中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (18): 3885-3896.doi: 10.12307/2025.658
• 组织构建综述 tissue construction review • 上一篇 下一篇
脊髓损伤后细胞自噬的调控治疗机制及策略
杨溢珂1,2,任亚锋2,李 冰2,尚文雅1,黄 靖1,郭 佳1,刘慧瑶1
- 1河南中医药大学康复医学院,河南省郑州市 450046;2河南中医药大学第一附属医院,河南省郑州市 450000
-
收稿日期:2024-06-07接受日期:2024-08-12出版日期:2025-06-28发布日期:2024-11-29 -
通讯作者:任亚锋,博士,主任医师,硕士生导师,河南中医药大学第一附属医院,河南省郑州市 450000 -
作者简介:杨溢珂,女,2001年生,河南省郑州市人,汉族,河南中医药大学在读硕士,主要从事脊髓损伤后的康复治疗研究。 -
基金资助:河南省中医药科学研究专项(2022JDZX015,2021JDZY022),项目负责人:任亚锋;河南省“双一流”创建学科中医学科学研究专项(HSRP-DFCTCM-2023-1-25),项目负责人:任亚锋
Regulatory mechanisms and therapeutic strategies for cellular autophagy after spinal cord injury
Yang Yike1, 2, Ren Yafeng2, Li Bing2, Shang Wenya1, Huang Jing1, Guo Jia1, Liu Huiyao1
- 1Rehabilitation Medicine College, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China; 2The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China
-
Received:2024-06-07Accepted:2024-08-12Online:2025-06-28Published:2024-11-29 -
Contact:Ren Yafeng, MD, Chief physician, Master’s supervisor, the First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
About author:Yang Yike, Master candidate, Rehabilitation Medicine College, Henan University of Chinese Medicine, Zhengzhou 450046, Henan Province, China; The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou 450000, Henan Province, China -
Supported by:Henan Province Traditional Chinese Medicine Science Research Special Project, Nos. 2022JDZX015 and 2021JDZY022 (to RYF); “Double First Class” Creation Engineering Traditional Chinese Medicine Discipline Project of Henan University of Chinese Medicine, No. HSRP-DFCTCM-2023-1-25 (to RYF)
摘要:
文题释义:
细胞自噬:是一种真核细胞特有的细胞内降解和再循环过程,在细胞稳态和防止神经变性中起重要作用。自噬缺陷会导致神经元损伤,进而导致脊髓损伤后神经修复能力受损。
脊髓损伤:是中枢神经系统的一种严重创伤,常导致损伤节段以下的神经功能障碍,从而显著影响患者的生活质量,其病理生理机制复杂,临床治疗效果有限。
背景:细胞自噬通过自噬体-溶酶体降解途径来维持代谢和体内平衡,其与脊髓损伤后远端神经元细胞死亡和功能恢复受损密切相关,靶向细胞自噬来促进脊髓损伤后功能恢复是比较有前景的治疗方向。
目的:对细胞自噬在脊髓损伤中的作用、细胞自噬的相关调控机制及治疗策略进行归纳总结。
方法:以“spinal cord injury,autophagy,regulatory mechanisms,autophagy pathway,therapeutic target”为检索词,检索PubMed数据库;以“脊髓损伤,细胞自噬,调控机制,自噬通路,治疗靶点”为检索词,检索中国知网,最终纳入133篇英文文献和4篇中文文献。
结果与结论:①细胞自噬作为细胞程序性死亡方式的一种,在脊髓损伤的进展和治疗中起着至关重要的作用。大多数研究表明,适度激活或促进自噬能够通过减少炎症反应和细胞凋亡来促进神经功能的恢复;少数研究表明,过度激活自噬相反,会阻碍脊髓损伤后的功能恢复。②脊髓损伤后,PI3K/AKT/mTOR、MAPK、AMPK、p53信号通路及Beclin-1、ATG和LC3等因子正向或负向调节细胞自噬的发生发展。③促进或抑制自噬可能是调控创伤性脊髓损伤发病机制的有前景的治疗策略,西药氨氯地平、二甲双胍、米诺环素,中医药山楂叶总黄酮、白桦脂酸、氧化苦参碱、针灸,以及细胞外囊泡、外泌体、活性氧响应的复合纤维等作为细胞自噬的激活剂,通过激活细胞自噬,减轻脊髓损伤的继发性损伤反应;而西药胰岛素样生长因子1、依拉达奉,中医药人参皂苷、针灸,以及水凝胶搭载碱性成纤维细胞生长因子作为细胞自噬的抑制剂,通过抑制过度的细胞自噬来促进脊髓损伤后的功能恢复。④细胞自噬的相关调控因子之间相互联系,而细胞自噬对于脊髓损伤的双向作用使得调控细胞自噬的主导因素尚需进一步探讨。⑤以自噬作为脊髓损伤治疗靶点的研究多在动物模型中进行,尚无应用于临床领域的自噬相关药物,其安全性和有效性还需进一步的临床研究。
https://orcid.org/0009-0002-5451-4739(杨溢珂)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号:
引用本文
杨溢珂, 任亚锋, 李 冰, 尚文雅, 黄 靖, 郭 佳, 刘慧瑶. 脊髓损伤后细胞自噬的调控治疗机制及策略[J]. 中国组织工程研究, 2025, 29(18): 3885-3896.
Yang Yike, Ren Yafeng, Li Bing, Shang Wenya, Huang Jing, Guo Jia, Liu Huiyao . Regulatory mechanisms and therapeutic strategies for cellular autophagy after spinal cord injury[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(18): 3885-3896.
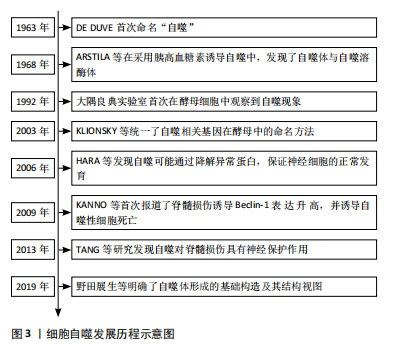
2.1.1 细胞自噬的研究历程 1963年,比利时科学家Christian de Duve在溶酶体国际会议上首次将细胞内细胞器和部分细胞质纳入溶酶体的过程称为 “自噬”[11],并因此与Albert Claude和George E.Palade共同获得了1974年的诺贝尔生理学或医学奖;紧接着,1968年ARSTILA等[12]在采用胰高血糖素诱导自噬的过程中,发现细胞内存在一种包裹了细胞组分却无水解酶的双层膜结构——自噬体,以及另一种含有多种水解酶的单层膜结构——自噬溶酶体。但在之后的将近30年里,这一领域却没有被科学家们广泛关注。直到1992年,大隅良典实验室发现一株由于突变导致液泡分解酶缺失的酵母细胞系[13],发现其在饥饿的情况下,在数个小时内酵母液泡中会形成大量的囊泡,即自噬小体,这项实验结果首次揭示了酵母细胞中存在自噬现象,这一发现在自噬研究领域掀起了一股新的浪潮。而后随着自噬相关基因及信号通路不断被揭示,Klionsky、大隅良典等专家[14]为促进自噬领域的发展,联合发表文章,统一了酿酒酵母中自噬相关基因的命名方式,即自噬相关基因(autophagy-related genes,ATG)。随后,在选择性自噬与疾病关系的研究中,HARA等[15]发现自噬可能通过清除异常蛋白质,防止不正常蛋白的积聚,来保证神经细胞的正常发育。KANNO等[16]在2009年首次报道了脊髓损伤后 Beclin-1蛋白表达的改变,并诱导自噬性细胞死亡,从而导致脊髓损伤后的神经组织损伤。而TANG等[17]在2014年发表的文章认为自噬的诱导可以通过抑制细胞凋亡对大鼠急性脊髓损伤产生神经保护作用。随着自噬研究的深入,自噬体形成的基础构造及其结构视图逐渐进入研究者的视野[18]。细胞自噬的研究历程见图3。
2.1.2 不同细胞群自噬在脊髓损伤中的表现 KANNO等[16]研究表明,脊髓损伤后自噬相关蛋白的表达从4 h开始增加,在3 d达到峰值,且在神经元、神经胶质细胞中均可观察到自噬相关蛋白的表达,表明这些细胞群自噬均参与脊髓损伤后的病理生理过程。近年来,随着自噬研究的不断深入,小胶质细胞自噬[19]、少突胶质细胞自噬[20]、神经元自噬等对脊髓损伤的功能修复作用受到了国内外学者的广泛关注[21]。现就以上3类细胞群的自噬对脊髓损伤的修复作用进行综述。
(1)小胶质细胞自噬:小胶质细胞在维持中枢神经系统平衡方面起着关键作用,它们构成了负责启动神经炎症反应的主要细胞类型[22]。脊髓损伤后,病变部位的小胶质细胞在损伤后1-3 d主要呈现促炎极化,表现为促炎因子肿瘤坏死因子α和白细胞介素6的释放[23],这些炎症递质可促进神经元凋亡,进而加重损伤。因此,在损伤早期阶段减少促炎性小胶质细胞对于脊髓损伤的修复具有重要意义。LIU等[24]研究表明,增强促炎小胶质细胞的自噬可减轻神经炎症,进而减少神经元的凋亡。而小胶质细胞自噬受损则会增强小胶质细胞的促炎性激活,进而加剧小鼠脊髓损伤后的神经炎症[25]。WU等[26]的研究同样表明增强小胶质细胞的自噬流,对于促进髓鞘碎片的清除进而促进脊髓损伤后髓鞘的保存和神经修复至关重要。
(2)少突胶质细胞自噬:在中枢神经系统中,少突胶质细胞负责髓鞘的形成和维持。脊髓损伤早期少突胶质细胞的大量丢失可能是神经功能严重受损的基础,而晚期少突胶质细胞的丢失可能进一步促进轴突损伤,导致神经功能更难恢复[27]。WANG等[28]研究证明,上调脊髓损伤后少突胶质细胞自噬水平可以加快炎症因子的清除,减轻神经元炎症,促进神经元存活,从而加速脊髓损伤后运动功能的恢复。MEI等[20]的研究同样表明增加脊髓损伤后LC3B、p62表达来促进少突胶质细胞的自噬与促进脊髓损伤后神经元再生相关。反之,特异性敲除少突胶质细胞中的自噬相关基因,则会导致少突胶质细胞因自噬流减少而表现出神经元增殖、分化减少,凋亡和内质网应激增加,进而限制脊髓损伤的功能恢复[29]。
(3)神经细胞自噬:脊髓损伤后,神经元的大量死亡严重影响神经再生及神经功能的恢复[30]。而适度的自噬可以清除受损的蛋白质和细胞器,并将其分解成小分子物质释放到细胞质中,实现物质的循环利用,减少细胞死亡[31]。若能在脊髓神经细胞损伤早期一定程度上促进自噬,也能在一定程度上减轻继发性脊髓神经细胞损伤[8]。CHANG等[32]研究表明促进脊髓损伤后神经细胞的自噬可以保护神经细胞免受机械损伤,其保护机制与Beclin-1和LC3 Ⅱ/Ⅰ的表达有关。闫鹏[33]发现脊髓损伤后激活腺苷酸活化蛋白激酶信号通路可以增强神经细胞内自噬流,进而抑制神经细胞凋亡,从而保护脊髓神经元免受体外机械损伤。LIU等[34]和WANG等[35]的研究同样表明,促进神经元自噬可以抑制神经细胞凋亡并促进其增殖,有效改善脊髓损伤大鼠的运动功能。
2.1.3 脊髓损伤中细胞自噬的作用 近年来,细胞自噬在脊髓损伤中的作用受到学者的广泛关注[36-37]。细胞自噬在脊髓损伤中既表现出减轻炎症反应、抑制细胞凋亡以及促进功能恢复的积极作用,也表现出抑制功能恢复或诱导细胞自噬性死亡的消极影响[38]。因此,自噬在脊髓损伤中是一把双刃剑。
(1)细胞自噬在脊髓损伤中的积极作用:自噬是维持健康神经元内稳态及质量调控的重要机制,是针对中枢神经系统疾病和损伤的细胞保护过程[39]。在中枢神经系统中,自噬通过清除受损的蛋白质、细胞器和细胞来对抗神经变性,对脊髓损伤发挥神经保护作用。细胞自噬在脊髓损伤中的积极作用主要表现为通过减轻损伤后的炎症反应和神经元细胞凋亡,从而促进脊髓损伤后的功能恢复。
脊髓损伤后的炎症反应是复杂的,主要由细胞因子、趋化因子等介导,包括肿瘤坏死因子α、白细胞介素1β、白细胞介素6、干扰素γ、趋化因子CX3C家族、趋化因子CXC家族等[40]。免疫细胞的广泛浸润是导致局部炎症并损伤轴突和神经元的主要原因[41]。因此,调节炎症因子、改善神经炎症对脊髓损伤的康复具有重要意义。病理状态下小胶质细胞由静息状态下的M0表型向M1和M2表型分化,其中M1表型小胶质细胞启动一系列神经毒性反应,而M2表型小胶质细胞主要与神经保护作用相关[42]。脊髓损伤后,M1表型小胶质细胞分化增加,导致破坏性促炎递质释放增加并加剧神经炎症,通过促进自噬使核苷酸结合寡聚化结构域样受体蛋白3炎症小体降解失活,可以靶向抑制小胶质细胞的分化,从而减轻脊髓损伤后的炎症反应[43]。LI等[44]研究表明,脊髓损伤后自噬的抑制增强了小胶质细胞向M1表型分化,从而诱导促炎反应,而在使用自噬增强剂增加自噬后,炎症反应明显减轻,可见自噬对于减轻脊髓损伤后炎症反应的重要作用。另有研究发现姜黄素通过诱导自噬,可以减少胶质瘢痕的形成和炎症反应的发生,促进脊髓髓鞘化、提高运动评分、改善组织损伤和减少神经元死亡从而促进脊髓损伤后的功能恢复[45]。
凋亡是细胞程序性死亡最常见的形式[46],脊髓损伤后的神经元细胞凋亡是导致继发性脊髓损伤的基本病理机制,细胞凋亡会阻断神经传导通路,从而加重继发性损伤[47]。抑制细胞凋亡是减轻继发性脊髓损伤的关键。研究表明,当细胞自噬标记蛋白 LC3B和Beclin-1的表达升高时,自噬体的形成明显增多,而促凋亡蛋白Bax、Caspase-3和促炎细胞因子肿瘤坏死因子α、白细胞介素1β和白细胞介素6表达下降,抗凋亡相关蛋白Bcl-2的表达升高,从而达到减轻细胞凋亡、促进功能恢复的目的[48]。在中等挫伤性脊髓损伤大鼠模型中,脊髓损伤后立即给予替勃龙治疗,替勃龙以时间依赖方式降低损伤部位的细胞凋亡和钙蛋白酶Ⅰ的活性,差异调节自噬,从而显著改善运动功能[49]。HU等[50]研究表明,在体内和体外脊髓损伤模型中,低剂量的脂多糖通过激活自噬通量,抑制细胞凋亡,从而在脊髓损伤中发挥神经保护作用。
膜联蛋白A7属于膜联蛋白家族,其可通过调节细胞周期、耐药性、凋亡等来调节心血管疾病和癌症的自噬[51-52]。溶酶体相关膜蛋白是一种位于溶酶体外膜上的高度糖基化蛋白质,在调节溶酶体的功能中发挥作用[53]。研究发现,膜联蛋白A7激活剂可以通过激活膜联蛋白A7的活性来促进膜联蛋白A7与溶酶体相关膜蛋白5的相互作用,通过稳定溶酶体的酸性环境和调节自噬相关信号通路来促进自噬,从而抑制细胞凋亡,修复受损神经元,促进脊髓损伤的修复[54]。
(2)细胞自噬在脊髓损伤中的消极作用:自噬在脊髓损伤中既有积极作用,也有消极作用。一方面,适度的诱导自噬可以增加危险环境中的细胞存活率,从而防止细胞死亡;另一方面,自噬的过度和长时间激活则会导致细胞的程序性死亡,即自噬性细胞死亡[55]。少突胶质细胞对形成髓鞘至关重要,髓鞘可促进脊髓损伤后神经功能的恢复[56]。但脊髓损伤后少突胶质细胞自噬的过度激活会导致自噬性少突胶质细胞死亡,而少突胶质细胞死亡可能导致脊髓脱髓鞘,并最终影响脊髓创伤修复[57]。CONG等[57]证实,神经营养因子3通过抑制脊髓损伤后少突胶质细胞的过度自噬来促进少突胶质细胞的存活和增殖以及脊髓损伤后运动功能的恢复。同时,WANG等[58]实验也表明,人参皂苷Rb1通过抑制神经元的自噬对脊髓损伤发挥神经保护作用。
2.2 细胞自噬的相关调控机制 在生理条件下自噬受到体内外多种因素的严格调控和影响。自噬是一个复杂的调节过程,涉及许多上游调节途径。文章就几个主要相关通路及蛋白对自噬的调控机制进行介绍。
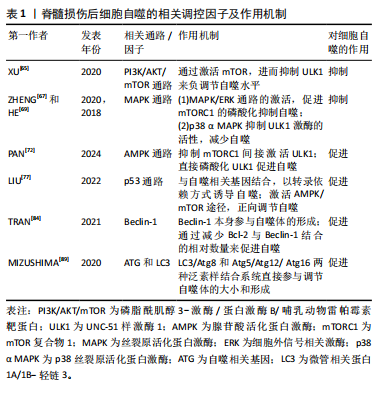
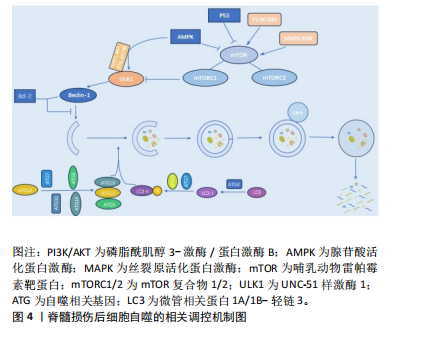
2.2.1 磷脂酰肌醇3-激酶/蛋白激酶B/哺乳动物雷帕霉素靶蛋白(phosphoinositide-3 kinase/protein kinase B/mammalian target of rapamycin,PI3K/AKT/mTOR)信号通路 PI3K/AKT/mTOR信号通路是经典的自噬调节通路,抑制该通路可激活自噬,促进受损脊髓的恢复,反之则抑制自噬的激活[59]。PI3K是自噬信号级联的触发器,也是该通路最上游的分子,PI3K根据其编码基因不同,可分为p110α催化亚基和p110β催化亚基。p110α催化亚基通过PI3K/AKT/mTOR通路实现抑制细胞自噬的作用[60],而p110β催化亚基一方面通过与RAS相关蛋白5(RAS-related protein 5,Rab5)结合实现对细胞自噬的促进作用;另一方面p110β催化亚基可以激活转录因子家族叉头盒蛋白O(Forkhead box protein O,FoxO),而FoxO蛋白参与ATG7等促进细胞自噬蛋白的表达,从而起到间接促进细胞自噬的作用[61]。
AKT也被称为蛋白激酶B,在PI3K下游被激活,AKT除了通过下游mTOR途径,还通过影响细胞转录水平和细胞自噬蛋白的表达途径实现对细胞自噬的调控。首先,FoxO蛋白可以通过与细胞自噬蛋白相结合(直接途径)或调节miR-Nas(间接途径)来促进细胞自噬,而AKT介导的FoxO3活性抑制作用会阻碍FoxO蛋白的表达,导致细胞自噬基因的表达下调,从而起到抑制细胞自噬的作用[62]。其次,AKT通过与S774结合磷酸化UNC-51样激酶1(UNC51-like kinase-1,ULK1),与S295结合磷酸化Beclin-1,导致细胞自噬蛋白ULK1和Beclin-1失活,进而起到抑制细胞自噬的作用[63]。
mTOR是一种丝氨酸/苏氨酸蛋白激酶,属于PI3K家族。mTOR作为一种负调节因子,可与多种蛋白结合,形成两种不同的复合物,即mTOR复合物1 (mTORC1)和2 (mTORC2) [64]。ULK是目前发现的仅有的一个具有丝氨酸/苏氨酸激酶活性的关键蛋白。ULK1是ULK复合物的核心激酶,也是合成自噬小泡不可或缺的关键成分。研究发现,PI3K激活Akt,进而促进mTOR的激活,后者通过抑制下游的ULK1复合物来发挥对自噬水平的负调节作用[65]。
2.2.2 丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)通路 MAPK是一种丝氨酸/苏氨酸激酶,MAPK信号通路可以将信号从细胞膜传递到细胞核,来响应各种不同的刺激。MAPK信号通路调节真核细胞中的基因表达,并将细胞外信号与控制基本细胞过程(如生长、增殖、分化、迁移、凋亡等)的机制联系起来[66]。MAPK信号通路是一条由细胞外信号相关激酶(extracellular-regulated kinase 1/2,ERK1/2)、c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)、p38丝裂原活化蛋白激酶(p38 mitogen-activated protein kinase,p38MAPK)和细胞外信号调节激酶5 (extracellular-regulated kinase 5,ERK5)4个不同的级联反应共同调控的信号转导途径[67]。其中,MAPK/ERK通路在细胞自噬平衡中起关键作用[67]。据报道,MAPK/ERK信号通路活化后, mTORC1的磷酸化增加,细胞自噬水平下降[68];其次,研究发现p38 α MAPK能够抑制ULK1激酶的活性,并使其与自噬起始复合物中自噬相关蛋白13的结合减少,从而降低自噬水平[69]。
2.2.3 腺苷酸活化蛋白激酶(adenosine monophosphate-activated protein kinase,AMPK)通路 AMPK是真核生物细胞和有机体内调控能量稳态的重要激酶,AMPK途径在调节自噬启动中发挥着重要作用[70]。AMPK通过直接或间接激活ULK1来调节体内的自噬[71]。研究表明,激活AMPK磷酸化,可以通过抑制mTOR途径促进自噬的起始[72]。
首先,AMPK严格依赖ATP,在饥饿情况下AMPK活化,通过抑制mTORC1的活性来促进或激活自噬;在营养充足情况下AMPK失活,对mTORC1的抑制作用减弱,mTORC1与ULK1的结合导致自噬抑制[73];其次,AMPK也能通过与ULK1上的S317,S467,S637和S777等相结合,使ULK1磷酸化,来诱导自噬的发生[9]。
2.2.4 P53 p53是人类癌症中最常见的突变肿瘤抑制因子,p53参与多种应激反应(如氧化应激、DNA损伤等),随后作为转录因子协调各种生物学过程,如短暂的细胞周期停滞、凋亡、衰老、分化、代谢以及自噬的调节[74]。P53可以以双重方式调节自噬,这主要取决于其亚细胞定位[75]。一方面,p53与自噬基因Atg2、Atg4、Atg7和Atg10结合[76],以转录依赖的方式诱导自噬,p53也可以通过激活AMPK途径[77],进而抑制mTOR的活化来减轻对自噬的抑制,或通过直接磷酸化ULK1来正向调节自噬。另一方面,当核定位序列缺失的p53在细胞质中积累时,则会抑制自噬的发生发展[78]。FLORIDDIA等[79]研究发现,p53在神经元和神经胶质水平发挥双重作用,p53不仅参与轴突萌发和可塑性,影响纤维化瘢痕的形成,还调节小胶质细胞和巨噬细胞的增殖,对脊髓损伤后运动功能的恢复起着关键作用。
2.2.5 Beclin-1 在哺乳动物的细胞自噬过程中,Beclin-1参与调节自噬体膜的形成和物质转运过程[80]。Beclin-1也是自噬通路的主要协调分子和中心环节[81]。其中,Beclin-1和抗凋亡Bcl-2蛋白家族之间的相互作用与自噬和凋亡过程相关。Bcl-2家族的成员——Bcl-2、Bcl-xl、Mcl-1和Bcl-B等均可与Beclin-1结合来影响自噬过程,其作用机制主要与Bcl-2和Beclin-1上的BH3结构域结合有关[82],该过程可影响Beclin-1的活性,进而抑制细胞自噬的发展[83]。
因此,Bcl-2与Beclin-1在细胞内结合的相对数量在一定程度上影响了细胞自噬的水平[84]。脊髓半切大鼠脊髓损伤后4 h,Beclin-1的表达明显增加,并在3 d后达到高峰,且这一现象至少持续21 d。同时,Beclin-1在神经元和神经胶质细胞(如星形胶质细胞和少突胶质细胞)中均有表达[85]。另一项研究,在脊髓损伤24 h后,Beclin-1的表达水平达到高峰;相反,Bcl-2水平则明显减低[86]。
2.2.6 ATG和LC3 自噬包括双膜囊泡的发育,该囊泡包裹细胞质和有缺陷的细胞器与溶酶体融合进而被降解[87]。在囊泡的形成过程中,LC3/Atg8和Atg5/Atg12/Atg16两种泛素样结合系统的作用更突出,它们参与调节自噬体的大小和形成[88]。在Atg5/Atg12/Atg16系统中,泛素样蛋白Atg12的C端甘氨酸以ATP依赖性方式被Atg7(E1样酶)激活,并依次与Atg7和Atg10(E2样酶)形成硫酯中间体[89]。而后Atg12通过异肽键与Atg5在泛素样反应中偶联,之后Atg12-Atg5偶联物与Atg16二聚体非共价形成更大的复合物[90]。在LC3/Atg8系统中,LC3/Atg8被Atg4蛋白酶在羧基端剪切后产生胞质LC3-Ⅰ。LC3-Ⅰ被Atg7(E1样酶)和Atg3(E2样酶)修饰和加工后与磷脂酰乙醇胺(PE)偶联,形成 LC3-磷脂酰乙醇胺偶联物(LC3-Ⅱ),后者被招募至自噬体膜上,成为连接LC3与自噬小泡的一种结构蛋白[91]。因此,LC3-Ⅰ和LC3-Ⅱ常作为反映细胞自噬发生和自噬活性的分子标志。脊髓损伤后,LC3根据时间、位置和细胞类型而变化。ZHANG等[92]通过Western blot检测发现脊髓损伤后脊髓中LC3-Ⅱ/LC3-Ⅰ的比值在第3天显著增加,在第7天达到峰值,并在脊髓损伤后21 d显著降低。
脊髓损伤后细胞自噬的相关调控机制,见表1和图4。
2.3 脊髓损伤中细胞自噬的治疗策略 目前脊髓损伤的治疗方法包括药物、手术、康复、护理等,而临床方面的突破主要集中在新药的研发、细胞疗法、生物材料移植的临床试验以及新型的物理调控手段和人工智能等方面。脊髓损伤急性期的治疗目标主要以稳定病情、保证患者生存为主;慢性期主要以减少并发症、恢复功能、鼓励患者回归社会和工作为主[93]。但这些治疗往往伴随着一系列的并发症,导致患者不得不承受二次痛苦,因此开发新的治疗靶点和治疗方法显得尤为重要。
靶向细胞自噬是一个有前景的治疗方向,通过促进细胞自噬发生或抑制细胞自噬的过度发展将会为治疗脊髓损伤提供更多的机会和方向,并且自噬已经被确定为包括脊髓损伤在内的多种疾病的治疗和干预靶点[36]。尽管现在有关靶向自噬化合物和靶向自噬治疗脊髓损伤的
临床试验较少,靶向细胞自噬治疗脊髓损伤仍是一项很有吸引力的治疗策略。
2.3.1 自噬激活剂 越来越多的动物实验研究表明,在创伤性脊髓损伤中,诱导自噬的发生发展对于保护中枢神经系统细胞和促进脊髓损伤后运动功能恢复至关重要。在创伤性脊髓损伤中,自噬可以促进细胞内容物的降解和循环,从而实现细胞内能量供应和细胞器更新,使神经元能够在缺乏营养因子的环境中生存[94]。
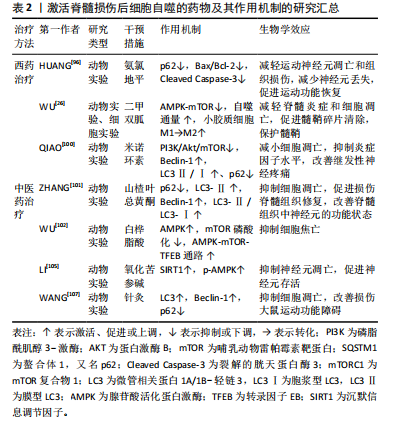
(1)西药疗法:近年来,国内外研究表明,脊髓损伤中细胞自噬的药理学激活仍是重要的神经保护方法。氨氯地平是长效二氢吡啶类钙通道阻滞剂,是目前治疗高血压的一线药物。除了降低血压,氨氯地平还展现出在抑制神经元凋亡和促进神经组织修复方面的潜力[95]。Huang等[96]研究发现脊髓损伤后自噬通量阻断,在使用氨氯地平处理后重新被激活,氨氯地平处理组Bax/Bcl-2和裂解caspase-3表达显著降低,苏木精-伊红染色显示氨氯地平组小鼠的脊髓瘢痕和空泡少于未干预脊髓损伤小鼠,提示氨氯地平通过上调自噬通量,下调凋亡蛋白的表达,减轻运动神经元的凋亡和组织损伤,促进脊髓损伤小鼠运动功能恢复。
二甲双胍是治疗2型糖尿病最常用的降糖药之一,除了降低血糖水平,二甲双胍已被证明是治疗各种中枢神经系统疾病的有效候选药物,包括帕金森病[97]、亨廷顿舞蹈症等[98]。研究表明,二甲双胍通过增强自噬流改善脊髓损伤后的运动功能[99]。此外,WU等[26]研究表明,二甲双胍改善了脊髓损伤后脊髓自噬流的阻断,通过激活AMPK-mTOR信号通路增强了自噬体和溶酶体的融合,使自噬水平显著提高,同时促进了小胶质细胞M1向M2表型极化,发挥了保护髓鞘以及促进脊髓损伤后运动功能恢复的作用。
米诺环素是一种广谱抗菌的四环素类抗生素,但它对脊髓损伤后的功能恢复也有作用。QIAO等[100]通过检测米诺环素治疗后受损脊髓内自噬相关蛋白Beclin-1和LC3Ⅱ/Ⅰ、p62的表达,以及分析PI3K/Akt/mTOR信号通路相关蛋白的表达,发现米诺环素以剂量依赖性方式缓解脊髓损伤大鼠的神经性疼痛,并通过抑制PI3K/Akt/mTOR通路的表达来激活自噬,从而防止细胞凋亡,促进脊髓损伤后的功能恢复。
(2)中医药治疗:目前,中医药领域内有关激活脊髓损伤后细胞自噬的研究不断增多。山楂叶总黄酮是几种黄酮的总称,是山楂叶的有效活性成分之一,ZHANG等[101]研究表明,与脊髓损伤组相比,山楂叶总黄酮治疗组的P62蛋白表达水平降低,LC3-Ⅱ、Beclin-1和LC3-Ⅱ/LC3-Ⅰ蛋白表达水平增加,体外研究进一步证明,山楂叶总黄酮保护受损的脊髓运动神经元并通过增强自噬来抑制细胞凋亡,改善神经元的功能状态。
白桦脂酸是一种天然的羽扇豆烷型五环三萜化合物,WU等[102]研究表明白桦脂酸可以增加AMPK的磷酸化,并抑制mTOR的磷酸化,通过激活AMPK-mTOR-TFEB信号通路来增强脊髓损伤的自噬水平,随后抑制细胞焦亡,最终促进脊髓损伤后的功能恢复。
氧化苦参碱是从苦参根中提取的主要喹啉西啶生物碱,具有抗炎、抗凋亡和抗氧化功能,对大鼠脑缺血或缺血再灌注损伤具有潜在的保护作用[103-104]。氧化苦参碱对脊髓损伤的治疗作用主要涉及AMPK信号通路介导的神经元自噬活性的增强,提高沉默信息调节因子(silent information regulator family protein 1,SIRT1)和p-AMPK表达水平,进而抑制神经元凋亡并促进神经元的存活,最终促进压迫性脊髓损伤大鼠的功能恢复[105]。
针灸是中医的重要组成部分,夹脊穴位于督脉与足太阳经之间,可通过督脉调理二经经气,并且夹脊穴的散布位置与神经节段的关系密切。因此,针刺夹脊穴常被认为是治疗脊髓损伤非常有效的传统疗法,对改善脊髓损伤后神经和运动功能疗效显著[106]。王奕鑫等[107]研究表明,在脊髓半切损伤大鼠模型中,与其他组相比,夹脊温针治疗组自噬相关蛋白LC3、Beclin-1的表达增加,而P62蛋白表达减少,细胞自噬明显被激活,最终改善了脊髓半切损伤大鼠的运动功能。
文章将促进脊髓损伤后细胞自噬的西药疗法、中医药疗法及相关作用机制研究进行汇总,见表2。
(3)组织工程技术:细胞移植是脊髓损伤最具前景的治疗方法,包括干细胞(神经干细胞、胚胎/多能干细胞、间充质/造血干细胞)和成熟体细胞(神经细胞、少突胶质细胞、星形胶质细胞、施万细胞和嗅鞘细胞)在内的不同细胞已被用于治疗不同阶段的脊髓损伤[108-109],可分化为神经元、胶质细胞及星形胶质细胞等[110-111]。神经干细胞移植对脊髓损伤有特殊的神经保护功能,其机制主要与促进神经再生、可塑性和抑制神经炎症有关[112]。然而,如何实现干细胞在靶组织的定向迁移,目前仍是一个难题。最新研究发现,干细胞移植后可通过旁分泌途径起到神经保护和促进损伤修复的作用,其中小细胞外囊泡和外泌体是目前的研究热点[113]。
小细胞外囊泡是目前发现的最小的内吞膜结合纳米囊泡[114],可通过传递多种信号分子来调控受体细胞的活
性。小细胞外囊泡包含大量功能性mRNAs、微小RNA 和特异性miRNAs,在生理和病理条件下介导多种功能[115]。神经干细胞来源小细胞外囊泡在组织损伤和修复中起重要作用。RONG等[48]在大鼠脊髓损伤模型中注射神经干细胞来源小细胞外囊泡后,观察到大鼠脊髓损伤面积明显减少,LC3B和Beclin-1的表达明显增加,而在使用自噬抑制剂后,逆转了神经干细胞来源小细胞外囊泡处理后Bax和裂解Caspase-3表达减少的现象,提示在脊髓损伤早期,神经干细胞来源小细胞外囊泡通过激活自噬来减少神经元凋亡、抑制神经炎症,最终促进脊髓损伤后的功能恢复。此外,RONG等[116]发现神经干细胞来源小细胞外囊泡中含有14-3-3t蛋白,14-3-3t蛋白过表达可增强细胞自噬,进而增强神经干细胞来源小细胞外囊泡在体外和体内的抗凋亡和抗炎作用,来缓解脊髓损伤后的细胞凋亡和神经炎症。
外泌体是由细胞分泌的囊泡,具有双层膜,直径为40-100 nm,其可代表细胞外衍生的磷脂纳米载体,携带蛋白质、脂质、编码RNA、非编码RNA、细胞因子和生长因子等生物活性分子传递到特定的受体细胞进行细胞间通讯[117],从而发挥细胞样生物学功能,改变效应细胞的微环境,调节靶细胞的生长和增殖。GU等[118]使用骨髓间充质干细胞来源外泌体治疗脊髓损伤大鼠,结果发现,与脊髓损伤组相比,骨髓间充质干细胞来源外泌体组的LC3BⅡ和Beclin-1蛋白表达在损伤后第3天增加,P62蛋白表达减少,而促凋亡蛋白Caspase-3表达显著降低,抗凋亡蛋白Bcl-2表达升高,BBB评分在损伤后2-4周继续增加,提示骨髓间充质干细胞来源外泌体能够通过促进早期自噬来减弱神经元凋亡,促进脊髓损伤大鼠运动功能的恢复。此外,施万细胞是外周神经系统轴突的重要组成部分。研究表明,施万细胞来源外泌体在体外和体内都促进轴突再生[119]。PAN等[120]研究表明施万细胞来源外泌体通过抑制EGFR/Akt/mTOR信号通路来上调自噬水平、减少细胞凋亡,进而诱导脊髓损伤后的轴突保护和运动功能的恢复。近年来,有研究发现外周巨噬细胞能有效改善病变部位的炎症微环境,这是促进脊髓损伤后修复的关键因素[121]。ZHANG等[122]研究表明,外周巨噬细胞来源外泌体通过抑制PI3K/AKT/mTOR途径增强自噬来促进抗炎型小胶质细胞极化,对脊髓损伤后神经元具有保护作用。白藜芦醇能够在多种神经系统疾病中激活自噬[123],但由于白藜芦醇不能穿过血脑屏障,因此其应用受到限制。FAN等[124]研究表明,与游离白藜芦醇相比,白藜芦醇介导的外泌体显著改善脊髓损伤大鼠的运动功能。此外,白藜芦醇介导的外泌体还促进神经元存活和自噬,同时降低凋亡水平,这主要与PI3K信号通路密切相关。
近年来,生物相容性材料已成为治疗脊髓损伤的有前途的新策略,其中,水凝胶因具有卓越的生物相容性而被广泛用作神经组织工程支架[125]。水凝胶可以作为装载干细胞或药物的运输系统递送相应治疗作用的药物和细胞,有效提高脊髓损伤的治疗效果[126]。ZHU等[127]制备了一种活性氧响应的复合纤维,即将负载雷帕霉素的聚乙烯醇水凝胶与包裹脑源性神经营养因子的微溶胶静电纺丝纤维通过酰胺键接枝,体内和体外实验证明,外层水凝胶实现了对过量活性氧的响应性清除,雷帕霉素的爆发式释放增强了神经元自噬,抑制了内源性活性氧的产生,并进一步提高了神经元抵抗氧化应激和凋亡的能力;内部的微溶胶纤维可用于引导轴突出芽和成熟,脑源性神经营养因子的持续释放可补充脊髓损伤后耗竭的神经营养因子,促进局部神经元生长,减少胶质瘢痕的形成,双向调节活性氧级联反应来实现神经功能恢复。
2.3.2 自噬抑制剂 当自噬增强创伤性脊髓损伤中的神经变性时,自噬的治疗性抑制将成为神经保护和功能恢复的有吸引力的替代治疗策略。
(1)西药疗法:胰岛素样生长因子1是一种由70个氨基酸组成的多肽,其在中枢神经系统局部产生的神经营养因子可促进轴突再生、保护运动神经元、抑制神经炎症[128]。既往研究发现,在脊髓损伤模型中,胰岛素样生长因子1可以使p-Akt和p-mTOR表达水平增高,增加存活神经细胞的数量,促进脊髓损伤大鼠的功能恢复[129],说明胰岛素样生长因子1通过调控PI3K/Akt/mTOR信号通路抑制过度自噬,并参与髓鞘碎片的清除,从而发挥对脊髓损伤后神经元的保护作用。
依达拉奉是一种众所周知的自由基清除剂,现在被广泛用于治疗创伤性脊髓损伤。XU等[130]对脊髓损伤大鼠采用依达拉奉治疗,显著改善了脊髓损伤大鼠功能、减少神经元损失以及保护血脊髓屏障完整性,这主要归因于依达拉奉治疗对LC3和Beclin-1蛋白表达的抑制,以及对P62蛋白表达的促进。
(2)中医药治疗:人参皂苷Rb1是人参的主要有效成分,具有抗炎、抗细胞凋亡、免疫调节及促进神经轴突生长等多种药理效应[131],在中枢神经系统疾病中发挥神经保护作用。WANG等[58]研究表明,人参皂苷Rb1增加了脊髓损伤动物模型的PC12细胞存活率,通过抑制细胞的过度自噬来抑制细胞的自噬性死亡,从而减少运动神经元的损失并促进功能恢复。
此外,针刺夹脊穴联合低频电刺激可以通过激活下游的PI3K/AKT/mTOR通路,促进该信号通路磷酸化水平,抑制细胞自噬的发生,来治疗脊髓损伤后的膀胱功能障碍[132]。
抑制脊髓损伤后细胞自噬的药物和中医药及其作用机制的研究汇总,见表3。
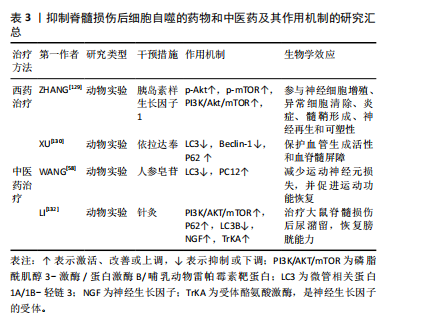
(3)组织工程技术:生物材料或生物支架已被证明在神经损伤修复中显示出巨大的前景[133]。海藻酸钠水凝胶具有生物相容性、无毒、无免疫原性等优点,可作为支架材料广泛应用于组织工程及细胞移植等领域[134]。研究表明,碱性成纤维细胞生长因子通过抑制过度自噬促进脊髓损伤恢复[135]。但全身施用碱性成纤维细胞生长因子具有半衰期短、难以通过血脊髓屏障以及致瘤性等缺点[136]。ZHANG等[137]使用海藻酸钠水凝胶负载碱性成纤维细胞生长因子,单次原位注射可使碱性成纤维细胞生长因子持续释放到受损脊髓中,对脊髓损伤有更好的治疗效果。同时,研究证实海藻酸钠水凝胶负载碱性成纤维细胞生长因子可通过调控PI3K/Akt/FOXO1/KLF4信号通路抑制自噬的过度激活,从而改善脊髓损伤后血脊髓屏障完整性,促进运动功能的恢复。

| [1] LIN A, SHAAYA E, CALVERT JS, et al. A Review of Functional Restoration From Spinal Cord Stimulation in Patients With Spinal Cord Injury. Neurospine. 2022;19(3):703-734. [2] ALIZADEH A, DYCK SM, KARIMI-ABDOLREZAEE S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front Neurol. 2019;10:282. [3] MÜLLER-JENSEN L, PLONER CJ, KRONEBERG D, et al. Clinical Presentation and Causes of Non-traumatic Spinal Cord Injury: An Observational Study in Emergency Patients. Front Neurol. 2021;12: 701927. [4] HE X, DENG B, MA M, et al. Bioinformatics Analysis of Programmed Cell Death in Spinal Cord Injury. World Neurosurg. 2023. doi: 10.1016/j.wneu.2023.06.043. [5] GALLUZZI L, VITALE I, AARONSON SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486-541. [6] LUO C, TAO L. The Function and Mechanisms of Autophagy in Spinal Cord Injury. Adv Exp Med Biol. 2020;1207:649-654. [7] PARZYCH KR, KLIONSKY DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20(3):460-473. [8] RAY SK. Modulation of autophagy for neuroprotection and functional recovery in traumatic spinal cord injury. Neural Regen Res. 2020; 15(9):1601-1612. [9] CAO W, LI J, YANG K, et al. An overview of autophagy: Mechanism, regulation and research progress. Bull Cancer. 2021;108(3):304-322. [10] KANNO H, OZAWA H, SEKIGUCHI A, et al. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine (Phila Pa 1976). 2011;36(22):E1427-E1434. [11] KAWAMATA T, KAMADA Y, KABEYA Y, et al. Organization of the pre-autophagosomal structure responsible for autophagosome formation. Mol Biol Cell. 2008;19(5):2039-2050. [12] ARSTILA AU, TRUMP BF. Studies on cellular autophagocytosis. The formation of autophagic vacuoles in the liver after glucagon administration. Am J Pathol. 1968;53(5):687-733. [13] TAKESHIGE K, BABA M, TSUBOI S, et al. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol. 1992;119(2):301-311. [14] KLIONSKY DJ, CREGG JM, DUNN WA JR, et al. A unified nomenclature for yeast autophagy-related genes. Dev Cell. 2003;5(4):539-545. [15] HARA T, NAKAMURA K, MATSUI M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885-889. [16] KANNO H, OZAWA H, SEKIGUCHI A, et al. Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death. Neurobiol Dis. 2009;33(2):143-148. [17] TANG P, HOU H, ZHANG L, et al. Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol. 2014;49(1):276-287. [18] NODA NN, WANG Z, ZHANG H. Liquid-liquid phase separation in autophagy. J Cell Biol. 2020;219(8):e202004062. [19] ZHAO X, SUN J, YUAN Y, et al. Zinc Promotes Microglial Autophagy Through NLRP3 Inflammasome Inactivation via XIST/miR-374a-5p Axis in Spinal Cord Injury. Neurochem Res. 2022;47(2):372-381. [20] MEI X, WANG H, ZHANG H, et al. Blockade of receptor for advanced glycation end products promotes oligodendrocyte autophagy in spinal cord injury. Neurosci Lett. 2019;698:198-203. [21] NIE BX, ZHAO G, YUAN XF, et al. Inhibition of CDK1 attenuates neuronal apoptosis and autophagy and confers neuroprotection after chronic spinal cord injury in vivo. J Chem Neuroanat. 2022;119:102053. [22] FANG YP, QIN ZH, ZHANG Y, et al. Implications of microglial heterogeneity in spinal cord injury progression and therapy. Exp Neurol. 2023;359:114239. [23] DAVID S, KRONER A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12(7):388-399. [24] LIU Y, CHU W, MA H, et al. Fisetin orchestrates neuroinflammation resolution and facilitates spinal cord injury recovery through enhanced autophagy in pro-inflammatory glial cells. Int Immunopharmacol. 2024;130:111738. [25] LI Y, LEI Z, RITZEL R, et al. Impairment of autophagy in microglia/macrophages exacerbates neuroinflammation after spinal cord injury in mice. Anesth Analg. 2022;134:1196-1197. [26] WU YQ, XIONG J, HE ZL, et al. Metformin promotes microglial cells to facilitate myelin debris clearance and accelerate nerve repairment after spinal cord injury. Acta Pharmacol Sin. 2022;43(6):1360-1371. [27] DUNCAN GJ, MANESH SB, HILTON BJ, et al. The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury. Glia. 2020;68(2):227-245. [28] WANG H, LIU C, MEI X, et al. Berberine attenuated pro-inflammatory factors and protect against neuronal damage via triggering oligodendrocyte autophagy in spinal cord injury. Oncotarget. 2017; 8(58):98312-98321. [29] SARASWAT OHRI S, BANKSTON AN, MULLINS SA, et al. Blocking Autophagy in Oligodendrocytes Limits Functional Recovery after Spinal Cord Injury. J Neurosci. 2018;38(26):5900-5912. [30] SALVADOR AFM, KIPNIS J. Immune response after central nervous system injury. Semin Immunol. 2022;59:101629. [31] ZHUANG XX, WANG SF, TAN Y, et al. Pharmacological enhancement of TFEB-mediated autophagy alleviated neuronal death in oxidative stress-induced Parkinson’s disease models. Cell Death Dis. 2020;11(2):128. [32] CHANG Y, YANG T, DING H, et al. Tauroursodeoxycholic acid protects rat spinal cord neurons after mechanical injury through regulating neuronal autophagy. Neurosci Lett. 2022;776:136578. [33] 闫鹏.AMPK-SIRT1信号通路在脊髓损伤后神经细胞自噬和细胞凋亡调节中的作用[D].沈阳:中国医科大学,2019. [34] LIU S, LIU H, GONG C, et al. MiR-10b-5p Regulates Neuronal Autophagy and Apoptosis Induced by Spinal Cord Injury Through UBR7. Neuroscience. 2024;543:13-27. [35] WANG P, XU D, ZHANG Y, et al. Netrin-1 promotes neuronal autophagy and inhibits neuronal apoptosis by activating ampk/mtor signaling pathway to play neuroprotective role of spinal cord injury in rats. Acta Medica Mediterranea. 2021;37(3):1377-1382. [36] SHEN Y, WANG YP, CHENG X, et al. Autophagy regulation combined with stem cell therapy for treatment of spinal cord injury. Neural Regen Res. 2023;18(8):1629-1636. [37] KIM KH, LEE MS. Autophagy--a key player in cellular and body metabolism. Nat Rev Endocrinol. 2014;10(6):322-337. [38] XIANG H, ZHANG J, LIN C, et al. Targeting autophagy-related protein kinases for potential therapeutic purpose. Acta Pharm Sin B. 2020; 10(4):569-581. [39] NIXON RA, YANG DS. Autophagy and neuronal cell death in neurological disorders. Cold Spring Harb Perspect Biol. 2012;4(10):a008839. [40] FREYERMUTH-TRUJILLO X, SEGURA-URIBE JJ, SALGADO-CEBALLOS H, et al. Inflammation: A Target for Treatment in Spinal Cord Injury. Cells. 2022;11(17):2692. [41] HELLENBRAND DJ, QUINN CM, PIPER ZJ, et al. Inflammation after spinal cord injury: a review of the critical timeline of signaling cues and cellular infiltration. J Neuroinflammation. 2021;18(1):284. [42] XU GY, XU S, ZHANG YX, et al. Cell-Free Extracts from Human Fat Tissue with a Hyaluronan-Based Hydrogel Attenuate Inflammation in a Spinal Cord Injury Model through M2 Microglia/Microphage Polarization. Small. 2022;18(17):e2107838. [43] JIANG F, XIA M, ZHANG Y, et al. Cannabinoid receptor-2 attenuates neuroinflammation by promoting autophagy-mediated degradation of the NLRP3 inflammasome post spinal cord injury. Front Immunol. 2022;13:993168. [44] LI Y, LEI Z, RITZEL RM, et al. Impairment of autophagy after spinal cord injury potentiates neuroinflammation and motor function deficit in mice. Theranostics. 2022;12(12):5364-5388. [45] LI W, YAO S, LI H, et al. Curcumin promotes functional recovery and inhibits neuronal apoptosis after spinal cord injury through the modulation of autophagy. J Spinal Cord Med. 2021;44(1):37-45. [46] SHI Z, YUAN S, SHI L, et al. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 2021;54(3):e12992. [47] ABBASZADEH F, FAKHRI S, KHAN H. Targeting apoptosis and autophagy following spinal cord injury: Therapeutic approaches to polyphenols and candidate phytochemicals. Pharmacol Res. 2020;160:105069. [48] RONG Y, LIU W, WANG J, et al. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019;10(5):340. [49] SÁNCHEZ-TORRES S, OROZCO-BARRIOS C, SALGADO-CEBALLOS H, et al. Tibolone Improves Locomotor Function in a Rat Model of Spinal Cord Injury by Modulating Apoptosis and Autophagy. Int J Mol Sci. 2023;24(20):15285. [50] HU J, HUANG K, BAO F, et al. Low-dose lipopolysaccharide inhibits spinal cord injury-induced neuronal apoptosis by regulating autophagy through the lncRNA MALAT1/Nrf2 axis. PeerJ. 2023;11:e15919. [51] HUANG S, LIU N, LI H, et al. TIA1 interacts with annexin A7 in regulating vascular endothelial cell autophagy. Int J Biochem Cell Biol. 2014;57:115-122. [52] LIU H, GUO D, SHA Y, et al. ANXA7 promotes the cell cycle, proliferation and cell adhesion-mediated drug resistance of multiple myeloma cells by up-regulating CDC5L. Aging (Albany NY). 2020;12(11):11100-11115. [53] ALESSANDRINI F, PEZZÈ L, CIRIBILLI Y. LAMPs: Shedding light on cancer biology. Semin Oncol. 2017;44(4):239-253. [54] LI N, CHEN L, ZHAO X, et al. Targeting ANXA7/LAMP5-mTOR axis attenuates spinal cord injury by inhibiting neuronal apoptosis via enhancing autophagy in mice. Cell Death Discov. 2023;9(1):309. [55] BURSCH W, ELLINGER A, KIENZL H, et al. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis. 1996;17(8):1595-1607. [56] FREI E, KLUSMAN I, SCHNELL L, et al. Reactions of oligodendrocytes to spinal cord injury: cell survival and myelin repair. Exp Neurol. 2000; 163(2):373-380. [57] CONG Y, WANG C, WANG J, et al. NT-3 Promotes Oligodendrocyte Proliferation and Nerve Function Recovery After Spinal Cord Injury by Inhibiting Autophagy Pathway. J Surg Res. 2020;247:128-135. [58] WANG P, LIN C, WU S, et al. Inhibition of Autophagy is Involved in the Protective Effects of Ginsenoside Rb1 on Spinal Cord Injury. Cell Mol Neurobiol. 2018;38(3):679-690. [59] LI Z, SONG Y, HOU W, et al. Atractylodin induces oxidative stress-mediated apoptosis and autophagy in human breast cancer MCF-7 cells through inhibition of the P13K/Akt/mTOR pathway. J Biochem Mol Toxicol. 2022;36(8):e23081. [60] YU X, LONG YC, SHEN HM. Differential regulatory functions of three classes of phosphatidylinositol and phosphoinositide 3-kinases in autophagy. Autophagy. 2015;11(10):1711-1728. [61] SINGH P, DAR MS, DAR MJ. p110α and p110β isoforms of PI3K signaling: are they two sides of the same coin? FEBS Lett. 2016;590(18):3071-3082. [62] YOGEV O, GOLDBERG R, ANZI S, et al. Jun proteins are starvation-regulated inhibitors of autophagy. Cancer Res. 2010;70(6):2318-2327. [63] TAKEUCHI H, KONDO Y, FUJIWARA K, et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65(8):3336-3346. [64] LIU GY, SABATINI DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21(4):183-203. [65] XU Z, HAN X, OU D, et al. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl Microbiol Biotechnol. 2020;104(2): 575-587. [66] SUN J, NAN G. The Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway as a Discovery Target in Stroke. J Mol Neurosci. 2016;59(1):90-98. [67] ZHENG S, SHU Y, LU Y, et al. Chloroquine Combined with Imatinib Overcomes Imatinib Resistance in Gastrointestinal Stromal Tumors by Inhibiting Autophagy via the MAPK/ERK Pathway. Onco Targets Ther. 2020;13:6433-6441. [68] CARRIERE A, ROMEO Y, ACOSTA-JAQUEZ HA, et al. ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1). J Biol Chem. 2011;286(1):567-577. [69] HE Y, SHE H, ZHANG T, et al. p38 MAPK inhibits autophagy and promotes microglial inflammatory responses by phosphorylating ULK1. J Cell Biol. 2018;217(1):315-328. [70] GONG CY, ZHANG HH. Autophagy as a potential therapeutic target in intervertebral disc degeneration. Life Sci. 2021;273:119266. [71] EGAN DF, SHACKELFORD DB, MIHAYLOVA MM, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331(6016):456-461. [72] PAN SM, WANG CL, HU ZF, et al. Baitouweng decoction repairs the intestinal barrier in DSS-induced colitis mice via regulation of AMPK/mTOR-mediated autophagy. J Ethnopharmacol. 2024;318(Pt A):116888. [73] ALERS S, LÖFFLER AS, WESSELBORG S, et al. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32(1):2-11. [74] BIEGING KT, MELLO SS, ATTARDI LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 2014;14(5):359-370. [75] MAIURI MC, GALLUZZI L, MORSELLI E, et al. Autophagy regulation by p53. Curr Opin Cell Biol. 2010;22(2):181-185. [76] KENZELMANN BROZ D, SPANO MELLO S, BIEGING KT, et al. Global genomic profiling reveals an extensive p53-regulated autophagy program contributing to key p53 responses. Genes Dev. 2013;27(9):1016-1031. [77] LIU Y, GU W. The complexity of p53-mediated metabolic regulation in tumor suppression. Semin Cancer Biol. 2022;85:4-32. [78] WANG H, GUO M, WEI H, et al. Targeting p53 pathways: mechanisms, structures, and advances in therapy. Signal Transduct Target Ther. 2023;8(1):92. [79] FLORIDDIA EM, RATHORE KI, TEDESCHI A, et al. p53 Regulates the neuronal intrinsic and extrinsic responses affecting the recovery of motor function following spinal cord injury. J Neurosci. 2012;32(40):13956-13970. [80] XU HD, QIN ZH. Beclin 1, Bcl-2 and Autophagy. Adv Exp Med Biol. 2019;1206:109-126. [81] RONG Y, FAN J, JI C, et al. USP11 regulates autophagy-dependent ferroptosis after spinal cord ischemia-reperfusion injury by deubiquitinating Beclin 1. Cell Death Differ. 2022;29(6):1164-1175. [82] ERLICH S, MIZRACHY L, SEGEV O, et al. Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy. 2007;3(6):561-568. [83] PATTINGRE S, TASSA A, QU X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927-939. [84] TRAN S, FAIRLIE WD, LEE EF. BECLIN1: Protein Structure, Function and Regulation. Cells. 2021;10(6):1522. [85] KANNO H, OZAWA H, SEKIGUCHI A, et al. The role of autophagy in spinal cord injury. Autophagy. 2009;5(3):390-392. [86] LYTLE JM, WRATHALL JR. Glial cell loss, proliferation and replacement in the contused murine spinal cord. Eur J Neurosci. 2007;25(6):1711-1724. [87] DAS G, SHRAVAGE BV, BAEHRECKE EH. Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb Perspect Biol. 2012;4(6):a008813. [88] YORIMITSU T, KLIONSKY DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12 Suppl 2(Suppl 2):1542-1552. [89] MIZUSHIMA N. The ATG conjugation systems in autophagy. Curr Opin Cell Biol. 2020;63:1-10. [90] GALLUZZI L, BAEHRECKE EH, BALLABIO A, et al. Molecular definitions of autophagy and related processes. EMBO J. 2017;36(13):1811-1836. [91] YU L, CHEN Y, TOOZE SA. Autophagy pathway: Cellular and molecular mechanisms. Autophagy. 2018;14(2):207-215. [92] ZHANG Q, HUANG C, MENG B, et al. Changes in autophagy proteins in a rat model of spinal cord injury. Chin J Traumatol. 2014;17(4):193-197. [93] HU X, XU W, REN Y, et al. Spinal cord injury: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8(1):245. [94] KLIONSKY DJ, PETRONI G, AMARAVADI RK, et al. Autophagy in major human diseases. EMBO J. 2021;40(19):e108863. [95] KAWAI H, DEGUCHI S, DEGUCHI K, et al. Synergistic benefit of combined amlodipine plus atorvastatin on neuronal damage after stroke in Zucker metabolic rat. Brain Res. 2011;1368:317-323. [96] HUANG Y, REN H, GAO X, et al. Amlodipine Improves Spinal Cord Injury Repair by Inhibiting Motoneuronal Apoptosis Through Autophagy Upregulation. Spine (Phila Pa 1976). 2022;47(17):E570-E578. [97] PATIL SP, JAIN PD, GHUMATKAR PJ, et al. Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience. 2014;277:747-754. [98] VÁZQUEZ-MANRIQUE RP, FARINA F, CAMBON K, et al. AMPK activation protects from neuronal dysfunction and vulnerability across nematode, cellular and mouse models of Huntington’s disease. Hum Mol Genet. 2016;25(6):1043-1058. [99] ZHANG D, XUAN J, ZHENG BB, et al. Metformin Improves Functional Recovery After Spinal Cord Injury via Autophagy Flux Stimulation. Mol Neurobiol. 2017;54(5):3327-3341. [100] QIAO L, TANG Q, AN Z, et al. Minocycline relieves neuropathic pain in rats with spinal cord injury via activation of autophagy and suppression of PI3K/Akt/mTOR pathway. J Pharmacol Sci. 2023;153(1):12-21. [101] ZHANG Q, LIU M, NONG H, et al. Total flavonoids of hawthorn leaves protect spinal motor neurons via promotion of autophagy after spinal cord injury. Front Pharmacol. 2022;13:925568. [102] WU C, CHEN H, ZHUANG R, et al. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int J Biol Sci. 2021;17(4):1138-1152. [103] DONG XQ, YU WH, HU YY, et al. Oxymatrine reduces neuronal cell apoptosis by inhibiting Toll-like receptor 4/nuclear factor kappa-B-dependent inflammatory responses in traumatic rat brain injury. Inflamm Res. 2011;60(6):533-539. [104] ZHAO P, ZHOU R, LI HN, et al. Oxymatrine attenuated hypoxic-ischemic brain damage in neonatal rats via improving antioxidant enzyme activities and inhibiting cell death. Neurochem Int. 2015;89:17-27. [105] LI J, CAO Y, LI LN, et al. Neuroprotective Effects of Oxymatrine via Triggering Autophagy and Inhibiting Apoptosis Following Spinal Cord Injury in Rats. Mol Neurobiol. 2023;60(8):4450-4471. [106] 胡华辉,黄小龙,刘飞,等.电针夹脊穴治疗脊髓损伤机制的实验研究进展[J].上海针灸杂志,2016,35(12):1480-1483. [107] 王奕鑫.夹脊温针对大鼠脊髓半切损伤后运动功能及细胞自噬的影响[D].长沙:湖南中医药大学,2024. [108] HUANG H, YOUNG W, SKAPER S, et al. Clinical Neurorestorative Therapeutic Guidelines for Spinal Cord Injury (IANR/CANR version 2019). J Orthop Translat. 2019;20:14-24. [109] TETZLAFF W, OKON EB, KARIMI-ABDOLREZAEE S, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28(8):1611-1682. [110] OUREDNIK J, OUREDNIK V, LYNCH WP, et al. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002;20(11):1103-1110. [111] TANG Y, YU P, CHENG L. Current progress in the derivation and therapeutic application of neural stem cells. Cell Death Dis. 2017; 8(10):e3108. [112] CHENG Z, ZHU W, CAO K, et al. Anti-Inflammatory Mechanism of Neural Stem Cell Transplantation in Spinal Cord Injury. Int J Mol Sci. 2016;17(9):1380. [113] RATAJCZAK MZ, JADCZYK T, PĘDZIWIATR D, et al. New advances in stem cell research: practical implications for regenerative medicine. Pol Arch Med Wewn. 2014;124(7-8):417-426. [114] THÉRY C, WITWER KW, AIKAWA E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [115] BARTENEVA NS, MALTSEV N, VOROBJEV IA. Microvesicles and intercellular communication in the context of parasitism. Front Cell Infect Microbiol. 2013;3:49. [116] RONG Y, LIU W, LV C, et al. Neural stem cell small extracellular vesicle-based delivery of 14-3-3t reduces apoptosis and neuroinflammation following traumatic spinal cord injury by enhancing autophagy by targeting Beclin-1. Aging (Albany NY). 2019;11(18):7723-7745. [117] YANG B, CHEN Y, SHI J. Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Adv Mater. 2019;31(2):e1802896. [118] GU J, JIN ZS, WANG CM, et al. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Improves Spinal Cord Function After Injury in Rats by Activating Autophagy. Drug Des Devel Ther. 2020;14:1621-1631. [119] CHING RC, WIBERG M, KINGHAM PJ. Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res Ther. 2018;9(1):266. [120] PAN D, ZHU S, ZHANG W, et al. Autophagy induced by Schwann cell-derived exosomes promotes recovery after spinal cord injury in rats. Biotechnol Lett. 2022;44(1):129-142. [121] TSAROUCHAS TM, WEHNER D, CAVONE L, et al. Dynamic control of proinflammatory cytokines Il-1β and Tnf-α by macrophages in zebrafish spinal cord regeneration. Nat Commun. 2018;9(1):4670. [122] ZHANG B, LIN F, DONG J, et al. Peripheral Macrophage-derived Exosomes promote repair after Spinal Cord Injury by inducing Local Anti-inflammatory type Microglial Polarization via Increasing Autophagy. Int J Biol Sci. 2021;17(5):1339-1352. [123] WANG N, HE J, PAN C, et al. Resveratrol Activates Autophagy via the AKT/mTOR Signaling Pathway to Improve Cognitive Dysfunction in Rats With Chronic Cerebral Hypoperfusion. Front Neurosci. 2019;13:859. [124] FAN Y, LI Y, HUANG S, et al. Resveratrol-primed exosomes strongly promote the recovery of motor function in SCI rats by activating autophagy and inhibiting apoptosis via the PI3K signaling pathway. Neurosci Lett. 2020;736:135262. [125] WANG H, XIA Y, LI B, et al. Reverse Adverse Immune Microenvironments by Biomaterials Enhance the Repair of Spinal Cord Injury. Front Bioeng Biotechnol. 2022;10:812340. [126] QIN C, QI Z, PAN S, et al. Advances in Conductive Hydrogel for Spinal Cord Injury Repair and Regeneration. Int J Nanomedicine. 2023;18:7305-7333. [127] ZHU H, ZHOU L, TANG J, et al. Reactive Oxygen Species-Responsive Composite Fibers Regulate Oxidative Metabolism through Internal and External Factors to Promote the Recovery of Nerve Function. Small. 2024:e2401241. doi: 10.1002/smll.202401241. [128] ZHAO L, ZHANG B, HUANG S, et al. Insulin-Like Growth Factor-1 Enhances Motoneuron Survival and Inhibits Neuroinflammation After Spinal Cord Transection in Zebrafish. Cell Mol Neurobiol. 2022; 42(5):1373-1384. [129] ZHANG D, YUAN Y, ZHU J, et al. Insulin-like growth factor 1 promotes neurological functional recovery after spinal cord injury through inhibition of autophagy via the PI3K/Akt/mTOR signaling pathway. Exp Ther Med. 2021;22(5):1265. [130] XU B, FANG J, WANG J, et al. Inhibition of autophagy and RIP1/RIP3/MLKL-mediated necroptosis by edaravone attenuates blood spinal cord barrier disruption following spinal cord injury. Biomed Pharmacother. 2023;165:115165. [131] CHEN W, GUO Y, YANG W, et al. Involvement of Connexin40 in the Protective Effects of Ginsenoside Rb1 Against Traumatic Brain Injury. Cell Mol Neurobiol. 2016;36(7):1057-1065. [132] 李格格.基于NGF-PI3K/AKT/mTOR轴调节自噬探讨低频电刺激联合针刺“夹脊穴”干预大鼠脊髓损伤尿潴留的影响[D].沈阳: 辽宁中医药大学,2021. [133] QIAN Y, YAO Z, WANG X, et al. (-)-Epigallocatechin gallate-loaded polycaprolactone scaffolds fabricated using a 3D integrated moulding method alleviate immune stress and induce neurogenesis. Cell Prolif. 2020;53(1):e12730. [134] RASTOGI P, KANDASUBRAMANIAN B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication. 2019; 11(4):042001. [135] ZHANG HY, WANG ZG, WU FZ, et al. Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury. Mol Neurobiol. 2013;48(3):452-464. [136] LI X, SUN H, LIN N, et al. Regeneration of uterine horns in rats by collagen scaffolds loaded with collagen-binding human basic fibroblast growth factor. Biomaterials. 2011;32(32):8172-8181. [137] ZHANG R, XIE L, WU F, et al. ALG-bFGF Hydrogel Inhibiting Autophagy Contributes to Protection of Blood-Spinal Cord Barrier Integrity via PI3K/Akt/FOXO1/KLF4 Pathway After SCI. Front Pharmacol. 2022;13: 828896. |
| [1] | 赖鹏宇, 梁 冉, 沈 山. 组织工程技术修复颞下颌关节:问题与挑战[J]. 中国组织工程研究, 2025, 29(在线): 1-9. |
| [2] | 周盼盼, 崔应麟, 张文涛, 王姝瑞, 陈佳慧, 杨 潼. 细胞自噬在脑缺血损伤中的作用及中药调控机制[J]. 中国组织工程研究, 2025, 29(8): 1650-1658. |
| [3] | 李帝均, 酒精卫, 刘海峰, 闫 磊, 李松岩, 王 斌. 明胶三维微球装载人脐带间充质干细胞修复慢性肌腱病[J]. 中国组织工程研究, 2025, 29(7): 1356-1362. |
| [4] | 孙玉婷, 吴家媛, 张 剑. 影响牙髓干细胞成骨及成牙本质分化的相关物理因素及作用机制[J]. 中国组织工程研究, 2025, 29(7): 1531-1540. |
| [5] | 赵瑞华, 陈思娴, 郭 杨, 石 磊, 吴承杰, 吴 毛, 杨光露, 张昊恒, 马 勇. 温肾通督方促进小鼠脊髓损伤的修复[J]. 中国组织工程研究, 2025, 29(6): 1118-1126. |
| [6] | 刘凌云, 何贵新, 秦伟彬, 宋 惠, 张利文, 唐伟智, 杨斐斐, 朱子一, 欧阳彬. 中药改善心肌损伤:线粒体钙稳态介导巨噬细胞自噬与焦亡的作用途径[J]. 中国组织工程研究, 2025, 29(6): 1276-1284. |
| [7] | 丁至立, 黄 杰, 蒋 强, 李土胜, 刘 江, 丁 宇. X射线透视引导下不同方式建立兔椎间盘退变模型的结果对比[J]. 中国组织工程研究, 2025, 29(5): 995-1002. |
| [8] | 李 帅, 刘 桦, 商永慧, 刘义琮, 赵启航, 刘 文. 安氏Ⅱ类错牙合畸形佩戴Twin-block矫治器时上颌骨的应力分布[J]. 中国组织工程研究, 2025, 29(5): 881-887. |
| [9] | 肖 放, 黄 雷, 王 琳. 磁性纳米材料与磁场效应加速骨损伤修复[J]. 中国组织工程研究, 2025, 29(4): 827-838. |
| [10] | 王思凡, 何惠宇, 杨 泉, 韩祥祯. miRNA-378a过表达巨噬细胞株复合胶原蛋白海绵:抗炎及促进组织修复[J]. 中国组织工程研究, 2025, 29(4): 789-799. |
| [11] | 孙现娟, 王秋花, 张锦艺, 杨杨杨, 王文双, 张晓晴. 不同静电纺丝膜上骨髓间充质干细胞的黏附、增殖与成血管平滑肌分化[J]. 中国组织工程研究, 2025, 29(4): 661-669. |
| [12] | 李明哲, 叶翔凌, 王 冰, 余 翔. 负载纳米钽的液晶显示光固化聚乳酸支架制备及促成骨性能[J]. 中国组织工程研究, 2025, 29(4): 670-677. |
| [13] | 余双奇, 丁 凡, 万 松, 陈 伟, 张学俊, 陈 东, 李 强, 林作丽. PLGA/赖氨酸接枝氧化石墨烯纳米粒子复合支架对MC3T3细胞成骨分化的影响[J]. 中国组织工程研究, 2025, 29(4): 707-712. |
| [14] | 党小雯, 黄海量, 黄 雷, 王亚洁. 生物医学领域碳纳米材料10年研究前沿与热点[J]. 中国组织工程研究, 2025, 29(4): 752-760. |
| [15] | 易小丁, 张 迪, 郭 鸿, 卿 亮, 赵田芋. 脱细胞肌腱支架:一种用于肌腱损伤修复的生物医学材料[J]. 中国组织工程研究, 2025, 29(34): 7385-7392. |
细胞自噬是一种细胞内降解和再循环过程,是真核细胞特有的现象。哺乳动物细胞中的自噬主要涉及微自噬、巨自噬和伴侣介导的自噬3种类型[7]。此文主要介绍巨自噬。巨自噬是在应激情况下,粗面内质网上无核糖体附着,使其部分双层膜脱落并逐渐覆盖受损的细胞器、有毒物质、衰老和不需要的蛋白质、脂肪、碳水化合物和核酸的大量胞质,形成自噬体,然后内容物被溶酶体酶降解,通过自噬体-溶酶体途径实现有效地周转、再循环和细胞稳态[8]。因此,自噬的过程主要分为自噬起始、自噬膜延伸和自噬溶酶体形成3个部分[9]。越来越多的证据表明,一方面自噬可以减少神经细胞的死亡以促进存活,在脊髓损伤后发挥神经保护作用;另一方面自噬损伤会导致受损细胞器和蛋白质聚集体的积累,从而干扰正常细胞功能,并可能导致细胞死亡[10]。
此文章对以往研究进行总结,阐述了细胞自噬在脊髓损伤中的作用,归纳了细胞自噬的相关调控机制,并从西药、中医药、组织工程3个层面,系统归纳了以自噬为靶点的脊髓损伤治疗新策略,以期为脊髓损伤后新的研究和临床治疗提供有价值的靶点。
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
1.1 资料来源
1.1.1 检索人及检索时间 第一作者在 2024年3-4月进行检索。
1.1.2 检索文献时限 各数据库在2024年4月之前发表的文献。
1.1.3 检索数据库 PubMed数据库和中国知网。
1.1.4 检索词 英文检索词为“spinal cord injury,autophagy,regulatory mechanisms,autophagy pathway,therapeutic target”,中文检索词为“脊髓损伤,细胞自噬,调控机制,自噬通路,治疗靶点”。
1.1.5 检索文献类型 研究原著和综述。
1.1.6 检索策略 PubMed数据库与中国知网检索策略,见图1。
1.1.7 检索文献量 PubMed数据库检索到3 775篇英文文献,中国知网检索到39篇中文文献。
1.2 入组标准
1.2.1 纳入标准 ①细胞自噬与脊髓损伤相关的文献;②细胞自噬中相关调控因子和作用机制的相关文献;③细胞自噬作为脊髓损伤治疗靶点的相关文献;④同一领域选择最新或在权威杂志发表的文献。
1.2.2 排除标准 ①发表时间久远的文献;②研究主题与细胞自噬无关或相关性低的文献;③研究重复的文献;④逻辑不严谨或结论不明确的文献。
1.3 文献质量评估及数据提取 计算机初步检索到3 814篇文献。阅读文献的题目、摘要、结果,排除过时、结论不明确的文献,而后通过阅读全文,再次排除侧重点不符及质量不高的文献,最终纳入137篇相关度高的文献进行综述,其中PubMed数据库133篇,中国知网4篇。文献筛选流程,见图2。
3.2 作者综述区别于他人他篇的特点 以往关于脊髓损伤后细胞自噬的综述多聚焦于单一信号通路或调节因子对脊髓损伤后细胞自噬的调控机制,单方面研究靶向抑制或激活细胞自噬对脊髓损伤的治疗作用,而没有综合总结细胞自噬对脊髓损伤后功能恢复的影响。而此综述详细归纳总结了脊髓损伤后细胞自噬的相关调控因子和信号通路,并探讨了靶向细胞自噬在脊髓损伤中的应用,除常规西医药物疗法,还综合归纳了用于靶向抑制或激活细胞自噬的组织工程技术,如小细胞外囊泡、外泌体、水凝胶搭载碱性成纤维细胞生长因子等,以及中医药治疗在靶向细胞自噬中的重要作用,以期为脊髓损伤的治疗提供新的靶点。
3.3 综述的局限性 近年来,细胞自噬在肿瘤、癌症中作用的研究相对较多,而关于细胞自噬在脊髓损伤中的作用机制以及相关靶向药物的研究相对较少,且由于篇幅有限,未能罗列出所有调节因子和通路的作用机制,以及靶向抑制或激活细胞自噬的方法;同时,由于各个信号通路及调节因子之间错综复杂、相互联系,不能确定在任意生理或病理环境中哪些调节因子处于主导地位,使文章的观点论述存在一定的主观意向。其次,目前脊髓损伤中自噬的检测主要包括生物标志物的表达和形态学观察。LC3、Beclin-1 通常用于评估自噬的激活,P62是自噬过程的底物蛋白,因此,常作为体内自噬的通量指示剂。此外,透射电子显微镜等形态学方法可以直接观察自噬体的形成。自噬在不同区域、不同时间的反应可能不同,但在大多数研究中,自噬的检测都集中在损伤区域。因此,在今后的研究中可以借助新兴的组织工程技术继续开发新的检测工具或指标,进一步探索自噬的时间模式及空间分布特点,以及细胞自噬的相关调控机制及靶向细胞自噬的方法。
3.4 综述的意义 文章以细胞自噬在脊髓损伤中的作用为切入点,详细论述了细胞自噬在脊髓损伤后的作用机制,并归纳了细胞自噬的相关信号通路和调节因子及近几年靶向调控细胞自噬的方法,以期为脊髓损伤后的靶向治疗提供新的思路。文章重点介绍了调节自噬的信号通路和调节因子,如PI3K/AKT/mTOR、MAPK、AMPK、p53通路抑制或激活自噬,而Beclin-1、ATG和LC3通过影响自噬体的合成来调节自噬,通过促进或抑制脊髓损伤后不同阶段的细胞自噬来减轻脊髓损伤后的神经炎症和细胞凋亡,进而促进损伤后功能恢复。接下来各位专家和学者可以继续围绕细胞自噬探索其在脊髓损伤后的时空表达,探索细胞自噬在脊髓损伤中确切的作用机制,同时围绕这些调控通路和调节因子,针对性实施相应的干预措施,以期在临床实践中取得更具体的成果。
3.5 课题专家组对未来的建议 细胞自噬在脊髓损伤的治疗和进展中起着至关重要的作用,促进或抑制自噬可能是控制创伤性脊髓损伤发病机制的一种有前途的治疗策略,虽然目前关于自噬和脊髓损伤的研究相对广泛,但仍有一些问题需要深入思考,未来可以从以下几个方面深入研究:①迫切需要研究新的工具来剖析细胞自噬水平对脊髓损伤的实际影响,包括对实际自噬能力通量、时间模式等测定;②脊髓损伤后细胞自噬的复杂性不能简化为单一细胞的作用结果,应深入研究自噬在不同细胞中的反应以及不同细胞间的相互作用;③细胞自噬的相关调控通路和蛋白有许多,应全面研究各调控组分之间是否存在相互作用,同时,阐明哪些调节因子在任何特定的生理或病理环境中占主导地位是有必要的;④以自噬作为脊髓损伤治疗靶点的研究仅在体外和动物模型中进行,未来可联合组织工程及各种新兴技术,加强开展临床试验,来确定靶向自噬治疗脊髓损伤的有效性和安全性;⑤脊髓损伤后不同阶段的细胞自噬水平存在差异,未来需要识别针对脊髓损伤不同阶段的调控细胞自噬水平的新药物。
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||