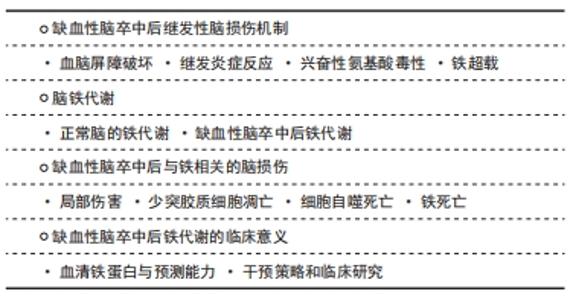
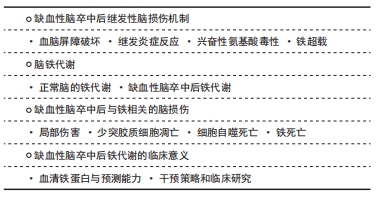
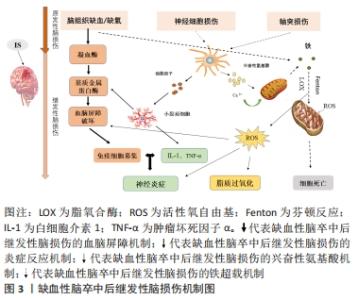
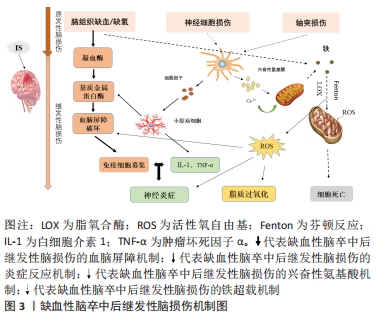
Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (32): 5223-5228.doi: 10.12307/2022.918
Previous Articles Next Articles
Relationship between iron metabolism and ischemic stroke
Fan Yongfu1, Su Kaiqi1, Yuan Jie1, Nie Chenchen1, Ruan Xiaodi1, Duan Zhaoyuan1, Feng Xiaodong1, 2
- 1Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China; 2Rehabilitation Center, the First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China
-
Received:2021-12-16Accepted:2022-01-29Online:2022-11-18Published:2022-05-14 -
Contact:Feng Xiaodong, Chief physician, Doctoral supervisor, Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China; Rehabilitation Center, the First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
About author:Fan Yongfu, Master candidate, Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
Supported by:the National Natural Science Foundation of China (General Project), No. 82174473 (to FXD); the National Natural Science Joint Fund Project, No. U2004131 (to FXD); Henan Provincial Traditional Chinese Medicine Scientific Research Special Project, No. 2018JDZX011 (to FXD); Henan Provincial Science and Technology Research Plan, No. 202102310167 (to FXD)
CLC Number:
Cite this article
Fan Yongfu, Su Kaiqi, Yuan Jie, Nie Chenchen, Ruan Xiaodi, Duan Zhaoyuan, Feng Xiaodong. Relationship between iron metabolism and ischemic stroke[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(32): 5223-5228.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
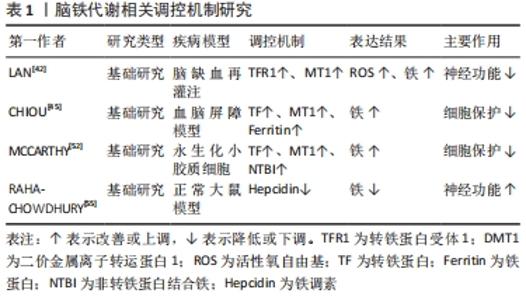
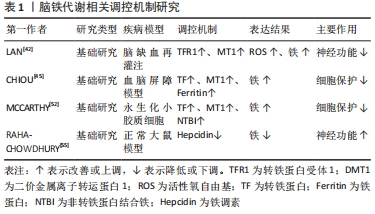
2.1.1 血脑屏障破坏 血脑屏障在维持大脑生化环境稳态中发挥着至关重要的作用[18],同时阻止血液中的有害物质如神经毒性物质、炎症因子、免疫细胞等进入中枢神经系统[19]。大量研究表明,血脑屏障的发育和功能维持离不开小胶质细胞的协同调控[20]。而脑缺血缺氧易导致小胶质细胞大量释放基质金属蛋白酶[21],将引起中枢神经系统中细胞外基质的降解,使紧密连接蛋白的表达和分布改变[22],从而使血脑屏障通透性增加,血液中的有害物质及大分子蛋白质可以通过血脑屏障从血液系统进入脑组织[23],进一步加重缺血性脑卒中后脑损伤。 2.1.2 继发炎症反应 神经炎症是缺血性脑卒中后继发性脑损伤的关键病理生理机制[24],并通过氧化应激等加剧神经功能损害[25]。当发生缺血性脑卒中时,小胶质细胞受到缺血刺激过度活化[26],引起肿瘤坏死因子α、白细胞介素1等促炎因子释放,诱导慢性炎症的渐进循环[27]。此外,除小胶质细胞活化外,脑缺血后巨噬细胞、中性粒细胞和淋巴细胞等免疫细胞通过破坏的血脑屏障聚集到受伤部位产生过量的脂质活性氧自由基[28],加重缺血性脑卒中后的炎症反应。 2.1.3 兴奋性氨基酸毒性 同样,兴奋性氨基酸的释放增加也与缺血性脑卒中后的神经功能损伤有关[29]。兴奋性氨基酸广泛存在于中枢神经系统中,其中以谷氨酸在大脑内含量最高。正常生理条件下,谷氨酸对维持中枢兴奋性突触传递信号起关键作用[30]。脑缺血时神经细胞的供血和供氧受到影响,能量耗竭,从而导致膜电位发生去极化[31],一方面引起突触前膜钙离子通道开放,大量释放谷氨酸至突触间隙[32];另一方面神经元对谷氨酸再摄取也受到抑制[33],细胞外谷氨酸进一步堆积,使得突触间隙内的谷氨酸维持在较高水平,对神经元直接产生毒性作用,造成神经元损伤甚至死亡[34]。由于持续性兴奋性氨基酸释放导致神经元死亡的现象也被称为“兴奋性氨基酸毒性”。 2.1.4 铁超载 缺血性脑卒中后大脑特定区域异常铁超载也是引起细胞死亡导致疾病进展的重要原因[35]。细胞正常生命活动的进行离不开铁的存在,因此维持大脑内铁稳态对细胞生存是格外重要的[36]。铁超载时,一方面Fe2+通过芬顿反应产生大量的脂质活性氧自由基[37];另一方面Fe2+参与合成脂氧合酶,进而催化脂质过氧化反应[38],二者都可诱导细胞发生铁死亡。在单侧、短暂的脑中动脉阻塞小鼠模型中,观察到缺血脑区铁的蓄积[39],同时在缺血性脑卒中患者血清中也观察到了铁离子水平明显升高[40]。此外,铁积累的增加也与缺血性脑卒中后神经损伤程度呈正相关[41]。因此,铁积累伴随着整个病理过程,铁被认为是缺血性脑卒中后继发性损伤中涉及的一个重要的病理生理因素[11,42]。 2.2 脑铁代谢 2.2.1 正常脑的铁代谢 铁是大脑新陈代谢所必需的,是大脑中含量最高的金属[13]。大脑的正常功能需要铁稳态的维持,而铁稳态取决于铁吸收、储存和释放[43],分为整体和细胞两个水平的调控。从整体水平看,循环系统中的铁通过血脑屏障进入大脑,主要有2种形式:转铁蛋白结合转铁蛋白受体复合物和非转铁蛋白结合的铁[44]。因此,血脑屏障在维持大脑铁稳态中发挥着极其重要的作用,保护大脑免受全身铁含量波动的影响。此外,研究报道脉络丛内皮细胞也是人体血脑屏障模型中铁运输的关键调节器,调节铁进入中枢神经系统[45]。而细胞水平中铁的吸收和储存则取决于细胞质内铁调节蛋白1和铁调节蛋白2的共同调节[46],当细胞缺铁时,铁调节蛋白与铁蛋白mRNA的铁反应元件结合来抑制铁蛋白的转化以减少铁的储存[47],同时其在核受体共激活因子 4的作用下,铁蛋白通过选择性自噬被运送到溶酶体降解来释放出自由铁用于细胞吸收[48];而当细胞内铁浓度高时,则进行相反的过程以降低细胞内铁含量,从而维持大脑内铁稳态。 2.2.2 缺血性脑卒中后铁代谢 研究表明,大脑的铁稳态在缺血性脑卒中后被破坏[49],继而阻碍缺血性脑卒中后神经功能恢复。缺血性脑卒中后脑铁代谢紊乱的可能原因是铁吸收和铁释放过程不平衡[50]。在脑组织处于缺血缺氧的病理条件下,脑内铁蛋白、转铁蛋白和转铁蛋白受体的表达将增加[42,51],引起神经元和少突胶质细胞摄取铁增多;同时缺血缺氧的酸性环境将引起小胶质细胞中二价金属离子转运蛋白1过表达[52],使非转铁蛋白结合铁的水平提高,引起脑铁含量增加。此外,研究发现缺血性脑卒中后患者血清中的铁调素和铁浓度会升高[40],而铁调素通过与细胞膜上铁转运蛋白1结合来调控细胞铁释放[53]。脑缺血时诱导白细胞介素6的表达增多,继而白细胞介素6通过 JAK/STAT3通路上调铁调素的表达[54],导致铁转运蛋白的下调,从而增强铁的吸收能力,减弱铁的释放能力,加剧了铁在细胞中的积累[55]。因此,在缺血性脑卒中后合理应用铁代谢抑制剂如去铁胺降低脑内铁离子含量,可以减少神经元死亡,促进缺血性脑卒中后神经功能恢复。 脑铁代谢相关调控机制研究总结,见表1。 "
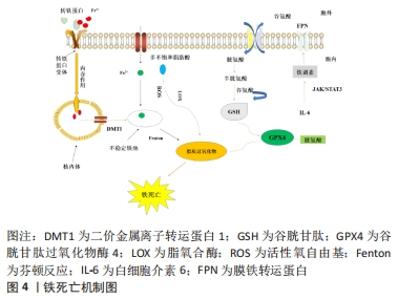
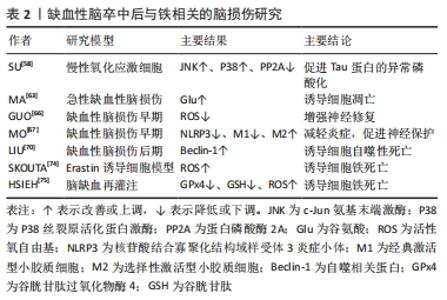
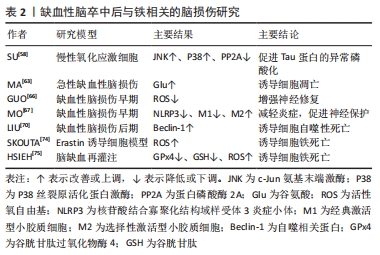
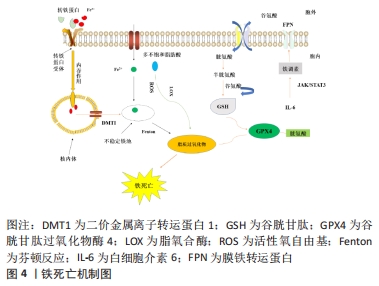
2.3 缺血性脑卒中后与铁相关的脑损伤 2.3.1 局部伤害 铁在大脑中的沉积可能会诱发局部病变。Tau蛋白是含量最丰富的微管相关蛋白[56],对于维持神经元、轴突和树突的生长发育起到了重要作用。而缺血缺氧会诱导Tau蛋白在神经元、星形胶质细胞和皮质深层突触的异常聚集[57],引起神经功能的损伤。同时在此过程中,积累的铁通过氧化应激和聚集体促进缺血性脑卒中患者大脑中Tau蛋白的异常磷酸化[39,58-59],形成神经纤维缠结,使其促进铁转运至细胞外的能力减弱,引起细胞内铁浓度增高,继而产生神经毒性甚至造成细胞死亡,这也是卒中损伤的新机制。故抑制铁沉积可能会通过维持Tau蛋白的正常功能来减少细胞死亡,促进损伤修复。 2.3.2 少突胶质细胞凋亡 脑组织内少突胶质细胞含大量铁和铁蛋白[60],由于铁离子浓度高导致其清除自由基能力削弱且需依赖于氧化磷酸化作用,故少突胶质细胞更易受到氧化应激损伤而凋亡[61-62]。当缺血性脑卒中发生时,脑组织因缺血缺氧使脑室周围谷氨酸浓度升高,接着与少突胶质细胞上的谷氨酸受体过度结合[63],造成少突胶质细胞损伤、凋亡,而铁可能在这一过程中发挥关键作用[64]。因此,在缺血性脑卒中早期通过调控铁代谢平衡和抑制少突胶质细胞凋亡将可能有助于促进受损脑组织修复,这也是减轻缺血性脑卒中后神经损伤的新治疗策略。 2.3.3 细胞自噬死亡 自噬主要依赖溶酶体进行细胞内降解和循环再利用,从而利于细胞存活或加速细胞消亡[65],因此自噬在维持细胞内稳态和促进细胞更新与代谢方面发挥着双重作用。一方面,在缺血性脑损伤早期的适当自噬有利于清除受损组织线粒体,减少活性氧自由基的产生[66],并通过抑制炎症小体的激活和调节小胶质细胞的表型交替来削弱神经炎症[67],从而起到对神经元的保护作用。另一方面,在缺血性脑损伤后期由于细胞中铁离子过量进而激活氧化应激反应,使活性氧自由基产生增多,诱导自噬的发生[68],最终造成细胞损伤乃至死亡。同时越来越多的证据表明缺血性脑卒中后铁稳态失衡会诱导脑内细胞如神经元、胶质细胞和脑微血管细胞发生自噬而死亡[69],加重缺血性脑卒中后继发性脑损伤。此外,LIU等[70]研究发现脑缺血会使脑内自噬相关蛋白Beclin-1表达增多,引起自噬性细胞的死亡,而抑制神经元的过度自噬,则能够缓解缺血性脑卒中后脑损伤[71]。故自噬对缺血性脑损伤的影响是一把双刃剑,适度自噬可以通过降低氧化应激反应和削弱神经炎症来保护神经元,但过度自噬则会导致神经元死亡进而加重缺血性脑卒中后神经功能损伤。因此,在缺血性脑卒中后及时通过促进铁代谢平衡来调节自噬活动可减少自噬介导的脑损伤,有助于神经修复。 2.3.4 铁死亡 铁死亡是近年来新发现的一种受调控的细胞死亡形式,其主要表现为细胞内铁离子蓄积、脂质过氧化、线粒体体积皱缩和膜密度增厚[72]。铁死亡是以活性氧自由基和铁离子介导的脂质过氧化为主要特征[73],其死亡形式具有铁离子依赖的特点,因此被称为“铁死亡”。3个因素与铁死亡发生密切相关:铁、多不饱和脂肪酸和氨基酸,见图4。研究表明,铁增多是铁死亡的必要条件,而缺血性脑卒中后铁离子浓度将迅速升高进而促进大量的活性氧自由基产生,最终导致脂质过氧化和细胞铁死亡[14]。大脑有丰富的多不饱和脂肪酸,多不饱和脂肪酸容易受到脂氧合酶催化和活性氧的影响继而促进脂质过氧化物的形成[74],诱发铁死亡。缺血性脑卒中后突触前神经元释放大量谷氨酸,高水平的细胞外谷氨酸可以抑制胱氨酸,而胱氨酸是合成半胱氨酸的原料。生理条件下,半胱氨酸参与合成谷胱甘肽[75],而谷胱甘肽可以通过调节谷胱甘肽过氧化物酶4的活性来减少脂质过氧化物蓄积[76]。相关地,谷胱甘肽不足也会影响谷胱甘肽过氧化物酶4发挥作用,从而诱发细胞铁死亡[77]。因此,在缺血性脑卒中的急性期调控铁代谢并促进谷胱甘肽过氧化物酶4的表达可以改善铁依赖性的神经元死亡,促进脑损伤的恢复。"
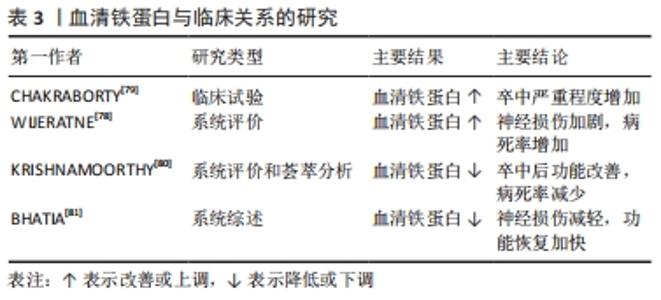
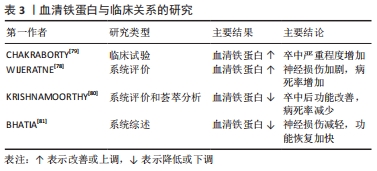
2.4 缺血性脑卒中后铁代谢的临床意义 2.4.1 血清铁蛋白与预测能力 血清铁蛋白水平对缺血性脑卒中的严重程度具有预测价值[78]。研究表明,缺血性脑卒中后血清铁蛋白水平与疾病进展相关[79]:研究人员招募了100例急性缺血性脑卒中患者和120名对照组,采用美国国立卫生研究院中风量表评估中风严重程度和免疫荧光法测定血清铁蛋白水平,结果发现,试验组的血清铁蛋白水平明显高于对照组,而血清铁蛋白水平越高,中风越严重,疾病进展越快。一项基于病例的系统评价显示[78]:急性缺血性脑卒中也正在成为新型冠状病毒肺炎的一项重要神经血管/神经系统并发症,同时研究人员发现较高的血清铁蛋白水平与新型冠状病毒肺炎合并急性缺血性脑卒中患者神经损伤有关,并造成75%的患者死亡或严重残疾。因此,合理应用血清铁蛋白指标检测缺血性脑卒中患者中风严重程度可能对后期神经损伤修复更有临床意义。 2.4.2 干预策略和临床研究 血清铁蛋白水平与缺血性脑卒中患者预后有关[80],神经功能恢复越差,其血清铁蛋白水平越高,故血清铁蛋白水平可作为缺血性脑卒中患者神经功能恢复的预测因子。以往研究发现,缺血性脑卒中后及时降低血清铁蛋白水平可以改善卒中后不良功能结局以及减少病死率[80]。同样,在一项系统综述中研究发现,缺血性脑卒中患者血清铁蛋白水平越低,预后越好[81]。因此,在临床应合理监测缺血性脑卒中患者血清铁蛋白水平,谨慎评估患者的预后风险,及时选用铁死亡抑制剂如铁螯合剂去铁酮能有效阻断铁诱导的脑损伤[82],促进缺血性脑卒中后神经功能恢复。 血清铁蛋白与临床关系的研究总结,见表3。 "
| [1] KIM SH, CHUNG DK, LEE YJ, et al. Neuroprotective effects of Danggui-Jakyak-San on rat stroke model through antioxidant/antiapoptotic pathway. J Ethnopharmacol. 2016;188:123-133. [2] HERPICH F, RINCON F. Management of Acute Ischemic Stroke. Crit Care Med. 2020;48(11):1654-1663. [3] GE JJ, XING YQ, CHEN HX, et al. Analysis of young ischemic stroke patients in northeast China. Ann Transl Med. 2020;8(1):3. [4] BARTHELS D, DAS H. Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol Basis Dis. 2020;1866(4):165260. [5] BENJAMIN EJ, BLAHA MJ, CHIUVE SE, et al. Heart Disease and Stroke Statistics- 2017 Update: A Report From the American Heart Association. Circulation. 2017; 135(10):e146-e603. [6] KHANDELWAL P, YAVAGAL DR, SACCO RL. Acute Ischemic Stroke Intervention. J Am Coll Cardiol. 2016;67(22):2631-2644. [7] YANG C, HAWKINS KE, DORé S, et al. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol. 2019;316(2): C135-C153. [8] MAGTANONG L, DIXON SJ. Ferroptosis and Brain Injury. Dev Neurosci. 2018; 40(5-6):382-395. [9] KIM JY, PARK J, CHANG JY, et al. Inflammation after Ischemic Stroke: The Role of Leukocytes and Glial Cells. Exp Neurobiol. 2016;25(5):241-251. [10] HERTELENDYP, TOLDI J, FüLöP F, et al. Ischemic Stroke and Kynurenines: Medicinal Chemistry Aspects. Curr Med Chem. 2018;25(42):5945-5957. [11] GUAN X, LI X, YANG X, et al. The neuroprotective effects of carvacrol on ischemia/reperfusion-induced hippocampal neuronal impairment by ferroptosis mitigation. Life Sci. 2019;235:116795. [12] RADAK D, KATSIKI N, RESANOVIC I, et al. Apoptosis and Acute Brain Ischemia in Ischemic Stroke. Curr Vasc Pharmacol. 2017;15(2):115-122. [13] ASHRAF A, CLARK M, SO PW. The Aging of Iron Man. Front Aging Neurosci. 2018; 10:65. [14] DATTA A, SARMAH D, MOUNICA L, et al. Cell Death Pathways in Ischemic Stroke and Targeted Pharmacotherapy. Transl Stroke Res. 2020;11(6):1185-1202. [15] PARK UJ, LEE YA, WON SM, et al. Blood-derived iron mediates free radical production and neuronal death in the hippocampal CA1 area following transient forebrain ischemia in rat. Acta Neuropathol. 2011;121(4):459-473. [16] VALDÉS HERNÁNDEZ MDC, CASE T, CHAPPELL FM, et al. Association between Striatal Brain Iron Deposition, Microbleeds and Cognition 1 Year After a Minor Ischaemic Stroke. Int J Mol Sci. 2019;20(6):1293. [17] SELIM M, FOSTER LD, MOY CS, et al. Deferoxamine mesylate in patients with intracerebral haemorrhage (i-DEF): a multicentre, randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2019;18(5):428-438. [18] NIAN K, HARDING IC, HERMAN IM, et al. Blood-Brain Barrier Damage in Ischemic Stroke and Its Regulation by Endothelial Mechanotransduction. Front Physiol. 2020;11:605398. [19] SWEENEY MD, ZHAO Z, MONTAGNE A, et al. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol Rev. 2019;99(1):21-78. [20] RONALDSON PT, DAVIS TP. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J Cereb Blood Flow Metab. 2020;40(1_suppl):S6-S24. [21] GURUSWAMY R, ELALI A. Complex Roles of Microglial Cells in Ischemic Stroke Pathobiology: New Insights and Future Directions. Int J Mol Sci. 2017;18(3):496. [22] ABDULLAHI W, TRIPATHI D, RONALDSON PT. Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol. 2018;315(3):C343-C356. [23] VARATHARAJ A, GALEA I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2017;60:1-12. [24] FADEN AI, WU J, STOICA BA, et al. Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br J Pharmacol. 2016;173(4):681-691. [25] ABBOTT NJ, PIZZO ME, PRESTON JE, et al. The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’system. Acta Neuropathol. 2018; 135(3):387-407. [26] JOLIVEL V, BICKER F, BINAMÉ F, et al. Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol. 2015;129(2):279-295. [27] LAN X, HAN X, LI Q, et al. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol. 2017;13(7):420-433. [28] AHMAD M, DAR NJ, BHAT ZS, et al. Inflammation in ischemic stroke: mechanisms, consequences and possible drug targets. CNS Neurol Disord Drug Targets. 2014; 13(8):1378-1396. [29] VALLON M, CHANG J, ZHANG H, et al. Developmental and pathological angiogenesis in the central nervous system. Cell Mol Life Sci. 2014;71(18):3489-3506. [30] ARCHIBALD J, MACMILLAN EL, ENZLER A, et al. Excitatory and inhibitory responses in the brain to experimental pain: A systematic review of MR spectroscopy studies. Neuroimage. 2020;215:116794. [31] MERELLI A, REPETTO M, LAZAROWSKI A, et al. Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases. J Alzheimers Dis. 2021;82(s1):S109-S126. [32] LIU Y, WANG S, KAN J, et al. Chinese Herbal Medicine Interventions in Neurological Disorder Therapeutics by Regulating Glutamate Signaling. Curr Neuropharmacol. 2020;18(4):260-276. [33] ZENDEDEL E, BUTLER AE, ATKIN SL, et al. Impact of curcumin on sirtuins: A review. J Cell Biochem. 2018;119(12):10291-10300. [34] FEDOROVICH SV, WASEEM TV. Metabolic regulation of synaptic activity. Rev Neurosci. 2018;29(8):825-835. [35] WEILAND A, WANG Y, WU W, et al. Ferroptosis and Its Role in Diverse Brain Diseases. Mol Neurobiol. 2019;56(7):4880-4893. [36] ALMUTAIRI MMA, XU G, SHI H. Iron Pathophysiology in Stroke. Adv Exp Med Biol. 2019;1173:105-123. [37] STOCKWELL BR, ANGELI JPF, BAYIR H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273-285. [38] LEI P, BAI T, SUN Y. Mechanisms of ferroptosis and relations with regulated cell death: a review. Front Physiol. 2019;10:139. [39] TUO Q, LEI P, JACKMAN K, et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry. 2017;22(11):1520-1530. [40] REICHE EMV, GELINKSI JR, ALFIERI DF, et al. Immune-inflammatory, oxidative stress and biochemical biomarkers predict short-term acute ischemic stroke death. Metab Brain Dis. 2019;34(3):789-804. [41] ZHANG Y, LU X, TAI B, et al. Ferroptosis and Its Multifaceted Roles in Cerebral Stroke. Front Cell Neurosci. 2021;15:615372. [42] LAN B, GE JW, CHENG SW, et al. Extract of Naotaifang, a compound Chinese herbal medicine, protects neuron ferroptosis induced by acute cerebral ischemia in rats. J Integr Med. 2020;18(4):344-350. [43] QIAN ZM, KE Y. Brain iron transport. Biol Rev Camb Philos Soc. 2019;94(5):1672-1684. [44] MCCARTHY RC, KOSMAN DJ. Iron transport across the blood-brain barrier: development, neurovascular regulation and cerebral amyloid angiopathy. Cell Mol Life Sci. 2015;72(4):709-727. [45] CHIOU B, NEAL EH, BOWMAN AB, et al. Endothelial cells are critical regulators of iron transport in a model of the human blood-brain barrier. J Cereb Blood Flow Metab. 2019;39(11):2117-2131. [46] YU P, CHANG YZ. Brain Iron Metabolism and Regulation. Adv Exp Med Biol. 2019; 1173:33-44. [47] ZHOU ZD, TAN EK. Iron regulatory protein (IRP)-iron responsive element (IRE) signaling pathway in human neurodegenerative diseases. Mol Neurodegener. 2017;12(1):75. [48] QUILES DEL REY M, MANCIAS JD. NCOA4-Mediated Ferritinophagy: A Potential Link to Neurodegeneration. Front Neurosci. 2019;13:238. [49] WAN J, REN H, WANG J. Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage. Stroke Vasc Neurol. 2019;4(2):93-95. [50] SPENCE H, MCNEIL CJ, WAITER GD. The impact of brain iron accumulation on cognition: A systematic review. PLoS One. 2020;15(10):e0240697. [51] ZHOU F, YU T, DU R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. [52] MCCARTHY RC, SOSA JC, GARDECK AM, et al. Inflammation-induced iron transport and metabolism by brain microglia. J Biol Chem. 2018;293(20):7853-7863. [53] PETROVA J, MANOLOV V, VASILEV V, et al. Ischemic stroke, inflammation, iron overload - Connection to a hepcidin. Int J Stroke. 2016;11(1):NP16-NP17. [54] KANAMORI Y, MURAKAMI M, SUGIYAMA M, et al. Hepcidin and IL-1β. Vitam Horm. 2019;110:143-156. [55] RAHA-CHOWDHURY R, RAHA AA, FOROSTYAK S, et al. Expression and cellular localization of hepcidin mRNA and protein in normal rat brain. BMC Neurosci. 2015;16:24. [56] LV HY, WU SJ, WANG QL, et al. Effect of erythropoietin combined with hypothermia on serum tau protein levels and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Neural Regen Res. 2017; 12(10):1655-1663. [57] CHEN X, JIANG H. Tau as a potential therapeutic target for ischemic stroke. Aging (Albany NY). 2019;11(24):12827-12843. [58] SU B, WANG X, LEE HG, et al. Chronic oxidative stress causes increased tau phosphorylation in M17 neuroblastoma cells. Neurosci Lett. 2010;468(3):267-271. [59] LIU Y, LIU J, LIU H, et al. Investigation of cerebral iron deposition in aged patients with ischemic cerebrovascular disease using susceptibility-weighted imaging. Ther Clin Risk Manag. 2016;12:1239-1247. [60] REINERT A, MORAWSKI M, SEEGER J, et al. Iron concentrations in neurons and glial cells with estimates on ferritin concentrations. BMC Neurosci. 2019;20(1):25. [61] MUKHERJEE C, KLING T, RUSSO B, et al. Oligodendrocytes Provide Antioxidant Defense Function for Neurons by Secreting Ferritin Heavy Chain. Cell Metab. 2020;32(2):259-272.e10. [62] ROTH AD, NúñEZ MT. Oligodendrocytes: Functioning in a Delicate Balance Between High Metabolic Requirements and Oxidative Damage. Adv Exp Med Biol. 2016;949:167-181. [63] MA Y, WANG J, WANG Y, et al. The biphasic function of microglia in ischemic stroke. Prog Neurobiol. 2017;157:247-272. [64] CHELI VT, CORREALE J, PAEZ PM, et al. Iron Metabolism in Oligodendrocytes and Astrocytes, Implications for Myelination and Remyelination. ASN Neuro. 2020;12:1759091420962681. [65] LIU Y, LEVINE B. Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ. 2015;22(3):367-376. [66] GUO QQ, WANG SS, ZHANG SS, et al. ATM-CHK2-Beclin 1 axis promotes autophagy to maintain ROS homeostasis under oxidative stress. EMBO J. 2020; 39(10):e103111. [67] MO Y, SUN YY, LIU KY. Autophagy and inflammation in ischemic stroke. Neural Regen Res. 2020;15(8):1388-1396. [68] JHELUM P, SANTOS-NOGUEIRA E, TEO W, et al. Ferroptosis Mediates Cuprizone- Induced Loss of Oligodendrocytes and Demyelination. J Neurosci. 2020;40(48): 9327-9341. [69] WANG P, SHAO BZ, DENG Z, et al. Autophagy in ischemic stroke. Prog Neurobiol. 2018;163-164. [70] LIU Y, SHOJI-KAWATA S, SUMPTER RM JR, et al. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc Natl Acad Sci U S A. 2013;110(51):20364-20371. [71] WANG LF, YOKOYAMA KK, CHEN TY, et al. Male-Specific Alleviation of Iron-Induced Striatal Injury by Inhibition of Autophagy. PLoS One. 2015;10(7):e0131224. [72] XIE Y, HOU W, SONG X, et al. Ferroptosis: process and function. Cell Death & Differentiation. 2016;23(3):369-379. [73] SMITH RE, TRAN K, SMITH CC, et al. The role of the Nrf2/ARE antioxidant system in preventing cardiovascular diseases. Diseases. 2016;4(4):34. [74] SKOUTA R, DIXON SJ, WANG J, et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136(12):4551-4556. [75] HSIEH CH, LIN YJ, CHEN WL, et al. HIF-1α triggers long-lasting glutamate excitotoxicity via system x in cerebral ischaemia-reperfusion. J Pathol. 2017; 241(3):337-349. [76] PHILPOTT CC, RYU MS. Special delivery: distributing iron in the cytosol of mammalian cells. Front Pharmacol. 2014;5:173. [77] HIRSCHHORN T, STOCKWELL BR. The development of the concept of ferroptosis. Free Radic Biol Med. 2019;133:130-143. [78] WIJERATNE T, SALES C, KARIMI L, et al. Acute Ischemic Stroke in COVID-19: A Case-Based Systematic Review. Front Neurol. 2020;11:1031. [79] CHAKRABORTY B, VISHNOI G, GOSWAMI B, et al. Lipoprotein(a), ferritin, and albumin in acute phase reaction predicts severity and mortality of acute ischemic stroke in North Indian Patients. J Stroke Cerebrovasc Dis. 2013;22(7):e159-167. [80] KRISHNAMOORTHY S, SINGH G, JOSE KJ, et al. Biomarkers in the Prediction of Hemorrhagic Transformation in Acute Stroke: A Systematic Review and Meta-Analysis. Cerebrovasc Dis. 2021:1-13. doi: 10.1159/000518570. [81] BHATIA R, PEDAPATI R, KOMAKULA S, et al. Stroke in Coronavirus Disease 2019: A Systematic Review. J Stroke. 2020;22(3):324-335. [82] DEVOS D, CABANTCHIK ZI, MOREAU C, et al. Conservative iron chelation for neurodegenerative diseases such as Parkinson’s disease and amyotrophic lateral sclerosis. J Neural Transm (Vienna). 2020;127(2):189-203. |
| [1] | Nong Fuxiang, Jiang Zhixiong, Li Yinghao, Xu Wencong, Shi Zhilan, Luo Hui, Zhang Qinglang, Zhong Shuang, Tang Meiwen. Bone cement augmented proximal femoral nail antirotation for type A3.3 intertrochanteric femoral fracturalysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(在线): 1-10. |
| [2] | Nie Chenchen, Su Kaiqi, Gao Jing, Fan Yongfu, Ruan Xiaodi, Yuan Jie, Duan Zhaoyuan, Feng Xiaodong. The regulatory role of circular RNAs in cerebral ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1286-1291. |
| [3] | Tian Qinyu, Tian Xinggui, Tian Zhuang, Sui Xiang, Liu Shuyun, Lu Xiaobo, Guo Quanyi. Protection of manganese oxide nanoparticles for bone marrow mesenchymal stem cell spreading against oxidative stress [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 821-826. |
| [4] | Li Qicheng, Deng Jin, Fu Xiaoyang, Han Na. Effects of bone marrow mesenchymal stem cells-derived exosomes on hypoxia-treated myoblasts [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 853-859. |
| [5] | Fan Xiao, Tao Jingwei, Jiang Shengyuan, Deng Bowen, Mu Xiaohong. Effect of tetramethylpyrazine on iron metabolism after spinal cord injury in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(22): 3561-3566. |
| [6] | Nong Fuxiang, Jiang Zhixiong, Li Yinghao, Xu Wencong, Shi Zhilan, Luo Hui, Zhang Qinglang, Zhong Shuang, Tang Meiwen. Application and role of exosome-regulated ferroptosis in disease diagnosis and treatment [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(15): 2443-2452. |
| [7] | Li Yong, Yuan Jianmei, Lu Danni, Ren Mihong, Deng Bowen, Wang Jiajun, Ma Rong, Xie Qian, Li Jinxiu, Xu Zhuo, Wang Jian. Comparison of cerebral microcirculation perfusion in rat models of middle cerebral artery occlusion prepared through common carotid artery insertion and external carotid artery insertion [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(11): 1683-1691. |
| [8] | Fu Xinya, Ma Jinjin, Li Meimei, Wang Xingran, Xie Jile, Saijilafu. Establishment of an in vitro model of H2O2-induced neuronal senescence [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(11): 1733-1738. |
| [9] | Hu Fei, Wang Jie. Safety and efficacy of mesenchymal stem cells in the treatment of ischemic stroke: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 76-82. |
| [10] | Jin Tao, Liu Lin, Zhu Xiaoyan, Shi Yucong, Niu Jianxiong, Zhang Tongtong, Wu Shujin, Yang Qingshan. Osteoarthritis and mitochondrial abnormalities [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1452-1458. |
| [11] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [12] | Yan Nan, Wu Yanlong, Tang Xiaohui, Zhang Xiaoyan, Wang Hui, Yang Tianze, Zhou Maochun, Wang Zhengdong, Yang Xiaoxia. Bone marrow mesenchymal stem cells may alleviate brain damage caused by the microglial overactivation in the cortex around ischemic site of stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3790-3795. |
| [13] | Chen Na, Wang Xiaohan, Zhang Yunke. Effect and mechanism of mesenchymal stem cells on aging-related ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3914-3920. |
| [14] | Xu Wenshan, Jiang Pingli, Liu Yulu, Ding Yanyi, Yu Yan, Yang Minguang, Liu Weilin, Chen Lidian. Salvianolic acid A effects on hippocampal protein expression in ischemic stroke rats: a tandem mass tag-based proteomic analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(23): 3714-3720. |
| [15] | Xiakeerzhati•Xiaohalati, Wang Xiaobei, Wang Lin. Nanoparticles: a novel strategy for the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(22): 3566-3572. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||