[1] XIANG M, YIN M, XIE S, et al. The molecular mechanism of neutrophil extracellular traps and its role in bone and joint disease. Heliyon. 2023;9(12):e22920.
[2] ZINDEL J, KUBES P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Ann Rev Pathol. 2020;15:493-518.
[3] NAKKALA JR, LI Z, AHMAD W, et al. Immunomodulatory biomaterials and their application in therapies for chronic inflammation-related diseases. Acta Biomater. 2021;123:1-30.
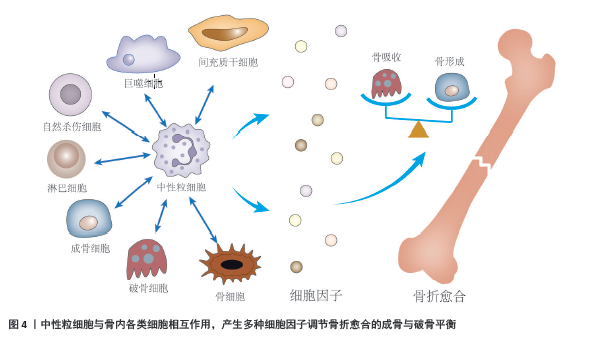
[4] KOVTUN A, BERGDOLT S, WIEGNER R, et al. The crucial role of neutrophil granulocytes in bone fracture healing. Eur Cell Mater. 2016; 32:152-162.
[5] XIAO B, ADJEI-SOWAH E, BENOIT DSW. Integrating osteoimmunology and nanoparticle-based drug delivery systems for enhanced fracture healing. Nanomedicine. 2024;56:102727.
[6] KOVTUN A, MESSERER DAC, SCHARFFETTER-KOCHANEK K, et al. Neutrophils in Tissue Trauma of the Skin, Bone, and Lung: Two Sides of the Same Coin. J Immunol Res. 2018;2018:8173983.
[7] TSCHAFFON-MÜLLER MEA, KEMPTER E, STEPPE L, et al. Neutrophil-derived catecholamines mediate negative stress effects on bone. Nat Commun. 2023;14(1):3262.
[8] HERATH TDK, LARBI A, TEOH SH, et al. Neutrophil-mediated enhancement of angiogenesis and osteogenesis in a novel triple cell co-culture model with endothelial cells and osteoblasts. J Tissue Eng Regen Med. 2018;12(2):e1221-e1236.
[9] HERATH TDK, SAIGO L, SCHALLER B, et al. In Vivo Efficacy of Neutrophil-Mediated Bone Regeneration Using a Rabbit Calvarial Defect Model. Int J Mol Sci. 2021;22(23):13016.
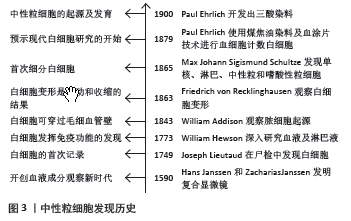
[10] KAY AB. Paul Ehrlich and the Early History of Granulocytes. Microbiol Spectr. 2016;4(4). doi: 10.1128/microbiolspec.MCHD-0032-2016.
[11] CAVAILLON JM. The historical milestones in the understanding of leukocyte biology initiated by Elie Metchnikoff. J Leukoc Biol. 2011; 90(3):413-424.
[12] ROSALES C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front Physiol. 2018;9:113.
[13] MCKENNA E, MHAONAIGH AU, WUBBEN R, et al. Neutrophils: Need for Standardized Nomenclature. Front Immunol. 2021;12:602963.
[14] MARTIN KR, WONG HL, WITKO-SARSAT V, et al. G-CSF - A double edge sword in neutrophil mediated immunity. Semin Immunol. 2021;54: 101516.
[15] JAKOVIJA A, CHTANOVA T. Neutrophil Interactions with the Lymphatic System. Cells. 2021;10(8):2106.
[16] SBIERSKI-KIND J, MROZ N, MOLOFSKY A B. Perivascular stromal cells: Directors of tissue immune niches. Immunol Rev. 2021;302(1):10-31.
[17] LO LW, CHANG CW, CHIANG MF, et al. Marginal Zone B Cells Assist With Neutrophil Accumulation to Fight Against Systemic Staphylococcus aureus Infection. Front Immunol. 2021;12:636818.
[18] YANG F, FENG C, ZHANG X, et al. The Diverse Biological Functions of Neutrophils, Beyond the Defense Against Infections. Inflammation. 2017;40(1):311-323.
[19] YANG P, LI Y, XIE Y, et al. Different Faces for Different Places: Heterogeneity of Neutrophil Phenotype and Function. J Immunol Res. 2019;2019:8016254.
[20] QUAIL DF, AMULIC B, AZIZ M, et al. Neutrophil phenotypes and functions in cancer: A consensus statement. J Exp Med. 2022;219(6): e20220011.
[21] CAPOBIANCO CA, HANKENSON KD, KNIGHTS AJ. Temporal dynamics of immune-stromal cell interactions in fracture healing. Front Immunol. 2024;15:1352819.
[22] BASTIAN OW, MROZEK MH, RAABEN M, et al. Serum from the Human Fracture Hematoma Contains a Potent Inducer of Neutrophil Chemotaxis. Inflammation. 2018;41(3):1084-1092.
[23] LU F, VERLEG SMNE, GROVEN RVM, et al. Is there a role for N1-N2 neutrophil phenotypes in bone regeneration? A systematic review. Bone. 2024;181:117021.
[24] CHRISTOFFERSSON G, VÅGESJÖ E, VANDOOREN J, et al. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120(23): 4653-4662.
[25] BASTIAN OW, KOENDERMAN L, ALBLAS J, et al. Neutrophils contribute to fracture healing by synthesizing fibronectin+ extracellular matrix rapidly after injury. Clin Immunol. 2016; 164: 78-84.
[26] SINGH P, CARRAHER C, SCHWARZBAUER JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010;26:397-419.
[27] BASTIAN OW, CROES M, ALBLAS J, et al. Neutrophils Inhibit Synthesis of Mineralized Extracellular Matrix by Human Bone Marrow-Derived Stromal Cells In Vitro. Front Immunol. 2018;9:945.
[28] GARCÍA-CULEBRAS A, DURÁN-LAFORET V, PEÑA-MARTÍNEZ C, et al. Role of TLR4 (Toll-Like Receptor 4) in N1/N2 Neutrophil Programming After Stroke. Stroke. 2019;50(10):2922-2932.
[29] ZHANG J, GU J, WANG X, et al. Engineering and Targeting Neutrophils for Cancer Therapy. Adv Mater. 2024;36(19):e2310318.
[30] DE LOS REYES AA, KIM Y. Optimal regulation of tumour-associated neutrophils in cancer progression. R Soc Open Sci. 2022;9(2):210705.
[31] OHMS M, MÖLLER S, LASKAY T. An Attempt to Polarize Human Neutrophils Toward N1 and N2 Phenotypes in vitro. Front Immunol. 2020;11:532.
[32] FRIDLENDER ZG, SUN J, KIM S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183-194.
[33] ANNEX BH, COOKE JP. New Directions in Therapeutic Angiogenesis and Arteriogenesis in Peripheral Arterial Disease. Circ Res. 2021;128(12): 1944-1957.
[34] MASSENA S, CHRISTOFFERSSON G, VÅGESJÖ E, et al. Identification and characterization of VEGF-A-responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood. 2015;126(17): 2016-2026.
[35] TURNER TC, SOK MCP, HYMEL LA, et al. Harnessing lipid signaling pathways to target specialized pro-angiogenic neutrophil subsets for regenerative immunotherapy. Sci Adv. 2020;6(44):eaba7702.
[36] LÖRCHNER H, CAÑES ESTEVE L, GÓES ME, et al. Neutrophils for Revascularization Require Activation of CCR6 and CCL20 by TNFα. Circ Res. 2023;133(7):592-610.
[37] ROSE-JOHN S. Interleukin-6 Family Cytokines. Cold Spring Harb Perspect Biol. 2018;10(2):a028415.
[38] SELLIN ML, KLINDER A, BERGSCHMIDT P, et al. IL-6-induced response of human osteoblasts from patients with rheumatoid arthritis after inhibition of the signaling pathway. Clin Exp Med. 2023;23(7): 3479-3499.
[39] YOSHITAKE F, ITOH S, NARITA H, et al. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kappaB signaling pathways. J Biol Chem. 2008;283(17):11535-11540.
[40] CAI L, LV Y, YAN Q, et al. Cytokines: The links between bone and the immune system. Injury, 2024;55(2):111203.
[41] JOHNSON RW, MCGREGOR NE, BRENNAN HJ, et al. Glycoprotein130 (Gp130)/interleukin-6 (IL-6) signalling in osteoclasts promotes bone formation in periosteal and trabecular bone. Bone. 2015;81:343-351.
[42] KING A, BALAJI S, LE LD, et al. Regenerative Wound Healing: The Role of Interleukin-10. Adv Wound Care (New Rochelle). 2014;3(4):315-323.
[43] MARUYAMA M, RHEE C, UTSUNOMIYA T, et al. Modulation of the Inflammatory Response and Bone Healing. Front Endocrinol (Lausanne). 2020;11:386.
[44] GAO X, GE J, ZHOU W, et al. IL-10 inhibits osteoclast differentiation and osteolysis through MEG3/IRF8 pathway. Cell Signal. 2022;95:110353.
[45] XIONG Y, YAN C, CHEN L, et al. IL-10 induces MC3T3-E1 cells differentiation towards osteoblastic fate in murine model. J Cell Mol Med. 2020;24(1):1076-1086.
[46] RUNDLE CH, MIYAKOSHI N, RAMIREZ E, et al. Expression of the fibroblast growth factor receptor genes in fracture repair. Clin Orthop Relat Res. 2002;(403):253-263.
[47] XIE Y, SU N, YANG J, et al. FGF/FGFR signaling in health and disease. Signal Transduct Target Ther. 2020;5(1):181.
[48] KAWAGUCHI H, JINGUSHI S, IZUMI T, et al. Local application of recombinant human fibroblast growth factor-2 on bone repair: a dose-escalation prospective trial on patients with osteotomy. J Orthop Res. 2007;25(4):480-487.
[49] ZHANG M, YU W, NIIBE K, et al. The Effects of Platelet-Derived Growth Factor-BB on Bone Marrow Stromal Cell-Mediated Vascularized Bone Regeneration. Stem Cells Int. 2018;2018:3272098.
[50] FRADE BB, DIAS RB, GEMINI PIPERNI S, et al. The role of macrophages in fracture healing: a narrative review of the recent updates and therapeutic perspectives. Stem Cell Investig. 2023;10:4.
[51] DIPIETRO LA, WILGUS TA, KOH TJ. Macrophages in Healing Wounds: Paradoxes and Paradigms. Int J Mol Sci. 2021;22(2):950.
[52] CHOW SKH, WONG CHW, CUI C, et al. Modulating macrophage polarization for the enhancement of fracture healing, a systematic review. J Orthop Translat. 2022;36:83-90.
[53] VI L, BAHT GS, WHETSTONE H, et al. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res. 2015;30(6):1090-1102.
[54] SU Y, GAO J, KAUR P, et al. Neutrophils and Macrophages as Targets for Development of Nanotherapeutics in Inflammatory Diseases. Pharmaceutics. 2020;12(12):1222.
[55] PALANO MT, GALLAZZI M, CUCCHIARA M, et al. Neutrophil and Natural Killer Cell Interactions in Cancers: Dangerous Liaisons Instructing Immunosuppression and Angiogenesis. Vaccines (Basel). 2021;9(12): 1488.
[56] MEDRANO-BOSCH M, SIMÓN-CODINA B, JIMÉNEZ W, et al. Monocyte-endothelial cell interactions in vascular and tissue remodeling. Front Immunol. 2023;14:1196033.
[57] HARJUNPÄÄ H, LLORT ASENS M, GUENTHER C, et al. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front Immunol. 2019;10:1078.
[58] BUONACERA A, STANCANELLI B, COLACI M, et al. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int J Mol Sci. 2022;23(7):3636.
[59] CHEN P, LIU Y, LIN X, et al. Diagnostic Value of the Blood Neutrophil-to-Lymphocyte Ratio and Monocyte-to-Lymphocyte Ratio in Tibia Fracture-Related Infection. Dis Markers. 2022;2022: 6119583.
[60] GOLSORKHTABARAMIRI M, MCKENZIE J, POTTER J. Predictability of Neutrophil to Lymphocyte Ratio in preoperative elderly hip fracture patients for post-operative short-term complications: a retrospective study. BMC Musculoskelet Disord. 2023;24(1):227.
[61] KÖNNECKE I, SERRA A, EL KHASSAWNA T, et al. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion. Bone. 2014;64:155-165.
[62] EL KHASSAWNA T, SERRA A, BUCHER CH, et al. T Lymphocytes Influence the Mineralization Process of Bone. Front Immunol. 2017; 8:562.
[63] CHEN R, HAO Z, WANG Y, et al. Mesenchymal Stem Cell-Immune Cell Interaction and Related Modulations for Bone Tissue Engineering. Stem Cells Int. 2022;2022:7153584.
[64] FENG B, FENG X, YU Y, et al. Mesenchymal stem cells shift the pro-inflammatory phenotype of neutrophils to ameliorate acute lung injury. Stem Cell Res Ther. 2023;14(1):197.
[65] SONG N, SCHOLTEMEIJER M, SHAH K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41(9):653-664.
[66] CAI B, LIN D, LI Y, et al. N2-Polarized Neutrophils Guide Bone Mesenchymal Stem Cell Recruitment and Initiate Bone Regeneration: A Missing Piece of the Bone Regeneration Puzzle. Adv Sci (Weinh). 2021; 8(19):e2100584.
[67] ANDO Y, TSUKASAKI M, HUYNH NCN, et al. The neutrophil-osteogenic cell axis promotes bone destruction in periodontitis. Int J Oral Sci. 2024;16(1):18.
[68] FISCHER V, HAFFNER-LUNTZER M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin Cell Dev Biol. 2022;123:14-21.
[69] NUMAZAKI K, TADA H, NISHIOKA T, et al. Neutrophil extracellular traps inhibit osteoclastogenesis. Biochem Biophys Res Commun. 2024;705: 149743.
[70] DELGADO-CALLE J, BELLIDO T. The osteocyte as a signaling cell. Physiol Rev. 2022;102(1):379-410.
[71] AZAB E, CHANDLER KB, UDA Y, et al. Osteocytes control myeloid cell proliferation and differentiation through Gsα-dependent and -independent mechanisms. FASEB J. 2020;34(8):10191-10211.
[72] XIAO M, ZHANG W, LIU W, et al. Osteocytes regulate neutrophil development through IL-19: a potent cytokine for neutropenia treatment. Blood. 2021;137(25):3533-3547. |