics of diamond-like carbon coated poly aryl-ether-ether-ketone. Biomaterials. 2010;31(32):8181-8187. [5] LIAO C, LI Y, TJONG SC. Polyetheretherketone and Its Composites for Bone Replacement and Regeneration. Polymers (Basel). 2020;12(12): 2858. [6] CHU L, LI R, LIAO Z, et al. Highly Effective Bone Fusion Induced by the Interbody Cage Made of Calcium Silicate/Polyetheretherketone in a Goat Model. ACS Biomater Sci Eng. 2019;5(5):2409-2416. [7] RAMESH N, MORATTI SC, DIAS GJ. Hydroxyapatite-polymer biocomposites for bone regeneration: A review of current trends. J Biomed Mater Res B Appl Biomater. 2018;106(5):2046-2057. [8] GUILLEN-ROMERO LD, OROPEZA-GUZMAN MT, LOPEZ-MALDONADO EA, et al. Synthetic hydroxyapatite and its use in bioactive coatings. J Appl Biomater Funct Mater. 2019;17(1):585949623. [9] 胡超然,邱冰,周祝兴,等.3D打印聚己内酯/纳米羟基磷灰石复合支架与骨髓间充质干细胞的体外生物相容性[J].中国组织工程研究,2020,24(4):589-595. [10] 王鹏,孙桂森,李红旗,等.高分子材料椎间融合器的应用现况[J].中国组织工程研究,2018,22(2):310-315. [11] AWASTHI S, PANDEY SK, ARUNAN E, et al. A review on hydroxyapatite coatings for the biomedical applications: experimental and theoretical perspectives. J Mater Chem B. 2021;9(2):228-249. [12] ARRES M, SALAMA M, RECHENA D, et al. Surface and mechanical properties of a nanostructured citrate hydroxyapatite coating on pure titanium. J Mech Behav Biomed Mater. 2020;108:103794. [13] 余文,孟昊业,孙逊,等.3D打印生物陶瓷在骨组织工程中的研究现状[J].中国矫形外科杂志,2018,26(14):1306-1310. [14] MANZOOR F, GOLBANG A, JINDAL S, et al. 3D printed PEEK/HA composites for bone tissue engineering applications: Effect of material formulation on mechanical performance and bioactive potential. J Mech Behav Biomed Mater. 2021;121:104601. [15] DURHAM JR, MONTELONGO SA, ONG JL, et al. Hydroxyapatite coating on PEEK implants: Biomechanical and histological study in a rabbit model. Mater Sci Eng C Mater Biol Appl. 2016;68:723-731. [16] HU X, MEI S, WANG F, et al. Implantable PEKK/tantalum microparticles composite with improved surface performances for regulating cell behaviors, promoting bone formation and osseointegration. Bioact Mater. 2021;6(4):928-940. [17] HU X, MEI S, WANG F, et al. A microporous surface containing Si3N4/Ta microparticles of PEKK exhibits both antibacterial and osteogenic activity for inducing cellular response and improving osseointegration. Bioact Mater. 2021;6(10):3136-3149. [18] SHIMIZU T, FUJIBAYASHI S, YAMAGUCHI S, et al. Bioactivity of sol-gel-derived TiO2 coating on polyetheretherketone: In vitro and in vivo studies. Acta Biomater. 2016;35:305-317. [19] THANIGACHALAM M, MUTHUSAMY SUBRAMANIAN AV. Evaluation of PEEK-TiO2- SiO2 nanocomposite as biomedical implants with regard to in-vitro biocompatibility and material characterization. J Biomater Sci Polym Ed. 2021;1-20. doi: 10.1080/09205063.2021.2014028. [20] HASEGAWA T, USHIROZAKO H, SHIGETO E, et al. The Titanium-coated PEEK Cage Maintains Better Bone Fusion With the Endplate Than the PEEK Cage 6 Months After PLIF Surgery: A Multicenter, Prospective, Randomized Study. Spine (Phila Pa 1976). 2020;45(15):E892-E902. [21] WANG H, WAN Y, LIU X, et al. The biomechanical effects of Ti versus PEEK used in the PLIF surgery on lumbar spine: a finite element analysis. Comput Methods Biomech Biomed Engin. 2021;24(10):1115-1124. [22] SCHULZ C, MAUER UM, MATHIEU R. PEEK cage fusion after anterior cervical corpectomy : Clinical and radiological results in patients with spondylotic myelopathy. Orthopade. 2017;46(3): 242-248. [23] SORPRESO RS, MARTINS DE, KANAS M, et al. Transforaminal intersomatic lumbar arthrodesis: comparison between autograft and cage in PEEK. Acta Ortop Bras. 2020;28(6):296-302. [24] 金贺荣,崔敬斌,邵苍.椎间融合器材料:临床应用的优势与关注热点[J].中国组织工程研究,2022,26(22):3592-3597. [25] KUSHIOKA J, KAITO T, MAKINO T, et al. Difference in the fusion rate and bone formationbetween artificial bone and iliac autograftinside an inter-body fusion cage - A comparison between porous hydroxyapatite/type 1 collagen composite and autologousiliac bone. J Orthop Sci. 2018;23(4):622-626. [26] JACKLE K, BRIX T, OBERTHUR S, et al. Cage or Pelvic Graft-Study on Bony Fusion of the Ventral Thoracic and Lumbar Spine in Traumatic Vertebral Fractures. Medicina (Kaunas). 2021;57(8):786. [27] BUSER Z, BRODKED S, YOUSSEFJ A, et al. Synthetic bone graft versus autograft or allograftforspinalfusion: a systematic review. J Neurosurg Spine. 2016;25(4):509-516. [28] PANAYOTOV IV, ORTI V, CUISINIER F, et al. Polyetheretherketone (PEEK) for medical applications. J Mater Sci Mater Med. 2016;27(7):118. [29] ABDULLAH MR, GOHARIAN A, ABDUL KM, et al. Biomechanical and bioactivity concepts of polyetheretherketone composites for use in orthopedic implants-a review. J Biomed Mater Res A. 2015;103(11): 3689-3702. [30] TURNBULL G, CLARKE J, PICARD F, et al. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018;3(3):278-314. [31] FATHI AM, AHMED MK, AFIFI M, et al. Taking Hydroxyapatite- Coated Titanium Implants Two Steps Forward: Surface Modification Using Graphene Mesolayers and a Hydroxyapatite-Reinforced Polymeric Scaffold. ACS Biomater Sci Eng. 2021;7(1):360-372. [32] LEE JH, JANG HL, LEE KM, et al. In vitro and in vivo evaluation of the bioactivity of hydroxyapatite-coated polyetheretherketone biocomposites created by cold spray technology. Acta Biomater. 2013; 9(4):6177-6187. [33] LEE JH, JANG HL, LEE KM, et al. Cold-spray coating of hydroxyapatite on a three-dimensional polyetheretherketone implant and its biocompatibility evaluated by in vitro and in vivo minipig model. J Biomed Mater Res B Appl Biomater. 2017;105(3):647-657. [34] CASAGRANDE RB, BALDIN EK, STEFFENS D, et al. HA-hybrid matrix composite coating on Ti-Cp for biomedical application. J Mater Sci Mater Med. 2020;31(10):82. [35] ADAMSKI R, SIUTA D. Mechanical, Structural, and Biological Properties of Chitosan/Hydroxyapatite/Silica Composites for Bone Tissue Engineering. Molecules. 2021;26(7):1976. [36] STEVANOVIC M, DJOSIC M, JANKOVIC A, et al. Antibacterial graphene-based hydroxyapatite/chitosan coating with gentamicin for potential applications in bone tissue engineering. J Biomed Mater Res A. 2020; 108(11):2175-2189. [37] NAKAMURA M, NAGAI A, TANAKA Y, et al. Polarized hydroxyapatite promotes spread and motility of osteoblastic cells. J Biomed Mater Res A. 2010;92(2): 783-790. [38] QIAO SC, DU J, ZHAO JM, et al. Effects of a hydroxyapatite-coated nanotube surface of titanium on MC3T3-E1 cells: an in vitro study. Implant Dent. 2015;24(2): 204-210. |
| [1] | Peng Zhixin, Yan Wengang, Wang Kun, Zhang Zhenjiang. Finite element analysis and structural optimization design of 3D printed forearm braces [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1340-1345. |
| [2] | He Yinhao, Li Xiaosheng, Chen Hongwen, Chen Tiezhu. 3D printed porous tantalum metal in the treatment of developmental dysplasia of the hip: current status and application prospect [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1455-1461. |
| [3] | Wang Yanjin, Zhou Yingjie, Chai Xubin, Zhuo Hanjie. Meta-analysis of the efficacy and safety of 3D printed porous titanium alloy fusion cage in anterior cervical discectomy and fusion [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(9): 1434-1440. |
| [4] | Zhang Tingting, Liu Juan, Zhang Xu. Bioactivity of phase-transition lysozyme for surface modification of zirconia all-ceramic implant material mediating hydroxyapatite coating [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(7): 1043-1049. |
| [5] | Chai Hao, Yang Deyong, Zhang Lei, Shu Li. 3D printing personalized osteotomy guide technology versus conventional total knee arthroplasty on the accuracy of lower limb force alignment: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(4): 646-654. |
| [6] | Jiang Haifang, Liu Rong, Hu Peng, Chen Wei, Wei Zairong, Yang Chenglan, Nie Kaiyu. Application of 3D printing technology in the precise and personalized treatment of cleft lip and palate [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 413-419. |
| [7] | Dai Xianglin, Zhang Wenfeng, Yao Xijun, Shang Jiaqi, Huang Qiujin, Ren Yifan, Deng Jiupeng. Barium titanate/polylactic acid piezoelectric composite film affects adhesion, proliferation, and osteogenic differentiation of MC3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 367-373. |
| [8] | Zhai Hongjie, Han Guanda, Li Lei, Dong Xiaohui, Jiang Zhiquan, Lou Feiyun. 3D printed polyetheretherketone material for skull defect repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 380-384. |
| [9] | Huang Dingan, Liu Chen. Three-dimensional finite element analysis of a new height adjustable cervical fusion cage [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(18): 2797-2803. |
| [10] | Jiang Chengming, Jiang Sainan, Tang Ye, Jiang Lin. Efficacy of 3D printed microporous titanium fusion device applied to anterior cervical decompression graft fusion and its effects on cervical spine anatomy and stress hormones [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(18): 2837-2841. |
| [11] | Zhang Hong, Wu Aimin, Li Junwei, Cai Xinyi, Ren Yanan, Du Chengfei. Biomechanical characteristics of integrated expandable repositionable cage through lateral anterior approach [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(13): 1975-1980. |
| [12] | Wu Di, Si Lina, Wu Lizhu, Wang Jianhua, Luo Jinwei, Chang Qiankun, Lyu Yongming, Du Yuanliang. Feasibility of three-dimensional printing technology combined with computer-aided design in total knee arthroplasty for severe knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(13): 2051-2057. |
| [13] | Yang Lian, Feng Haiyang, Xu Yuanjing. Current status of 3D printing medical applications and center construction [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(13): 2110-2115. |
| [14] | Huang Yanni, Yang Hua, Yang Dongmei, Hu Xulin, Gao Hong, Huang Yina. Rat bone defect repaired with polytrimethylene carbonate/beta-tricalcium phosphate microsphere scaffold [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1856-1862. |
| [15] | Li Xiaoxue, Hou Xiaowei. Finite element analysis of maxillary defect reconstruction based on polyetheretherketone meshes [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1805-1810. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||