中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (17): 3537-3547.doi: 10.12307/2025.708
• 软骨组织构建 cartilage tissue construction • 上一篇 下一篇
腺相关病毒介导p65shRNA和骨形态发生蛋白4协同表达促进软骨细胞的再生
余洋溢1,宋卓悦2,廉 强1,丁 康3,李广恒1
- 1 深圳市肌肉骨骼组织重建与功能恢复重点实验室,深圳市人民医院(暨南大学第二临床医学院,南方科技大学第一附属医院)骨科,成人关节重建与运动医学科,广东省深圳市 518020;2郑州市骨科医院骨科,河南省郑州市 450000;3深圳市平乐骨科医院骨科,广东省深圳市 518000
-
收稿日期:2024-06-11接受日期:2024-09-19出版日期:2025-06-18发布日期:2024-10-30 -
通讯作者:李广恒,博士,主任医师,深圳市肌肉骨骼组织重建与功能恢复重点实验室,深圳市人民医院(暨南大学第二临床医学院,南方科技大学第一附属医院)骨科,成人关节重建与运动医学科,广东省深圳市 518020 -
作者简介:余洋溢,男,1987年生,河南省人,汉族,2019年郑州大学毕业,博士,主治医师,主要从事骨软骨再生和生长板损伤修复研究。 并列第一作者:宋卓悦,郑州市骨科医院骨科,河南省郑州市 450000 -
基金资助:国家自然科学基金面上项目(81472136),项目负责人:李广恒
AAV-mediated expression of p65shRNA and bone morphogenetic protein 4 synergistically enhances chondrocyte regeneration
Yu Yangyi1 , Song Zhuoyue2, Lian Qiang1, Ding Kang3, Li Guangheng1
- 1Shenzhen Key Laboratory of Musculoskeletal Tissue Reconstruction and Function Restoration, Division of Adult Joint Reconstruction and Sports Medicine, Department of Orthopedic Surgery, Shenzhen People’s Hospital (The Second Clinical Medical College Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen 518020, Guangdong Province, China; 2Department of Orthopedics, Zhengzhou Orthopedics Hospital, Zhengzhou 450000, Henan Province, China; 3Department of Orthopedics, Shenzhen Pingle Orthopedics Hospital, Shenzhen 518000, Guangdong Province, China
-
Received:2024-06-11Accepted:2024-09-19Online:2025-06-18Published:2024-10-30 -
Contact:Li Guangheng, MD, Chief physician, Shenzhen Key Laboratory of Musculoskeletal Tissue Reconstruction and Function Restoration, Division of Adult Joint Reconstruction and Sports Medicine, Department of Orthopedic Surgery, Shenzhen People’s Hospital (The Second Clinical Medical College Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen 518020, Guangdong Province, China -
About author:Yu Yangyi, Attending physician, Shenzhen Key Laboratory of Musculoskeletal Tissue Reconstruction and Function Restoration, Division of Adult Joint Reconstruction and Sports Medicine, Department of Orthopedic Surgery, Shenzhen People’s Hospital (The Second Clinical Medical College Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen 518020, Guangdong Province, China Song Zhuoyue, Department of Orthopedics, Zhengzhou Orthopedics Hospital, Zhengzhou 450000, Henan Province, China Yu Yangyi and Song Zhuoyue contributed equally to this work. -
Supported by:the National Natural Science Foundation of China (General Program), No. 81472136 (to LGH)
摘要:
文题释义:
腺相关病毒:一种小型、非致病性的单链DNA病毒,属于细小病毒科(Parvoviridae)。尽管腺相关病毒能够感染人类及其他灵长类动物,但它自身无法引起疾病,因此广泛用于基因治疗研究中
软骨再生:作为运动学最重要的一环,软骨起着支持关节、缓冲冲击以及减少骨之间摩擦的关键作用。但由于软骨是一个无血管组织,治疗软骨损伤一直是一个巨大的挑战。然而,随着科学技术的不断更新,软骨再生技术也在不断发展。
摘要
背景:近年来,腺相关病毒(Adeno-associated virus,AAV)基因治疗已被证明是治疗骨关节炎的可靠和安全的方法。然而,鉴于骨关节炎发病机制的复杂性,单一基因操作治疗骨关节炎可能不能产生令人满意的效果。先前的研究表明,核转录因子κB可以促进骨关节炎软骨细胞中的炎症通路,而骨形态发生蛋白4可以促进软骨再生。
目的:利用一种可以特异性靶向核转录因子κB的p65短发夹RNA(p65shRNA)与骨形态发生蛋白4一起治疗骨关节炎。
方法:制备包含AAV-p65-shRNA和AAV-骨形态发生蛋白4的病毒颗粒,通过转染AAV-p65-shRNA或AAV-骨形态发生蛋白4进入细胞,评估其抑制软骨细胞炎症和促进软骨形成的效果,并进行体内和体外实验。实验按干预方式分为5组:PBS组、骨关节炎组、AAV-骨形态发生蛋白4组、AAV-p65shRNA组、骨形态发生蛋白4-p65shRNA 1∶1组,然后分别于术后4,12,24周采集标本。采集关节组织后进行番红O和阿利新蓝染色。通过免疫荧光染色检测关节腔内注射病毒颗粒对软骨修复的影响。进一步研究两种转染病毒颗粒的最佳比例,以提高混合细胞在体内的软骨形成潜能。
结果与结论:AAV-p65shRNA和AAV-骨形态发生蛋白4联合应用对软骨再生和骨关节炎治疗有协同作用。以1∶1的比例转染
AAV-p65shRNA和AAV-骨形态发生蛋白4的混合细胞产生最多的细胞外基质合成(P < 0.05)。体内实验结果也显示两种病毒的组合对骨关节炎软骨的再生潜力是所有组中最高的(P < 0.05)。上述结果证实,当两种病毒的比例相同时,联合疗法的效果最佳。减少p65shRNA 或骨形态发生蛋白4 转染细胞会导致胶原蛋白Ⅱ合成减少。p65shRNA抑制炎症和骨形态发生蛋白4促进再生对治疗骨关节炎同样重要。实验结果为同时抑制软骨炎症和促进软骨修复治疗早期骨关节炎提供了一种新策略。
https://orcid.org/0000-0003-0292-0954(Yu Yangyi)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号:
引用本文
余洋溢, 宋卓悦, 廉 强, 丁 康, 李广恒. 腺相关病毒介导p65shRNA和骨形态发生蛋白4协同表达促进软骨细胞的再生[J]. 中国组织工程研究, 2025, 29(17): 3537-3547.
Yu Yangyi , Song Zhuoyue, Lian Qiang, Ding Kang, Li Guangheng . AAV-mediated expression of p65shRNA and bone morphogenetic protein 4 synergistically enhances chondrocyte regeneration[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(17): 3537-3547.
In vitro study of human OA chondrocytes
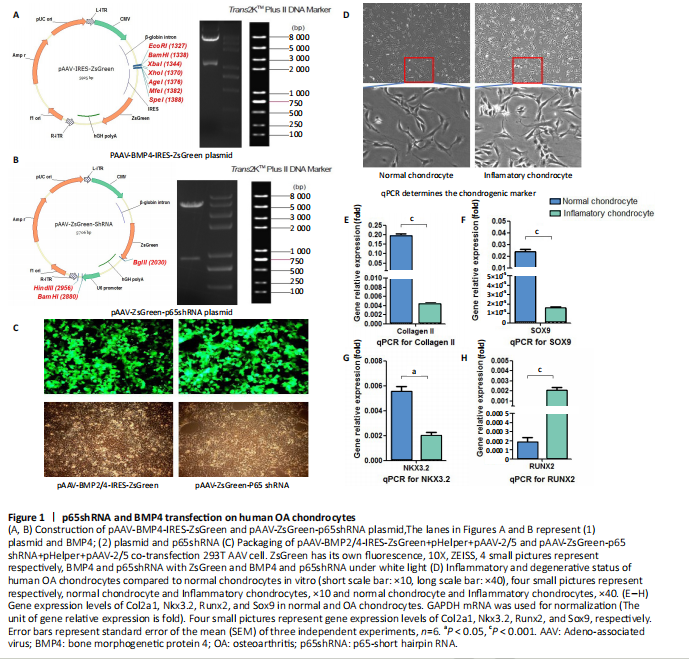
Chondrocytes from OA patients were isolated and cultured in vitro (Figure 1D). The related gene expression of chondrocytes was analyzed by real-time quantitative PCR (Figure 1E?H). The results showed that chondrocyte marker genes Col II, Nkx3.2, RUNX2 and Sox9 in OA chondrocytes were significantly downregulated, indicating the degenerative status of OA chondrocytes. In addition, the expression level of Runx2 was significantly increased in OA chondrocytes. Therefore, the OA chondrocytes presented an inflammatory, degenerative, hypertrophic status.
Effects of p65shRNA and BMP4 transfection on human OA chondrocytes
OA chondrocytes were transfected by AAV containing p65shRNA or BMP4. The success of the transfection was confirmed by real-time quantitative PCR and western blot/ELISA. The results show that p65 was significantly downregulated in p65shRNA transfected cells and significantly upregulated in BMP4 transfected cells. When AAV-BMP4 was implanted with gelfoam into the muscle pocket of nude rat, more bone formation could be found on X-ray, which was further confirmed by micro-CT analysis (Figure 2A?H).
Combination effect of BMP4 and p65 and optimization of the mixed ratio in vitro
The cell numbers were the same for the combined group and the three unmixed cell groups, which were composed of non-transfected OA chondrocytes, p65shRNA trans-fected cells, and BMP4 transfected cells. On days 14, 21, and 28, cell culture medium and cell pellets were collected for analysis.
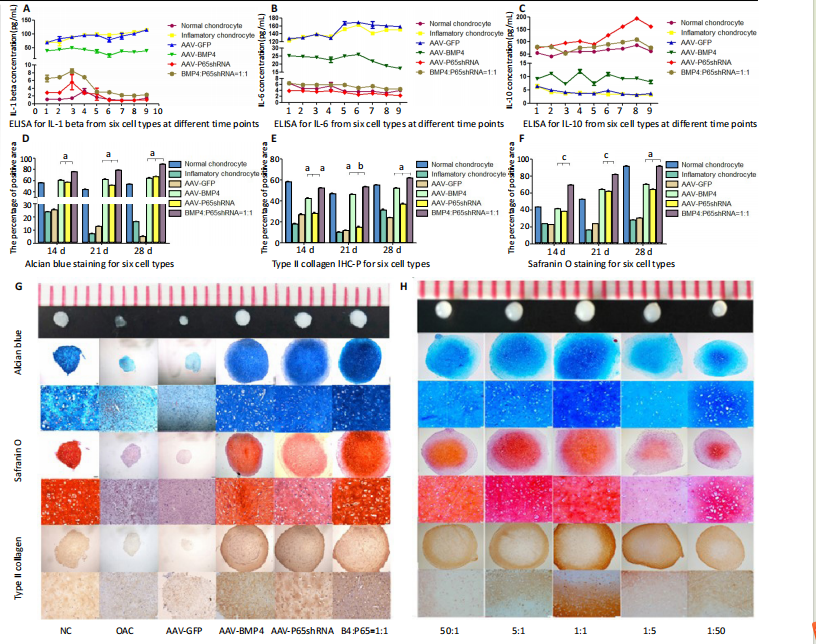
The concentrations of IL-1β, IL-6, and IL-10 in the culture medium were measured to reveal the modulation of inflammation. As shown in Figure 2C (day 21), OA chondrocytes presented a pro-inflammatory status with high levels of IL-1β and IL-6, but a low level of IL-10. Compared to the non-transfected OA chondrocytes, the p65shRNA transfected cells presented an improved inflammatory status. For the combined group, the pro-inflammatory factors were significantly decreased, but slightly higher than the p65shRNA alone group. Thus, transfection with both p65shRNA and BMP4 could suppress the inflammatory reaction of OA chondrocytes. The combined group showed slightly less suppression than the p65shRNA group, which resulted from the proportion of BMP4 transfected cells.
The effects of p65shRNA and BMP4 on OA chondrocytes were further analyzed based on the promotion of anabolic activity. Growing from the same number of cells, the cell pellets in different groups clearly had various sizes after 21 days (Figure 2A). The non-transfected OA chondrocytes formed very small aspheric pellets, while the transfected cells formed large pellets. The combined group was the largest among the cell groups. These observations indicate that combined p65shRNA and BMP4 transfection could promote the proliferation or extracellular matrix (ECM) synthesis of OA chondrocytes, and the combination of the two viruses exert a synergistic effect. The amount of ECM synthesized in each cell group was then assessed by Alcian blue, Safranin O, and type II collagen immunohistochemical staining (Figure 2B?E). The non-transfected control group had the lightest staining. In p65shRNA and BMP4 transfected cells, the staining strength increased, and the combined group had the heaviest staining. The quantified statistical analysis of staining (Figure 2E) shows that both p65shRNA and BMP4 transfection increased the ECM synthesis activity of OA chondrocytes.
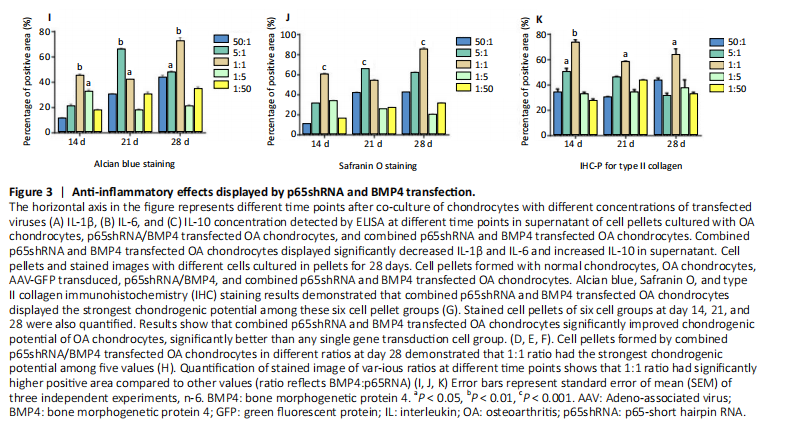
After confirming the significant effect of the combined strategy, we further optimized the repair effects by altering the mixture ratio of the two transfected cells. We tested five different mixture combinations (Figure 3). After 21 days, cell pellets in different groups demonstrated various sizes; the 1:1 group had the largest sizes, the 5:1 and 1:5 groups had the second largest, and the 50:1 and 1:50 groups had the smallest (Figure 3H). With Alcian blue and Safranin O staining, the 1:5 group showed the heaviest staining, while the 1:1 group showed the second heaviest (Figure 3H). This indicated that BMP4 promoted the synthesis of proteoglycan. For type II collagen immunohistochemistry staining, the 1:1 group had significantly heavier staining than the others, indicating that the presence of p65shRNA BMP4 promoted the synthesis
of type II collagen, which is a marker of hyaline cartilage formation. The statistical analysis (Figure 3I?K) confirmed our observations of the staining images. BMP4 contributed more to ECM synthesis than p65shRNA. However, with the same extent of inflammation modulation by p65shRNA, BMP4 synthesized the largest amount of type II collagen.
Therapeutic effects of combined AAV-p65shRNA and AAV-BMP4 injection in the rat OA model in vivo
The synergistic effect of AAV-p65shRNA and AAV-BMP4 in vivo was also tested by injecting the processed virus into the joints of SD rats with OA. As mixing an equal amount of p65shRNA and BMP4 transfected OA chondrocytes presented the best re-generative effects in vitro, we injected equal amounts of AAV-p65shRNA and AAV-BMP4 virus particles (0.5×1010) into the rat joints. The rats were sacrificed 12 weeks after injection, and hind limb knee joints were harvested. By gross observation, we found that the OA control rats had severely destroyed joints (Figure 4). Injection of AAV-p65shRNA virus particles (1×1010) greatly inhibited the inflammation but did not generate more cartilage when compared to the BMP4 and combined groups. With AAV-BMP4, there was regenerated hyaline-like cartilage. For the combined group, joints showed less inflammation and obvious hyaline-like cartilage formation. Therefore, the same as the in vitro results, the application of both viruses combined also presented the strongest regenerative potential in vivo.
We further performed histological staining of the cartilage specimen (Figure 4). It showed that the OA rats had no cartilage left. The rats injected with virus showed obvious


cartilage regeneration, and the combined group had the most robust ECM synthesis. At higher magnification, many chondrocytes were found to be distributed in the cartilage layer in samples from the AAV-BMP4 and combined groups; only the combined group had chondrocytes distributed all around the whole cartilage layer. Twelve weeks after virus injection, robust cartilage regenerative potential was observed in all virus-injected groups; the combined group had the best effect and BMP4 exhibited a better repair effect than p65shRNA.
| References [1] MANIVONG S, CULLIER A, AUDIGIé F, et al. New trends for osteoarthritis: biomaterials, models and modeling. Drug Discov Today. 2023;28(3):103488. [2] EMAMI A, NAMDARI H, PARVIZPOUR F, et al. Challenges in osteoarthritis treatment. Tissue Cell. 2023;80:101992. [3] HERMANN W, LAMBOVA S, MULLER-LADNER U. Current treatment options for osteoarthritis. Curr Rheumatol Rev. 2018;14(2):108-116. [4] CHULAY JD, YE GJ, THOMAS DL, et al. Preclinical evaluation of a recombinant adeno-associated virus vector expressing human alpha-1 antitrypsin made using a recombinant herpes simplex virus production method. Hum Gene Ther. 2011;22(2):155-165. [5] KAPLITT MG, FEIGIN A, TANG C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007; 369(9579):2097-2105. [6] MADRY H, CUCCHIARINI M, TERWILLIGER EF, et al. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum Gene Ther. 2003;14(4):393-402. [7] MADRY H, VENKATESAN JK, SCHMITT G, et al. rAAV vectors as safe and efficient tools for the stable delivery of genes to primary human chondrosarcoma cells in vitro and in situ. Sarcoma. 2012;2012:347417. [8] WATSON LEVINGS RS, BROOME TA, SMITH AD, et al. Gene therapy for osteoarthritis: pharmacokinetics of intra-articular self-complementary adeno-associated virus interleukin-1 receptor antagonist delivery in an equine model. Hum Gene Ther Clin Dev. 2018;29(2):90-100. [9] LIM CL, LEE YJ, CHO JH, et al. Immunogenicity and immunomodulatory effects of the human chondrocytes, hChonJ. BMC Musculoskelet Disord. 2017;18(1):199. [10] SOKOLOVE J, LEPUS CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77-94. [11] RIGOGLOU S, PAPAVASSILIOU AG. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45(11):2580-2584. [12] HENROTIN Y, CLUTTERBUCK AL, ALLAWAY D, et al. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18(2):141-149. [13] SHAKIBAEI M, JOHN T, SCHULZE-TANZIL G, et al. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73(9):1434-1445. [14] ROMAN-BLAS JA, JIMENEZ SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14(9):839-848. [15] CHOI MC, JO J, PARK J, et al. NF-κB signaling pathways in osteoarthritic cartilage destruction. Cells. 2019;8(7):734. [16] EVANS CH, GHIVIZZANI SC, ROBBINS PD. Osteoarthritis gene therapy in 2022. Curr Opin Rheumatol. 2023;35(1):37-43. [17] KURODA R, USAS A, KUBO S, et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54(2):433-442. [18] MARCU KB, OTERO M, OLIVOTTO E, et al. NF-kappaB signaling: multiple angles to target OA. Curr Drug Targets. 2010;11(5):599-613. [19] MATSUMOTO T, COOPER GM, GHARAIBEH B, et al. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60(5):1390-1405. [20] XIAO X, LI J, SAMULSKI RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72(3):2224-2232. [21] LI J, SAMULSKI RJ, XIAO X. Role for highly regulated rep gene expression in adeno-associated virus vector production. J Virol. 1997;71(7): 5236-5243. [22] WANG B, LI J, FU FH, et al. Systemic human minidystrophin gene transfer improves functions and life span of dystrophin and dystrophin/utrophin-deficient mice. J Orthop Res. 2009;27(4):421-426. [23] AISENBREY EA, BILOUSOVA G, PAYNE K, et al. Dynamic mechanical loading and growth factors influence chondrogenesis of induced pluripotent mesenchymal progenitor cells in a cartilage-mimetic hydrogel. Biomater Sci. 2019;7(12):5388-5403. [24] TIAN K, QI M, WANG L, et al. Two-stage therapeutic utility of ectopically formed bone tissue in skeletal muscle induced by adeno-associated virus containing bone morphogenetic protein-4 gene. J Orthop Surg Res. 2015;10:86. [25] JEON OH, KIM C, LABERGE RM, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23(6): 775-781. [26] CHAGANTI RK, LANE NE. Risk factors for incident osteoarthritis of the hip and knee. Curr Rev Musculoskelet Med. 2011;4(3):99-104. [27] HARRIS H, CRAWFORD A. Recognizing and managing osteoarthritis. Nursing. 2015;45(1):36-42; quiz 42-33. [28] KHEZRI K, MALEKI DIZAJ S, RAHBAR SAADAT Y, et al. Osteogenic differentiation of mesenchymal stem cells via curcumin-containing nanoscaffolds. Stem Cells Int. 2021;2021:1520052. [29] VAHEDI P, MOGHADDAMSHAHABI R, WEBSTER TJ, et al. The use of infrapatellar fat pad-derived mesenchymal stem cells in articular cartilage regeneration: a review. Int J Mol Sci. 2021;22(17):9215. [30] MARTEL-PELLETIER J, PELLETIER JP, FAHMI H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin Arthritis Rheum. 2003; 33(3):155-167. [31] YANG Q, TANG Y, IMBROGNO K, et al. AAV-based shRNA silencing of NF-κB ameliorates muscle pathologies in mdx mice. Gene Ther. 2012;19(12):1196-1204. [32] ZHANG Y, PIZZUTE T, PEI M. Anti-inflammatory strategies in cartilage repair. Tissue Eng Part B Rev. 2014;20(6):655-668. [33] MILJKOVIC ND, COOPER GM, MARRA KG. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthritis Cartilage. 2008;16(10):1121-1130. [34] JANE JA JR, DUNFORD BA, KRON A, et al. Ectopic osteogenesis using adenoviral bone morphogenetic protein (BMP)-4 and BMP-6 gene transfer. Mol Ther. 2002;6(4):464-470. [35] BRAMLAGE CP, HäUPL T, KAPS C, et al. Decrease in expression of bone morphogenetic proteins 4 and 5 in synovial tissue of patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2006; 8(3):R58. [36] WANG D, PRAKASH J, NGUYEN P, et al. Bone morphogenetic protein signaling in vascular disease: anti-inflammatory action through myocardin-related transcription factor A. J Biol Chem. 2012;287(33): 28067-28077. [37] VAN DER KRAAN PM, VAN DEN BERG WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20(3):223-232. [38] BEHRENDT P, FELDHEIM M, PREUSSE-PRANGE A, et al. Chondrogenic potential of IL-10 in mechanically injured cartilage and cellularized collagen ACI grafts. Osteoarthritis Cartilage. 2018;26(2):264-275. |
| [1] | 赖鹏宇, 梁 冉, 沈 山. 组织工程技术修复颞下颌关节:问题与挑战[J]. 中国组织工程研究, 2025, 29(在线): 1-9. |
| [2] | 尹 路, 蒋川锋, 陈俊杰, 易 明, 王子赫, 石厚银, 汪国友, 沈骅睿. 沙苑子苷A对关节软骨细胞凋亡的影响[J]. 中国组织工程研究, 2025, 29(8): 1541-1547. |
| [3] | 徐田杰, 樊佳欣, 郭小玲, 贾 祥, 赵兴旺, 刘凯楠, 王 茜. 二甲双胍抑制PI3K/AKT/mTOR信号通路保护骨关节炎模型大鼠关节软骨[J]. 中国组织工程研究, 2025, 29(5): 1003-1012. |
| [4] | 吴广涛, 秦 刚, 何凯毅, 范以东, 李威材, 朱宝刚, 曹 英. 免疫细胞与膝骨关节炎之间因果作用:一项两样本双向孟德尔随机化分析[J]. 中国组织工程研究, 2025, 29(5): 1081-1090. |
| [5] | 水 晶, 何 宇, 江 楠, 徐 坤, 宋丽娟, 丁智斌, 马存根, 李新毅. 星形胶质细胞调节中枢神经系统的髓鞘再生[J]. 中国组织工程研究, 2025, 29(36): 7889-7897. |
| [6] | 张晓宇, 韦善文, 方佳炜, 倪 莉. 普鲁士蓝纳米粒子抗氧化恢复退变髓核细胞线粒体功能[J]. 中国组织工程研究, 2025, 29(34): 7318-7325. |
| [7] | 赵雪梅, 王 睿, 奥·乌力吉, 包书茵, 江小华. 蒙药沙蓬粗寡糖对小鼠滑膜细胞炎症和凋亡的影响[J]. 中国组织工程研究, 2025, 29(32): 6939-6946. |
| [8] | 吴振桦, 张锡玮, 王一品, 李倩倩. 血清血脂7项与骨关节炎的关系:IEU OPEN GWAS数据库欧洲人群的大样本分析[J]. 中国组织工程研究, 2025, 29(32): 7004-7014. |
| [9] | 樊佳欣, 贾 祥, 徐田杰, 刘凯楠, 郭小玲, 张 辉, 王 茜. 二甲双胍抑制铁死亡改善骨关节炎模型大鼠的软骨损伤[J]. 中国组织工程研究, 2025, 29(30): 6398-6408. |
| [10] | 王万春, 易 军, 严张仁, 杨 悦, 董德刚, 李玉梅. 717解毒合剂重塑细胞外基质稳态促进蝮蛇伤大鼠局部损伤组织的修复[J]. 中国组织工程研究, 2025, 29(30): 6457-6465. |
| [11] | 宋雨婷, 文春雷, 李 奕, 柏 雪, 高 鸿, 胡廷菊, 王子君, 严 旭. 心肌细胞外基质重塑对缝隙连接蛋白43及其Ser368位点磷酸化和电传导的影响[J]. 中国组织工程研究, 2025, 29(29): 6212-6218. |
| [12] | 孙雅蕙, 王宇峰, 郭 超, 姚俊杰, 纪媛媛, 李中旭, 娄惠娟, 江晶蕾, 孙一萍, 徐 婧, 丛德毓. 推拿对2型糖尿病大鼠骨骼肌细胞外基质胶原沉积的影响[J]. 中国组织工程研究, 2025, 29(26): 5549-5555. |
| [13] | 郝茂辰, 马 超, 刘 凯, 柳可心, 孟令婷, 王杏如, 王建忠. 骨关节炎内质网应激关键基因的生物信息学筛选及实验验证[J]. 中国组织工程研究, 2025, 29(26): 5632-5641. |
| [14] | 纪雅琼, 宁忠平. 芍药苷对血管紧张素Ⅱ诱导心肌成纤维细胞纤维化的保护作用[J]. 中国组织工程研究, 2025, 29(25): 5382-5389. |
| [15] | 周丽君, 张克远, 徐飞虎, 王 茜, 俞 丽, 董士铭, 徐俊宇, 郭宇沨, 马海蓉, 丁 红. 环状RNA SEC24A对骨关节炎滑膜成纤维细胞增殖和凋亡的影响及机制[J]. 中国组织工程研究, 2025, 29(24): 5086-5092. |
However, OA is a complex disease with many therapeutic genes responsible for its initiation and progression. Therefore, selecting an appropriate target gene is a key concern for clinical success. During OA progression, nuclear factor κB (NF-κB) is considered to be a possible target, because both interleukin (IL)-1β and tumor necrosis factor α (TNF-α) induce cartilage catabolism and amplify the inflammatory response by activating the NF-κB signaling pathway[9-15]. Besides the anti-inflammatory strategy, gene therapy with several growth factors presents excellent regenerative effects, with anabolic effects on cartilage homeostasis, such as bone morphogenetic proteins (BMPs)[16]. Previously, skeletal muscle derived stem cells transduced with retrovirus containing BMP4 gene were used for treatment of knee OA model rats and displayed great potential for inducing cartilage regeneration[17].
In a recent review, some studies noted that NF-κB signaling is extensively involved in OA pathology through a variety of functions, including the secretion of degrading enzymes, cytokines and chemokines, cyclooxygenase-2, and prostaglandin E2. Inhibition of NF-κB signaling has been accepted as a therapeutic target in OA[18-19]. Our previous studies found that BMP4 retrovirus gene shows therapeutic promise for repairing articular cartilage defects and OA in animal models by promoting chondrogenesis[19]. Based on these considerations, we chose p65 and BMP4 as our targets. They may play a synergistic role in reducing inflammation and promoting chondrogenesis.
In the present study, AAV containing short hairpin RNA specifically targeting the messenger RNA of p65 (p65shRNA) was constructed to inhibit the NF-κB signaling pathway, and AAV containing the BMP4 gene was constructed to enhance the chondrocyte anabolic process. The chondrogenic potential of gene-transduced OA chondrocytes and their combination in various ratios was investigated in vitro. Finally, mixtures of the two viruses in certain ratios were injected into rats with OA induced by the administration of iodoacetic acid, and their synergistic effect on OA was assessed in vivo.
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
Design
After the preparation of viral particles including AAV-p65-shRNA and AAV-BMP4, their efficacy in inhibiting inflammation in chondrocytes and promoting chondrogenesis was assessed by transfecting AAV-p65-shRNA or AAV-BMP4 into cells, with both in vitro and in vivo evaluation. Then, the optimal ratio between the two types of transfected viral particles was further investigated to improve the chondrogenic potential of mixed cells in vivo.
Time and settings
The experiment was completed in the Orthopedic Laboratory of Shenzhen People’s Hospital from January 2021 to January 2023.
Materials
Sixty 10-week-old male Sprague-Dawley rats (GuangDong GemPharmatech Co.) were assigned to five groups; All animal research adhered to the institution’s or the National Research Council’s guidelines for the Care and Use of Laboratory Animals. This investigation was approved by the Institutional Animal Care and Use Committee of Shenzhen People’s Hospital at Jinan University in 2021 (approval No. LL-KY-2020024). A 6-mm×6-mm piece of sterile gelatin sponge, bovine serum albumin, and the other materials were purchased from Sinopharm Chemical Reagent (Shenzhen, China).
Methods
Separation and culture of articular cartilage in human OA During knee arthroplasty for patients with osteoarthritis, approximately 30 g of cartilage tissue was cut with an osteotome at the junction of normal and degraded cartilage tissue on the femoral condyle. The tissue was chopped with scissors and placed in type I collagenase solution (100 mg/mL: Abcam, Shenzhen, China), type VI collagenase solution (100 mg/mL: Abcam, Shenzhen, China), or neutral protease solution (1.2 mg/mL; Abcam) and digested for 60 minutes at 37 °C. The digested tissue was resuspended in DMEM medium containing 10% fetal bovine serum and 1% penicillin/streptomycin (Gibco) after centrifugation. After soaking, the suspension was passed through a 40 μm filter to obtain single-cell suspension.
The chondrocytes were cultured on a 24-well cell culture plate with type I collagen adsorbed on the surface, and the cell density was 5×103/well. Cell culture medium was made with 10% fetal bovine serum in DMEM. The cells were passaged to ~70% confluency, and trypsin containing ethylene-diamine-tetraacetic acid (Gibco, Shenzhen, China) was used for passage. The cells were sub-cultured and placed on a culture plate at a density of 2.5×103/cm2.
Preparation of viral particles
A classical method of co-transfecting 293 packaging cells with three plasmids was used[20-22]. After the 293 cells were trypsinized, they were passaged into a 225 cm2 culture dish. The cell confluency of the monolayer culture was about 20%−40%. Then 40 mL of cell culture medium was added to ensure even distribution of cells in the dish. The cells were placed in an incubator until cell confluency was close to 80% and transfection started. One hour before transfection, half of the fresh cell culture medium was replaced, and 23 μg of the AAV plasmid (Hanbio, Shenzhen, China) of p65 shRNA or BPM4 and 23 μg of the two helper plasmids were added to 4 mL of a 300 mmol/L calcium chloride solution, and these solutions were uniformly added to the 293 cells after 3 days of transfection (Figure 1A−C).
Purification of AAV particles
The AAV particles were purified by high-speed centrifugation using a cesium chloride gradient. For this procedure, 11 mL of a 40% sucrose solution containing 0.01% BSA (Sigma-Aldrich, Shenzhen, China) was added to a sterile ultrafiltration tube, to which 48 mL of crude virus extract was added, and the mixture was ultracentrifuged at 100, 000 r/minute at 4 °C for 16 hours. After the addition of cesium chloride, the cells were repeatedly centrifuged several times to obtain purified AAV particles, and then subjected to DNA dot-blot hybridization to determine their concentration.
Transfection of chondrocytes
Chondrocytes were cultured in 10% fetal bovine serum DMEM medium to 85% confluency. AAV-p65-shRNA, AAV-BMP4, and Adeno-Associated Virus carrying Green Fluorescent Protein (AAV-GFP) suspensions with a virus titer of approximately
5×1012/mL were separately added to the cell culture medium. After 48 hours of transfection, they were replaced in the DMEM containing 10% fresh fetal bovine serum.
In vitro 3D culture of chondrocytes
After successful transfection of chondrocytes, the culture reached about 85% confluency, and the chondrocytes were grouped and counted again. The groups were set as follow: PBS; OA; AAV-BMP4; AAV-p65shRNA; BMP4-p65shRNA (1:1 mixed). Each group of 2×105 cells was set as a 3D cell culture system and transferred to a 15 mL cell culture tube containing 2 mL of chondrocyte culture medium consisting of DMEM-HG, 50 μg/mL L-ascorbic acid 2-phosphate (Sigma-Aldrich), 40 μg/mL DL-proline (Sigma-Aldrich), 1% ITS+ Premix (Becton-Dickinson, Franklin Lakes, NJ, USA), 1% P/S with either no growth factors or with 100 ng/mL BMP2 (Peprotech, Shenzhen, China) and/or 10 ng/mL TGF-β3 (Peprotech). Cells were then pelleted at 500×g for 5 minutes. All pellets were incubated at 37 °C in 5% CO2 for 21 days, with the culture medium refreshed every 2−3 days[23-24]. During the 3D culture of chondrocytes, cell culture fluid and cell specimens were collected at 14, 21, and 28 days for biochemical, molecular biology, and tissue index detection. The concentrations of IL-1, IL-6, and BMP4 in the cell culture medium were detected by ELISA. The expression of type I, II collagen, SOX9, and type X collagen at different time points was detected by RT-PCR.
Histology and immunochemistry
As previously described[25], paraffin sections were dewaxed in an oven at 60 °C for 4 hours and dewaxed in xylene (Sigma, Shenzhen, China) for 20 minutes; the xylene was replaced and the sections were soaked for another 20 minutes, then underwent gradient hydration (high to low concentration of 100%−70%, 5 minutes each) and placed in distilled water for 5 minutes. After staining of type II collagen (anti-type II collagen antibody, 1:50; Abcam) and corresponding horseradish peroxidase conjugated secondary antibody (1:100; Abcam) with Alcian blue solution, safranin O, and immunohistochemistry, the ratio of positive area to total field area was determined by ImageJ (NIH) in a blinded manner. Quantification was performed by determining the percentage of area that contained positive-stained area. Specifically, area% mineralization was quantified using NIH ImageJ. For the Alcian blue assay, cell laden films were washed at their respective time points with PBS and stained using Alcian Blue 8GX (1%). Films were then washed with DI water and remaining bound Alcian Blue dye was extracted with 4M guanidine HCl. Absorbance of the extracted Alcian Blue dye was read at optical density 615 nm and dye extracted from cell-free nHA-PCLUR films served as blank controls. Images for all matrix stains were taken with a reflected-light microscope (Zeiss Axioscope 5). For quantitative histomorphometry for Safranin O or immunohistochemistry, images were taken at 4X via bright-field microscopy (Olympus BX41). The area of interest was defined as a 2 mm × 3 mm rectangular region. Bone was thresholded as black stain in the von Kossa sections and cartilage was thresholded as orange in the Safranin O sections. Bone and cartilage within the defect were reported as an area percentage of the total area of interest.
ELISA
The concentrations of IL-1B, IL-6, IL-10, and BMP4 in cell culture medium were determined by ELISA (Thermo Fisher, Shenzhen, China), according to the manufacturer’s instruction, with detection limits of 7 pg/mL for IL-1, 3 pg/mL for IL-6 and IL-10, and 15 pg/mL for BMP4. The test sample was added to the microplate wells coated with antigen or antibody. The sample containing the target molecule (such as an antibody or antigen) bound to the fixed molecules on the plate. A blocking solution (usually a protein solution such as bovine serum albumin) was added to block the non-specific binding sites on the microplate. This step prevents non-specific proteins from binding in subsequent steps, reducing background noise. A secondary antibody conjugated with an enzyme that specifically recognizes and binds to the target antigen or antibody was added. The enzyme was usually horseradish peroxidase or alkaline phosphatase. Multiple washes of the microplate were performed to remove any unbound antibodies or antigens, ensuring that only specifically bound complexes remain on the plate. Absorbance at 450 nm was measured using a microplate spectrophotometer (DS Pharma Biomedical).
Cell combinations at different ratios in vitro
Osteoarthritic chondrocytes transfected with AAV-p65-shRNA and AAV-BMP4 virus were prepared beforehand. The mix ratios of the two cells were 50:1, 5:1, 1:1, 1:5, and 1:50, and different combinations of cells were cultured in chondrocyte culture medium in vitro by a 3D cell culture technique. Cell culture medium was collected on days 14, 21, and 28, and cell pellets were collected for testing of biochemical, molecular biology, and tissue indicators in order to analyze and compare the changes in the biological behavior of chondrocytes between groups. Alcian blue, safranin O, and type II collagen immunohistochemistry staining results were analyzed for chondrogenic potential among these groups. The method was as described above.
Animal study
In this experiment, 60 10-week-old male Sprague-Dawley rats (GuangDong GemPharmatech Co.) were assigned to 5 group with 12 animals in each group: PBS; OA; AAV-BMP4; AAV-p65shRNA; AAV-BMP4-p65shRNA (1:1 mixed); to be consistent, the same hybrid ratio was applied in the animal study. At each time point (0, 4, and 12 weeks after knee joint injection), eight knee joints from four rats were collected for further analysis. The rats in the control group were treated with iodoacetic acid (Thermo Fishe, Shenzhen, China) to induce knee osteoarthritis without any viral particle injection. The other four groups were treated with iodoacetic acid to induce knee osteoarthritis and two kinds of virus particles in optimal ratios (1×108, 10×108, 100×108, and 1 000×108 virus particles) were injected into the knee joint. The degeneration process of articular chondrocytes in knee osteoarthritis was observed at each time point.
Specimen collection and processing
Animals were sacrificed at each time point. The gross specimen of removed hind limb knee joint was photographed, and a preliminary general assessment was made. Some specimens were fixed in formalin solution for 48 hours, and decalcification was performed in 10% ethylene-diamine-tetraacetic acid (Sigma) solution for 2 weeks, then paraffin-embedded specimens were used as conventional histochemical staining samples. Some specimens were quickly embedded in OCT liquid and frozen in liquid nitrogen. Frozen specimens were stored at −80 °C. A deep-temperature refrigerator was used for frozen immunohistochemical staining. Tissue staining, including safranin O and Alcian blue, was applied after collecting articular tissue. The effect of virus particles injected into the joint cavity on cartilage repair was detected by immunofluorescence staining.
Main observing index
(1) Purified AAV particles were obtained and then subjected to DNA dot-blot hybridization to determine their concentration. (2) Tissue staining, including safranin O and Alcian blue, was applied after collecting articular tissue. The effect of virus particles injected into the joint cavity on cartilage repair was detected by immunofluorescence staining.
Statistical analysis
All data were statistically processed using SPSS 26.0 software (IBM, USA). The values were in accordance with normal distribution. The measurement data that were in accordance with normal distribution were expressed as the mean ± standard deviation. The statistical method for comparing the differences between the groups was two independent sample t-test. P < 0.05 was considered significantly different. The statistical methods of the article had been reviewed by biostatistical experts from Jinan University.
OA is one of the most common degenerative diseases worldwide, and there are no effective medications or treatments to reverse the pathological process and regenerate hyaline cartilage[26-27]. In the present study, we demonstrated an excellent regenerative effect of the combination of AAV-p65shRNA and AAV-BMP4 for the treatment of OA. These two factors also demonstrated an almost equal contribution to restraining OA chondrocytes and yielded a synergistic regenerative effect.
Chondrocytes are the predominant target cells for OA treatment, as they are the only cell type resident in articular cartilage and are responsible for ECM turnover. However, it is difficult for systemically administered drugs to reach chondrocytes, as cartilage is avascular tissue. Intra-articular injection of drugs could solve the delivery problem and increase local concentration, but multiple injections are needed. Stem cells, such as various mesenchymal stem cells, have proved helpful for osteochondral defects, as they have multidirectional differentiation ability, including osteogenic differentiation and chondrogenic regeneration, and have been considered as an alternative treatment strategy[28-29]. Therefore, intra-articular gene therapy could generate a long-term effect and avoid the risks of systemic administration. Currently, several vectors are employed to deliver therapeutic genes for OA treatment. Among them, AAV is superior, as it can penetrate into the cartilage matrix and transduce chondrocytes in situ. It has been reported that genes delivered via AAV could be efficiently transfected into chondrocytes and damaged cartilage tissue with efficiency exceeding 70%, and the impact on the targeted gene could remain for more than 5 months.
The NF-κB signaling pathway is of pivotal importance in OA initiation, progression, and symptom development. In joints with inflammation arising from aging, obesity, trauma, overuse of alcohol, metabolic disorders, or genetic elements, NF-κB is activated by pro-inflammatory factors, such as IL-1β and TNF-α, and the activated NF-κB can accelerate ECM degradation by activating ECM degrading enzymes, including matrix metalloproteinase 13 and a disintegrin and metalloproteinase with thrombospodin motifs. It can also upregulate cyclooxygenase-2 and prostaglandin E2, leading to inflammatory injury aggravation and joint pain[30]. Moreover, activated NF-κB can promote the secretion of several inflammatory factors, including IL-1β, TNF-α, IL-6, IL-8, IL-17, and IL-18, which in turn promotes NF-κB signaling and aggravates the disease. Therefore, both the progression and symptoms will be alleviated by blocking the NF-κB signaling pathway, and this has become a therapeutic target for OA treatment. Of note, subunit p65 is a key element associated with the inflammatory reaction in OA and is an effective target for gene therapy to treat OA. Small interfering RNA of p65 delivered by AAV was found to effectively block NF-κB signaling and promote tissue regeneration in mice with Duchenne muscular dystrophy[31]. In the present study, p65shRNA effectively inhibited the inflammation process and built up an anti-inflammatory environment, manifested as low levels of IL-1β and IL-6, and a high level of IL-10. The ECM increments after p65shRNA transfection further confirmed that this anti-inflammatory environment supports cartilage regeneration[32].
BMP4 is used in promoting mesenchymal stem cell differentiation into cartilage[33]. It has also been reported that BMP4 can maintain the chondrocytic phenotype and increase the secretory activity of chondrocytes[34]. In addition, it was also reported that BMP4 was downregulated in synovial tissue from OA patients, and it was downregulated in OA chondrocytes[35]. This verifies that external supple-mentation with BMP4 through gene transfer could facilitate cartilage tissue repair. In addition to its anabolic effect on chondrocytes, BMP4 may modulate the inflammatory reaction, based on the results of our study. It has also been reported that BMP signaling might block the inflammation process by inhibiting the activation of NF-κB[36].
In vitro, we tested five different mixture combinations. After 21 days, cell pellets in different groups demonstrated various sizes; the 1:1 group had the largest sizes, the 5:1 and 1:5 groups had the second largest, and the 50:1 and 1:50 groups had the smallest. in vivo, the synergistic effects of AAV-p65shRNA and AAV-BMP4 were also tested by injecting the processed virus into the joints of Sprague-Dawley rats with OA. As mixing an equal amount of p65shRNA and BMP4 transfected OA chondrocytes presented the best re-generative effects in vitro, we injected equal amounts of AAV-p65shRNA and AAV-BMP4 virus particles into the rat joints. We found that the OA control rats had severely destroyed joints. Injection of AAV-p65shRNA virus particles (1×1010) greatly inhibited the inflammation but did not generate more cartilage when compared to the BMP4 and combined groups. With AAV-BMP4, there was regenerated hyaline-like cartilage. For the combined group, joints showed less inflammation and obvious hyaline-like cartilage formation. Therefore, the same as the in vitro result, the application of both viruses combined also presented the strongest regenerative potential in vivo. Synergistic gene therapy has good effects both in vivo and in vitro, among which the same ratio gene therapy has the best effect among different ratio gene therapy.The limitation of this article is that it was only verified in animals and the therapeutic effect has not been tested in human experiments.
Suppressing inflammation has always been at the core of cartilage repair, and it was found that inflammatory factors such as IL-1β impede chondrogenic differentiation, and anti-inflammatory factors such as IL-10 promote chondrogenesis[37-38]. Considering the fact that OA chondrocytes have very low BMP4 expression, we thought that AAV-BMP4 transfection might play an important role in supporting cartilage growth in our experiment.
OA has a complex pathogenesis, which makes it unlikely that we can optimally modulate all of the cells with a single gene modulation. In this regard, two or more transgenes in favor of cartilage homeostasis applied together could reinforce the improvement, unless the genes inhibit each other. Usually, to achieve the optimal effect, two or more genes responsible for important but different signal pathways are selected in combined gene therapy. In the present study, we also discovered that the combined therapy had the maximum effect when the two viruses were administered in equal proportions. Decreasing either p65shRNA or BMP4 transfected cells resulted in less type II collagen synthesis. This implies that inhibiting inflammation by p65shRNA and promoting regeneration by BMP4 are equally important for OA treatment, and fully demonstrates the effectiveness of our therapeutic strategy of targeting the two critical pathways simultaneously.
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
近年来,腺相关病毒基因治疗已被证明是治疗骨关节炎的可靠和安全的方法。然而,鉴于骨关节炎的发病机制的复杂性,单一基因操作治疗骨关节炎可能无法产生令人满意的结果。先前的研究表明,核转录因子kB可以抑制骨关节炎软骨细胞中的炎症通路,而骨形态发生蛋白4可以促进软骨再生。实验旨在使用一种可以特异性靶向核转录因子kB的p65短发夹RNA(p65shRNA)与骨形态发生蛋白4一起治疗骨关节炎。#br#
#br#
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程#br#
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||