中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (10): 2189-2200.doi: 10.12307/2025.407
• 生物材料综述 biomaterial review • 上一篇
富血小板血浆及水凝胶治疗脊髓损伤
赵文琪1,于海驰2,宋艺儒2,袁天阳2,刘钦毅2
- 1吉林大学白求恩第二临床医学院,吉林省长春市 130012;2吉林大学第二医院,吉林省长春市 130041
-
收稿日期:2024-03-15接受日期:2024-04-23出版日期:2025-04-08发布日期:2024-08-26 -
通讯作者:刘钦毅,主任医师,教授,吉林大学第二医院,吉林省长春市 130041 袁天阳,主治医师,吉林大学第二医院,吉林省长春市 130041 -
作者简介:赵文琪,男,2003年生,山东省临沂市人,汉族,吉林大学白求恩第二临床医学院本科生。 -
基金资助:吉林省自然科学基金项目(20210101265JC),项目负责人:于海驰
Platelet-rich plasma and hydrogel for spinal cord injury
Zhao Wenqi1, Yu Haichi2, Song Yiru2, Yuan Tianyang2, Liu Qinyi2
- 1Bethune Second Clinical College of Medicine, Jilin University, Changchun 130012, Jilin Province, China; 2Second Hospital of Jilin University, Changchun 130041, Jilin Province, China
-
Received:2024-03-15Accepted:2024-04-23Online:2025-04-08Published:2024-08-26 -
Contact:Liu Qinyi, Chief physician, Professor, Second Hospital of Jilin University, Changchun 130041, Jilin Province, China Yuan Tianyang, Attending physician, Second Hospital of Jilin University, Changchun 130041, Jilin Province, China -
About author:Zhao Wenqi, Bethune Second Clinical College of Medicine, Jilin University, Changchun 130012, Jilin Province, China -
Supported by:Natural Science Foundation of Jilin Province, No. 20210101265JC (to YHC)
摘要:
文题释义:
继发性脊髓损伤:是在脊髓原发性损伤的基础上,经血管、氧自由基、兴奋性氨基酸、一氧化氮等因素机制造成的继发性损伤。由于继发性损伤常常发生较快、窗口期短,后果严重,从而导致临床上对于继发性脊髓损伤的修复性治疗效果不甚理想,改善继发性损伤是治疗脊髓损伤的重点。
神经生长因子:广泛分布于机体各组织器官中(包括脑),在靶组织中的浓度与交感神经和感觉神经在靶区分支的密度和mRNA含量有关。神经生长因子能促进中枢和外周神经元的生长、发育、分化、成熟,维持神经系统的正常功能,加快神经系统损伤后的修复。
背景:大量文献报道了富血小板血浆、水凝胶治疗脊髓损伤的作用及其机制,但较少文章归纳总结它们治疗脊髓损伤的策略。
目的:归纳总结脊髓损伤的病理进程,富血小板血浆和水凝胶单独及联合应用修复脊髓损伤的策略。
方法:应用计算机检索PubMed和中国知网数据库建库至2024年3月之前发表的文献,中文检索词为“脊髓损伤,富血小板血浆,水凝胶”,英文检索词为“spinal cord injury,spinal cord,Platelet-rich plasma,hydrogel,angiogenesis,neuralgia,combination therapy”,按照纳入和排除标准对文献进行筛选,最终纳入128篇文献进行综述分析。
结果与结论:①富血小板血浆的分类复杂多样,在脊髓损伤的修复性治疗应用中的效果也是各有不同,但都表现出一定的积极的效果,即具有一定的促进轴突再生、刺激血管生成、治疗神经性疼痛等作用;②富血小板血浆的作用主要得益于其所含的生长因子等成分;③水凝胶的种类也很多,在脊髓损伤的修复性治疗中主要起到填充、模拟细胞外基质、搭载药物与生物产品、作为支架搭载细胞等作用;④与单一的治疗方式相比,富血小板血浆和水凝胶联合治疗可更有效地促进神经再生和脊髓功能的恢复。
https://orcid.org/0009-0000-8578-637X (赵文琪)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
中图分类号:
引用本文
赵文琪, 于海驰, 宋艺儒, 袁天阳, 刘钦毅. 富血小板血浆及水凝胶治疗脊髓损伤[J]. 中国组织工程研究, 2025, 29(10): 2189-2200.
Zhao Wenqi, Yu Haichi, Song Yiru, Yuan Tianyang, Liu Qinyi. Platelet-rich plasma and hydrogel for spinal cord injury[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(10): 2189-2200.
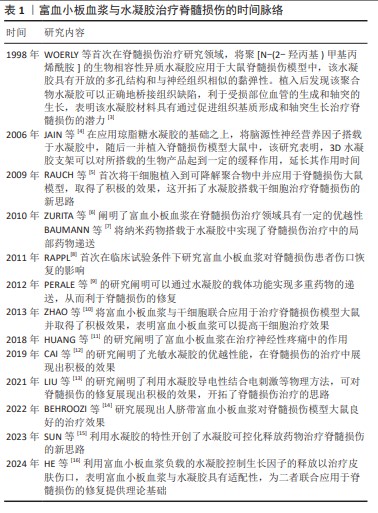
2.2 脊髓损伤的机制与治疗
2.2.1 脊髓的解剖与生理 脊髓是连接躯体和大脑的最为重要的沟通渠道,从大脑底部的延髓延伸至颅骨的枕骨大叶,到达L1/L2腰椎,在那里终止为延髓圆锥,远端为马尾神经。脊髓受到脊柱的骨性保护,此外,硬脑膜、蛛网膜、软脑膜、蛛网膜下腔的脑脊液、硬膜外腔的纤维和脂肪组织等对脊髓亦有一定的保护作用[17]。
脊髓由灰质和白质组成。脊髓的灰质被白质包围,灰质中神经细胞的轴突主要是无髓鞘的,负责接收和传导冲动;与灰质不同,白质是主要由有髓鞘的感觉轴突和运动轴突组成的相互连接的纤维集合[18]。脊髓作为人的低级中枢可以通过反射弧独立地响应感受器所传入的信息,而无需大脑的信息输入就可以完成一些非条件反射[19]。
2.2.2 脊髓损伤的分类及机制 脊髓损伤是一种对人体功能造成毁灭性打击的创伤性疾病,会导致强烈的神经性疼痛以及运动、感觉和自主神经功能障碍[20]。脊髓损伤损伤的复杂性以及缺乏有效的治疗方法使脊髓损伤成为一个世界性的问题。
根据发病原因,脊髓损伤可分为创伤性和非创伤性,当急性或慢性疾病(如肿瘤、感染或退行性疾病)对脊髓造成损害时就会发生非创伤性脊髓损伤[21]。创伤性脊髓损伤通常由椎骨骨折或脱位创伤性撞击引起的,根据发病的时程又可分为原发性损伤和继发性损伤,其中损伤时对脊髓的初始机械冲击损伤称为原发性损伤。原发性脊髓损伤往往会直接损害神经元、神经胶质细胞和脊髓的神经血管系统[22]。总体而言,原发性损伤的程度决定了脊髓损伤的严重程度和结局。继发性脊髓损伤是在原发性脊髓损伤后发生的,这种衍生的退行性过程通常在几分钟和几小时内开始,发生的快慢与初始损伤程度呈正比。由此引起的继发性损伤包括血管通透性的改变、电解质紊乱、谷氨酸兴奋性毒性作用、代谢改变、功能失调的炎症反应以及神经胶质瘢痕形成等[23],这一系列的反应或可发生级联效应,相互不断促进,最终形成了神经轴突再生的抑制环境,使损伤进入慢性期,这也是脊髓损伤难以治疗与恢复的原因之一。
2.2.3 脊髓损伤的治疗 目前脊髓损伤的治疗相对有限,侧重于损伤原发阶段的早期诊断和直接手术干预,如早期手术减压以减小血管机械压力,限制神经功能的潜在丧失[24],急性损伤后及时治疗对于防止发生级联继发性损伤至关重要,但若未及时干预或干预结果不理想,脊髓损伤会进入预后较差的继发性脊髓损伤阶段。除了手术干预外,在目前的临床实践中还通常使用类固醇、脱水剂、改善微循环的药物和神经营养物质等药物;另外,高压氧治疗、脉冲电疗和局部冷冻疗法也经常在临床中应用[25]。
但目前临床上脊髓损伤干预的窗口期较短,因此预后往往不是十分理想[26],虽然脊髓损伤并无修复性治疗的实际临床应用,但在基础实验方面研究成果颇为丰硕。
在脊髓损伤治疗的实验性研究中,损伤部位的神经保护和神经再生是两大主要目的。细胞移植作为目前脊髓损伤治疗研究中的常用手段,是将有增殖分化潜能的细胞和组织移植到损伤部位,移植的细胞和组织会取代丢失和受损的细胞和组织,因为它们分泌调节免疫应答和局部微环境的神经营养因子,同时提供轴突再生和髓鞘再生所需的底物和支持。目前细胞移植治疗脊髓损伤常用的细胞有神经膜细胞、间充质干细胞、神经干细胞、嗅鞘细胞等[27-28],另外转基因细胞也是目前研究的热点之一,它可以更有效地表达所需的保护性神经生长因子,增强神经干细胞或祖细胞分化为神经元,提高移植细胞的存活率,从而提高细胞治疗的疗效,促进脊髓损伤的再生和修复[29-30]。除了细胞移植,生物产品也常用于脊髓损伤的治疗研究之中,比如将富血小板血浆、某些细胞外泌体、microRNA、槲皮素或其他富含生长因子的生物制品等注入相应部位促进组织再生,旨在重新促进受伤神经元组织的连接,促进轴突和神经元再生,通过脊髓损伤部位的再细胞化来恢复神经损失[31-32]。目前研究人员正在积极探索不同的递送技术和策略,主要是利用一些材料(如导管、片材、支架、纤维、颗粒或水凝胶等[33])将纳米颗粒、营养因子或者具有增殖分化潜能的细胞定向投送到相应部位[34],以提高移植的存活率或精准化释放施加药物或生物制品、防止神经受到外界环境的抑制和损害[35]。虽然脊髓损伤的修复性治疗在基础实验中取得了一定的成效,但每一种疗法还是存在着各种各样的局限性,因此联合应用成为研究的一大焦点之一;并且由于动物模型实验与人类脊髓损伤的机制复杂性不完全匹配,因此还面临着与物种间变异和临床前实验模型相关的重大挑战。
2.3 富血小板血浆 富血小板血浆是将动物或人的全血经过离心后得到的富含高浓度血小板的血浆[高于(150.0-350.0)×106 L-1],最初用作治疗血小板减少症患者的输血产品。因为富血小板血浆是通过全血离心产生的,其自体性质具有极好的安全性[36]。此外,血小板在伤口修复中具有十分重要的生物学作用,而富血小板血浆通过将超生理浓度的血小板输送到靶组织促进组织愈合,被广泛用于外科和牙科领域等医疗领域[37]。近年来,富血小板血浆对骨骼、软骨、皮肤、肌腱和肌肉的再生作用也引起了其他医学领域的兴趣,如创伤和骨科、眼科、整形外科以及运动和美容医学[38]。在创伤学中,富血小板血浆已在各种病理学(肌腱病、关节病、骨移植物)领域中进行了一定研究,并且结果显示了不同程度的有效性。在脊髓损伤治疗的研究中富血小板血浆也有着一定的应用。
2.3.1 富血小板血浆的分类 富血小板血浆的纯化方法多种多样,根据离心条件和提取方法的不同,血小板、白细胞和生长因子的浓度会有所不同。富血小板血浆主要有开放和封闭技术2种纯化方法。在开放式技术纯化富血小板血浆的过程中,血液与工作区域环境接触,移液器和试管分别灭菌,用于富血小板血浆纯化过程;相比之下,封闭技术使用市售设备和试剂盒,血液和富血小板血浆在制备过程中不会暴露在环境中,这两种方法各有优缺点:开放式技术的优点是成本低,但存在细菌污染的风险。封闭技术的污染风险较低,但成本更高[39]。富血小板血浆的各物质含量被认为对治疗效果有重大影响,评估富血小板血浆中各物质含量和质量对于验证其疗效至关重要。DOHAN、DELONG等提出了一系列基于血小板浓度、活化与否以及白细胞等浓度的分类系统,可用于快速评估多项研究和临床实践中使用的富血小板血浆制剂[40]。具体分类及特点见表2。 现在文献中最常看到的是Dohan简单分类,将富血小板血浆产品分为4类:纯富血小板血浆、富含白细胞的富血小板血浆、纯富血小板纤维蛋白以及富含白细胞和血小板的纤维蛋白。

2.3.2 富血小板血浆中各有效成分
生长因子:作为富血小板血浆在脊髓损伤修复性治疗中最重要的物质,种类十分多样,包括血管内皮细胞生长因子、脑源性神经营养因子、胶质母细胞瘤衍生的神经营养因子、神经生长因子、肝细胞生长因子、血小板源性生长因子、胰岛素样生长因子和转化生长因子β等,这些因子具体发挥的作用见表3。

纤维蛋白与纤连蛋白:富血小板血浆含有纤维蛋白、纤连蛋白等,它们形成纤维网络执行组织修复细胞的支架功能,促进细胞黏附并防止细胞丢失[48],致密的纤维蛋白网络在受伤部位可以起到充当组织生长因子储存库的作用[49]。
ATP酶: ATP二磷酸水解酶是一种存在于血小板中的酶,可能与二磷酸腺苷依赖性血小板聚集的控制有关[50]。富血小板血浆作为血小板浓缩的产物成为胞外ATP酶的丰富来源,ATP可作为损伤部位使疼痛敏感化的传导分子,而富血小板血浆中的ATP酶可阻断此过程,从而达到减缓疼痛的作用。
其他成分:富血小板血浆中的成分复杂多样,还包括黏附蛋白、凝血因子和抑制剂、纤溶因子和抑制剂等,具体成分及举例见表4。
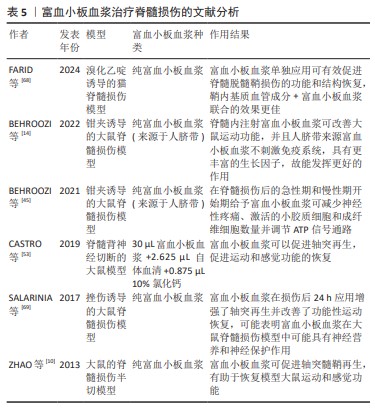
2.3.3 富血小板血浆治疗脊髓损伤的作用 富血小板血浆在脊髓损伤治疗中主要发挥促进轴突再生、刺激血管生成、治疗神经性疼痛的作用,具体见图3。 促进轴突再生:改善损伤的重要步骤之一是轴突再生,但是成人中枢神经系统中的轴突很难再生,这是迄今为止许多治疗失败的原因。据报道,生长因子尤其是神经营养因子在促进轴突再生方面有十分显著的作用[14]。富血小板血浆每微升含有数百万个血小板,超过正常血液的血小板密度。血小板α颗粒具有丰富的生长因子,包括血管内皮细胞生长因子、脑源性神经营养因子、胶质母细胞瘤衍生的神经营养因子、神经生长因子、肝细胞生长因子、血小板源性生长因子、胰岛素样生长因子和转化生长因子β;另一方面,富血小板血浆的各种生长因子可以协同放大彼此的结果,并使彼此更具可持续性。有研究表明,富血小板血浆在坐骨神经等一系列神经损伤的轴突再生修复中表现出显著的促进作用[51-52],表明富血小板血浆作为一种生物制剂具有促进轴突再生的作用,可以作为改善脊髓损伤的策略之一。事实上,富血小板血浆已经成为脊髓损伤恢复性治疗中促进轴突再生的热门,根据已有的研究成果,研究人员在脊髓损伤大鼠模型中发现,应用富血小板血浆可以促进脊髓背角内轴突的再生,从而显著改善大鼠感觉和运动功能,并且对中枢神经系统/周围神经系统的界面损伤有一定修复作用[14,47,53],表明富血小板血浆在脊髓损伤的修复中具有促进轴突再生的功能,是一种十分有前景的轴突再生的黏合剂和诱导剂。 刺激血管生成:在脊髓损伤继发性病变发展的过程中,血管损伤及血管通透性的改变起着十分明显的消极作用,这种损伤性的改变会进一步引起电解质紊乱、组织缺血坏死等一系列不良反应,进而形成了不利于轴突再生的消极环境,导致胶质瘢痕的形成。在修复性治疗过程中,血管的损伤甚至缺损可能导致氧气和营养供应不足,进而影响了组织修复的效果[54]。有研究表明,脊髓损伤后可发生双相血管生成反应,这被认为是一种内源性保护反应,因此微血管系统可以作为脊髓损伤后神经保护的治疗靶点。血小板含有大量关键生长因子,如血管内皮生长因子可刺激相关细胞的生理活动(如增殖、分化和迁移[55]),这意味着可以在有缺陷的位置募集相应的再生细胞,而血管内皮生长因子和血小板源性生长因子在脊髓损伤后会下调,由此研究者们猜测上调血管生成相关因子可能会促进脊髓损伤后的恢复。这种思路先前早已应用于拔牙等临床手术[56],而目前在组织工程方法研究中也成了一大焦点;另一方面,生长因子在血管内先天免疫系统中起着关键作用[57]。富血小板血浆作为血小板浓缩后形成的生物产品,富含大量的血小板和相关生长因子,因此,富血小板血浆在组织工程领域研究组织血管生成中占有十分重要的地位[3],富血小板血浆可促进血管生成、调节血管通透性,修复血脊髓屏障,营造了有利于神经保护的环境,从而起到促进脊髓损伤修复的作用[47]。
治疗神经性疼痛:神经性疼痛是脊髓损伤的常见后果之一,特征是自发性疼痛、痛觉过敏、异常疼痛和感觉异常[58]。最近的数据表明,30%-50%的脊髓损伤患者在受伤后1年内患有神经性疼痛,与一般人群(7%-10%)相比这一比例明显更高[59],分为自发性或诱发性疼痛,表现为疼痛分布区内的感觉障碍、异常性疼痛或痛觉过敏,或表现为灼热、刺痛、射痛、挤压、寒感和电击样感觉[60],这些症状可能会严重影响患者的生活质量并导致抑郁。在过去的10年中,各种疗法在恢复运动功能、提供神经保护、抑制炎症和替换病变部位丢失的神经元方面取得了令人鼓舞的结果,但大多数并没有表现出显著的镇痛作用。抗抑郁药、抗癫痫药和阿片类药物等多种药物可用于减轻神经性疼痛,但身体和心理依赖、肝肾损伤和耐受性等不良反应限制了它们的使用[61]。
研究表明,ATP在神经胶质网络内或神经元和神经胶质细胞之间充当传递信号分子,除神经元和星形胶质细胞外,小胶质细胞还可释放大量细胞外ATP,特别是在神经炎症期间,受伤神经元释放的炎症因子导致小胶质细胞激活和分子分泌,使脊髓中的感觉神经元敏感,由受损神经元分泌的ATP抑制γ-氨基丁酸介导的信号传导受体,最终导致疼痛细胞的启动或增强[62]。富血小板血浆是胞外ATP酶(一种将细胞外ATP水解为ADP和无机磷酸盐的跨膜酶)的丰富来源[50],可以减少脊髓损伤部位的高水平细胞外ATP,从而减小脊髓中感觉神经元的敏感度,减小疼痛感;另外,血小板是生长因子的主要来源,在血管生成和损伤组织愈合等许多过程中起着重要作用。有研究表明,许多生长因子(如胰岛素样生长因子1和血管内皮生长因子)可以通过影响哺乳动物雷帕霉素靶蛋白分子通路的信号转导来影响脊髓损伤中的神经性疼痛[44,63]。在实际研究中不乏富血小板血浆可以缓解神经性疼痛的相关信息,如烧伤所致的神经性疼痛[11,64]。而在最近一项研究中,研究者通过分析脊髓内注射人脐带富血小板血浆对脊髓压迫损伤模型中疼痛以及ATP受体和哺乳动物雷帕霉素靶蛋白表达的影响发现,在脊髓损伤后急性期和慢性期开始期脊髓内注射给予人脐带血富血小板血浆可减轻神经性疼痛[45]。
其他作用:关于富血小板血浆在脊髓损伤修复性治疗中作用的研究仍在进行之中,目前还发现富血小板血浆可能在改善微环境、促进骨生成和组织再生中发挥着作用[65-67]。
2.3.4 前景与局限 富血小板血浆作为一种自体来源的血液制品一般不会导致免疫反应,具有较高的安全性,并且具有较好的促进轴突再生和血管生成作用,还可以减轻脊髓损伤中的神经性疼痛,因此是治疗脊髓损伤的一种潜力型生物制品。
虽然富血小板血浆在脊髓损伤治疗的基础研究中已经有了不错成果,但在临床上还是缺乏相应的试验,并且富血小板血浆的作用时间较短,治疗效果也存在着一定的个体差异,甚至观察到富血小板血浆可能会加重炎症反应,因此单独应用富血小板血浆治疗脊髓损伤的效果是相对有限的,与其他材料或药物联合治疗可能会成为一种新的策略和思路。富血小板血浆治疗脊髓损伤的文献分析见表5。
2.4 水凝胶 水凝胶由于其独特的化学、物理和生物学特性和功能,如较好的细胞生物相容性、自愈性、抗菌活性、注射性、生物黏附性、生物降解性等,常被用于各种损伤的修复之中。在脊髓损伤修复性治疗中,水凝胶不仅用于促进组织修复,还作为生物活性载体(搭载细胞、药物或生物活性分子)进行局部治疗[70-71]。由于损伤的大小、形状和程度不同,脊髓损伤的病情非常复杂。在复杂的临床病例中,通过植入预制支架或药物输送装置对脊髓进行手术操作可能会对脊髓组织造成进一步的损伤,因此,靶向注射水凝胶到脊髓损伤部位与临床个体化治疗非常一致。注射后,水凝胶能很好地与脊髓损伤组织结合,缓慢释放干细胞/药物/生物活性分子,并且表现出导电性、抗炎性、黏附性、吸收性、温度变性和自愈性等特殊功能[72],使水凝胶成为脊髓损伤修复和再生有吸引力的材料。
2.4.1 用于脊髓损伤的水凝胶种类 水凝胶是具有水分子和亲水性聚合物网络的高度复合材料,包括注射水凝胶和非注射水凝胶。应用较多的为可注射水凝胶,可注射水凝胶是液体,可以很容易地运输到受伤部位组织。可注射性、固有的生物相容性、细胞相互作用、亲水性、渗透性和生物降解性使水凝胶成为模拟天然分子微环境的合适底物。脊髓是一种柔软的水状生物结构,刚度范围为3-
300 kPa。水凝胶作为一种生物纳米材料具有高亲水性等物理性质,在修复脊髓损伤方面具有独特的优势[73]。用于治疗脊髓损伤的水凝胶多是一些具有生物活性的水凝胶,其中按材料划分主要包括脱氧核糖核酸类水凝胶、蛋白质类水凝胶、肽类水凝胶、生物质多糖类水凝胶、复合材料水凝胶等,具体分类举例见表6。

2.4.2 水凝胶治疗脊髓损伤的作用 水凝胶在脊髓损伤修复中有较为广泛的应用,主要发挥填充、模拟细胞外基质、搭载药物与生物产品、作为支架搭载细胞等作用,具体见图4。
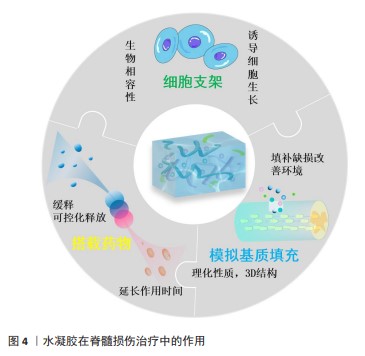
填充、模拟细胞外基质:脊髓损伤发生以后局部微环境十分复杂,往往会产生抑制神经再生的消极环境,特别是脊髓损伤后形成的囊性腔是中枢神经系统组织修复的主要障碍,而囊性腔则由富含纤连蛋白的细胞外基质连接。在脊髓损伤修复中,水凝胶系统填充损伤后的囊肿腔并通过模拟细胞外基质的复杂结构来支持细胞迁移和轴突生长,为神经组织再生提供合适的局部微环境。研究表明,咪唑聚合物水凝胶可以促进细胞外基质连接,脊髓内注射后可以完全消除脊髓损伤大鼠模型中的囊肿[90]。
为了使水凝胶拥有更接近真实细胞外基质的空间结构,一些研究人员采用3D打印方法制备水凝胶,对其整体结构进行分层设计,从而使脊髓损伤修复过程中的细胞可以更好地增殖分裂,有利于脊髓损伤治疗中的神经和组织再生。最新研究表明,分层设计的3D打印水凝胶明显改善了脊髓损伤部位的微环境,明显提升了损伤部位神经干细胞的活力,有利于脊髓损伤治疗过程中的神经再生[91]。
搭载药物与生物产品:搭载药物和生物产品是目前水凝胶的主要用途之一。为了治疗脊髓损伤,有研究使用聚赖氨酸基水凝胶搭载氨基胍纳米颗粒注入脊髓损伤模型大鼠相应部位,使氨基胍纳米颗粒得到持续释放,这样就使炎症细胞因子的产生持续受到抑制并形成抗炎环境,更有利于脊髓损伤的修复[92]。也有研究将人脐带间充质干细胞衍生的小细胞外囊泡封装于可注射的明胶丙烯酸甲酯水凝胶中用于治疗脊髓损伤,发现小细胞外囊泡在明胶丙烯酸甲酯水凝胶中持续存在14 d,从而使小细胞外囊泡具有了长期抗炎和抗纤维化的功效,有利于脊髓损伤的修复性治疗[93]。随着研究的不断加深,水凝胶搭载药物或生物产品时对药物释放的可控性逐渐成为热点之一,有研究者设计了一种具有可控给药系统的水凝胶,用于脊髓损伤的修复性治疗,该修复基于一种叫做“egg”纳米颗粒进行模块化设计,通过诱导信号级联反应响应外部和内部刺激,利用红外照射与内源性神经干/祖细胞迁移过程相匹配来实现精准可控的药物释放,最终促进了神经发生和运动功能的恢复[15]。这种水凝胶的出现展示了时空控制药物释放的革命性策略,为脊髓损伤修复性治疗之中可控给药系统的设计提供了指导。
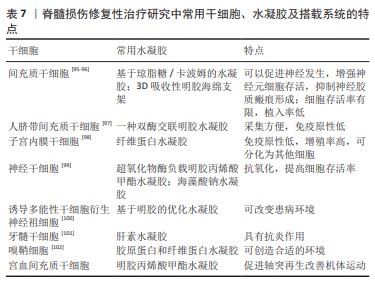
作为支架搭载细胞:基于细胞的治疗方法是研究治疗脊髓损伤的常用策略之一。然而最近的研究表明,由于植入的细胞不能存活很长时间,特别是当细胞植入到受伤和高反应性环境时存活率会显著降低,因此在细胞移植中常出现细胞植入后存活时间有限且植入率较低的问题。将细胞与基于生物材料的载体相结合被认为是治疗脊髓损伤等具有挑战性疾病新兴且有潜力的方法。目前基于细胞疗法的常用选择是通过应用水凝胶作为细胞载体来应对这些挑战,以增强细胞活力和提高在受伤区域的植入率[94],而干细胞作为具有增殖分化能力的细胞,就成为细胞移植的首选。常用的干细胞、所用水凝胶举例及搭载系统的特点见表7。
2.4.3 前景与局限 各种生物材料被广泛用于脊髓损伤修复的初步和临床前研究,其中生物活性水凝胶表现出3D多孔结构、高生物相容性、可注射性、易操作性以及与细胞外基质相似的性能等优势。生物活性水凝胶可以通过各种生物分子的化学和物理交联来制造,包括脱氧核糖核酸、蛋白质、肽、生物质多糖和其他类型的生物聚合物,这些天然和合成的生物分子为水凝胶修复脊髓损伤提供了潜在的生物活性和生物功能。此外,细胞、药物或者其他具有活性生物产品的负载促进了复合水凝胶的多功能性,进一步提高了注射水凝胶在脊髓损伤部位的修复效率。
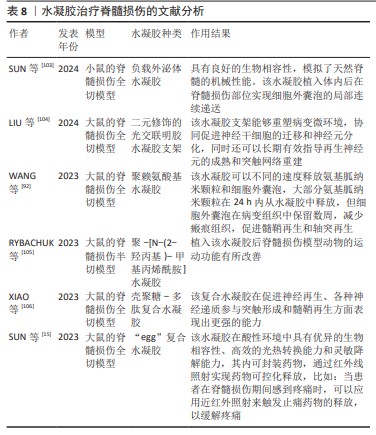
虽然水凝胶在脊髓损伤修复中的应已经取得了不错的成果,但目前仍存在各种各样的局限,比如:干细胞在水凝胶中的嵌入和分化存在挑战,水凝胶3D结构还待优化等,而且水凝胶的研究效果仍缺乏临床试验验证,这也是水凝胶应用的一大难点。水凝胶治疗脊髓损伤的文献分析见表8。
2.5 联合应用
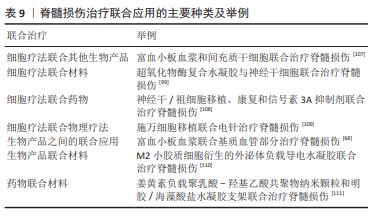
2.5.1 联合应用治疗脊髓损伤 在脊髓损伤治疗研究中,单独应用某一种材料或物质都有着或多或少的局限性,很难达到理想的效果,故目前多种疗法联合应用成为研究的一大焦点。联合治疗大致可以分为细胞疗法联合其他生物产品、细胞疗法联合材料、细胞疗法联合药物、细胞疗法联合物理疗法、生物产品之间的联合应用、生物产品联合材料、药物联合材料等。具体分类举例见表9。
2.5.2 水凝胶与富血小板血浆在脊髓损伤治疗中联合应用 富血小板血浆的局限之一就是作用时间较短和效果有限,用水凝胶搭载富血小板血浆可起到缓释效果,延长富血小板血浆作用时间。
已有研究表明,多功能水凝胶搭载富血小板血浆构成的纳米纤维支架具有细胞屏障和成骨功能,可用于引导组织再生/引导骨再生[112-113],搭载冻干富血小板纤维蛋白渗出液的明胶丙烯酸甲酯水凝胶可有效修复骨缺损[114],复合水凝胶导管搭载富血小板血浆可改善周围神经再生的微环境[115],搭载富血小板血浆的复合有机水凝胶可加速伤口愈合[116-119],利用3D打印水凝胶搭载富血小板血浆可以促进组织的血管化,这一系列研究表明水凝胶和富血小板血浆具有一定的适配性。
许多研究表明,搭载一些生长因子(如脑源性神经营养因子、酸性成纤维细胞生长因子、肝细胞生长因子等)的水凝胶在脊髓损伤修复中表现出比单独应用更好的效果,包括改善轴突生长、促进神经干细胞的形成、减少凋亡神经元的数量和炎症反应、减少神经胶质瘢痕形成等[120-122]。而富血小板血浆中包含这一系列的生长因子,故推断若水凝胶搭载富血小板血浆在脊髓损伤修复性治疗中会起到一个相互促进的效果。最近一项研究表明,当明胶丙烯酸甲酯水凝胶搭载富血小板血浆时会表现出促进细胞分化的效果,这是单独应用富血小板血浆或水凝胶所无法表现出来的。
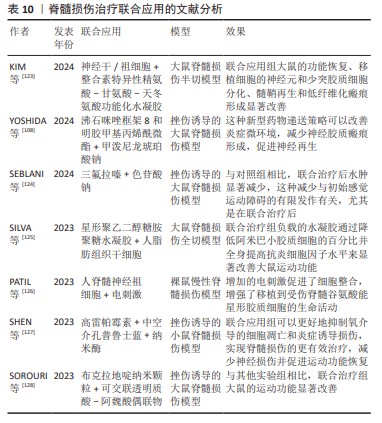
综上,搭载富血小板血浆的水凝胶可增强富血小板血浆作用的时间,促进组织和骨的再生、诱导血管的生成、减少瘢痕的形成、加快伤口的愈合和促进脊髓损伤部位神经相关细胞的增殖与分化。文献分析见表10。
| [1] LIMA R, MONTEIRO A, SALGADO AJ, et al. Pathophysiology and Therapeutic Approaches for Spinal Cord Injury. Int J Mol Sci. 2022; 23(22):13833. [2] FIANI B, ARSHAD MA, SHAIKH ES, et al. Current updates on various treatment approaches in the early management of acute spinal cord injury. Rev Neurosci. 2021;32(5):513-530. [3] BLATT S, SCHRÖGER SV, PABST A, et al. Biofunctionalization of Xenogeneic Collagen Membranes with Autologous Platelet Concentrate-Influence on Rehydration Protocol and Angiogenesis. Biomedicines. 2022;10(3):706. [4] JAIN A, KIM YT, MCKEON RJ, et al. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials. 2006;27(3):497-504. [5] RAUCH MF, HYNES SR, BERTRAM J, et al. Engineering angiogenesis following spinal cord injury: a coculture of neural progenitor and endothelial cells in a degradable polymer implant leads to an increase in vessel density and formation of the blood-spinal cord barrier. Eur J Neurosci. 2009;29(1):132-145. [6] ZURITA M, OTERO L, AGUAYO C, et al. Cell therapy for spinal cord repair: optimization of biologic scaffolds for survival and neural differentiation of human bone marrow stromal cells. Cytotherapy. 2010;12(4): 522-537. [7] BAUMANN MD, KANG CE, TATOR CH, et al. Intrathecal delivery of a polymeric nanocomposite hydrogel after spinal cord injury. Biomaterials. 2010;31(30):7631-7639. [8] RAPPL LM. Effect of platelet rich plasma gel in a physiologically relevant platelet concentration on wounds in persons with spinal cord injury. Int Wound J. 2011;8(2):187-195. [9] PERALE G, ROSSI F, SANTORO M, et al. Multiple drug delivery hydrogel system for spinal cord injury repair strategies. J Control Release. 2012; 159(2):271-280. [10] ZHAO T, YAN W, XU K, et al. Combined treatment with platelet-rich plasma and brain-derived neurotrophic factor-overexpressing bone marrow stromal cells supports axonal remyelination in a rat spinal cord hemi-section model. Cytotherapy. 2013;15(7):792-804. [11] HUANG SH, WU SH, LEE SS, et al. Platelet-Rich Plasma Injection in Burn Scar Areas Alleviates Neuropathic Scar Pain. Int J Med Sci. 2018; 15(3):238-247. [12] CAI Z, GAN Y, BAO C, et al. Photosensitive Hydrogel Creates Favorable Biologic Niches to Promote Spinal Cord Injury Repair. Adv Healthc Mater. 2019;8(13):e1900013. [13] LIU W, LUO Y, NING C, et al. Thermo-sensitive electroactive hydrogel combined with electrical stimulation for repair of spinal cord injury. J Nanobiotechnology. 2021;19(1):286. [14] BEHROOZI Z, RAMEZANI F, NASIRINEZHAD F. Human umbilical cord blood-derived platelet -rich plasma: a new window for motor function recovery and axonal regeneration after spinal cord injury. Physiol Behav. 2022;252:113840. [15] SUN X, XIONG T, YANG K, et al. Individually Tailored Modular “Egg” Hydrogels Capable of Spatiotemporally Controlled Drug Release for Spinal Cord Injury Repair. Adv Healthc Mater. 2023;12(27):e2301169. [16] HE Y, YANG W, ZHANG C, et al. ROS/pH dual responsive PRP-loaded multifunctional chitosan hydrogels with controlled release of growth factors for skin wound healing. Int J Biol Macromol. 2024;258(Pt 2):128962. [17] NEMETH C, BANIK NL, HAQUE A. Disruption of Neuromuscular Junction Following Spinal Cord Injury and Motor Neuron Diseases. Int J Mol Sci. 2024;25(6):3520. [18] NUARA A, BAZZINI MC, CARDELLICCHIO P, et al. The value of corticospinal excitability and intracortical inhibition in predicting motor skill improvement driven by action observation. Neuroimage. 2023;266:119825. [19] QI L, LIN SH, MA Q. Spinal VGLUT3 lineage neurons drive visceral mechanical allodynia but not sensitized visceromotor reflexes. Neuron. 2023;111(5):669-681.e5. [20] WIDERSTRöM-NOGA E. Neuropathic Pain and Spinal Cord Injury: Management, Phenotypes, and Biomarkers. Drugs. 2023;83(11): 1001-1025. [21] MÜLLER-JENSEN L, PLONER CJ, KRONEBERG D, et al. Clinical Presentation and Causes of Non-traumatic Spinal Cord Injury: An Observational Study in Emergency Patients. Front Neurol. 2021;12: 701927. [22] ALIZADEH A, DYCK SM, KARIMI-ABDOLREZAEE S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front Neurol. 2019;10:282. [23] HACHEM LD, FEHLINGS MG. Pathophysiology of Spinal Cord Injury. Neurosurg Clin N Am. 2021;32(3):305-313. [24] LAMBRECHTS MJ, ISSA TZ, HILIBRAND AS. Updates in the Early Management of Acute Spinal Cord Injury. J Am Acad Orthop Surg. 2023;31(17):e619-e632. [25] LI AH, BHATIA A, GULATI A, et al. Role of peripheral nerve stimulation in treating chronic neuropathic pain: an international focused survey of pain medicine experts. Reg Anesth Pain Med. 2023;48(6):312-318. [26] QUDDUSI A, PEDRO KM, ALVI MA, et al. Early surgical intervention for acute spinal cord injury: time is spine. Acta Neurochir (Wien). 2023; 165(9):2665-2674. [27] HU X, XU W, REN Y, et al. Spinal cord injury: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8(1):245. [28] HOSSEINI SM, BORYS B, KARIMI-ABDOLREZAEE S. Neural stem cell therapies for spinal cord injury repair: an update on recent preclinical and clinical advances. Brain. 2024;147(3):766-793. [29] GRIJALVA-OTERO I, DONCEL-PÉREZ E. Traumatic Human Spinal Cord Injury: Are Single Treatments Enough to Solve the Problem? Arch Med Res. 2024;55(1):102935. [30] SHANG WY, REN YF, LI B, et al. Efficacy of growth factor gene-modified stem cells for motor function after spinal cord injury in rodents: a systematic review and meta‑analysis. Neurosurg Rev. 2024;47(1):87. [31] SHANG Z, WANYAN P, WANG M, et al. Stem cell-derived exosomes for traumatic spinal cord injury: a systematic review and network meta-analysis based on a rat model. Cytotherapy. 2024;26(1):1-10. [32] HWANG J, JANG S, KIM C, et al. Role of Stem Cell-Derived Exosomes and microRNAs in Spinal Cord Injury. Int J Mol Sci. 2023;24(18):13849. [33] KAO Y, ZHU H, YANG Y, et al. CREB1 Facilitates GABAergic Neural Differentiation of Human Mesenchymal Stem Cells through BRN2 for Pain Alleviation and Locomotion Recovery after Spinal Cord Injury. Cells. 2023;13(1):67. [34] BUZOIANU-ANGUIANO V, TORRES-LLACSA M, DONCEL-PÉREZ E. Role of Aldynoglia Cells in Neuroinflammatory and Neuroimmune Responses after Spinal Cord Injury. Cells. 2021;10(10):2783. [35] TIN P, LIANG W, HAN B, et al. Hydrogel and Nanomedicine-Based Multimodal Therapeutic Strategies for Spinal Cord Injury. Small Methods. 2024;8(1):e2301173. [36] PAGANELLI A, CONTU L, CONDORELLI A, et al. Platelet-Rich Plasma (PRP) and Adipose-Derived Stem Cell (ADSC) Therapy in the Treatment of Genital Lichen Sclerosus: A Comprehensive Review. Int J Mol Sci. 2023;24(22):16107. [37] ACEBES-HUERTA A, ARIAS-FERNÁNDEZ T, BERNARDO Á, et al. Platelet-derived bio-products: Classification update, applications, concerns and new perspectives. Transfus Apher Sci. 2020;59(1):102716. [38] EVERTS P, ONISHI K, JAYARAM P, et al. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int J Mol Sci. 2020;21(20):7794. [39] ALVES R, GRIMALT R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Skin Appendage Disord. 2018;4(1):18-24. [40] ROSSI LA, MURRAY IR, CHU CR, et al. Classification systems for platelet-rich plasma. Bone Joint J. 2019;101-b(8):891-896. [41] BALLMER-HOFER K. Vascular Endothelial Growth Factor, from Basic Research to Clinical Applications. Int J Mol Sci. 2018;19(12):3750. [42] KARALI E, BELLOU S, STELLAS D, et al. VEGF signaling, mTOR complexes, and the endoplasmic reticulum: Towards a role of metabolic sensing in the regulation of angiogenesis. Mol Cell Oncol. 2014;1(3):e964024. [43] PINEDA-CORTEL MR, SUAREZ C, CABRERA JT, et al. Biotherapeutic Applications of Platelet-Rich Plasma in Regenerative Medicine. Tissue Eng Regen Med. 2023;20(6):811-828. [44] BOND P. Regulation of mTORC1 by growth factors, energy status, amino acids and mechanical stimuli at a glance. J Int Soc Sports Nutr. 2016;13:8. [45] BEHROOZI Z, RAMEZANI F, JANZADEH A, et al. Platelet-rich plasma in umbilical cord blood reduces neuropathic pain in spinal cord injury by altering the expression of ATP receptors. Physiol Behav. 2021;228: 113186. [46] LI SY, JOHNSON R, SMYTH LC, et al. Platelet-derived growth factor signalling in neurovascular function and disease. Int J Biochem Cell Biol. 2022;145:106187. [47] CHEN NF, SUNG CS, WEN ZH, et al. Therapeutic Effect of Platelet-Rich Plasma in Rat Spinal Cord Injuries. Front Neurosci. 2018;12:252. [48] PACHITO DV, BAGATTINI ÂM, DE ALMEIDA AM, et al. Technical Procedures for Preparation and Administration of Platelet-Rich Plasma and Related Products: A Scoping Review. Front Cell Dev Biol. 2020;8: 598816. [49] MIRON RJ, ZUCCHELLI G, PIKOS MA, et al. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig. 2017; 21(6):1913-1927. [50] ROSSATTO ER, DA SILVA LB, PEREIRA GS, et al. ATP diphosphohydrolase in human platelets from patients with coronary arteries heart disease. Platelets. 2003;14(1):47-52. [51] VARES P, DEHGHAN MM, BASTAMI F, et al. Effects of Platelet-Rich Fibrin/Collagen Membrane on Sciatic Nerve Regeneration. J Craniofac Surg. 2021;32(2):794-798. [52] KUFFLER DP. Platelet-Rich Plasma Promotes Axon Regeneration, Wound Healing, and Pain Reduction: Fact or Fiction. Mol Neurobiol. 2015;52(2):990-1014. [53] CASTRO MV, SILVA M, CHIAROTTO GB, et al. Reflex arc recovery after spinal cord dorsal root repair with platelet rich plasma (PRP). Brain Res Bull. 2019;152:212-224. [54] MASSON-MEYERS DS, TAYEBI L. Vascularization strategies in tissue engineering approaches for soft tissue repair. J Tissue Eng Regen Med. 2021;15(9):747-762. [55] VAN DER BIJL I, VLIG M, MIDDELKOOP E, et al. Allogeneic platelet-rich plasma (PRP) is superior to platelets or plasma alone in stimulating fibroblast proliferation and migration, angiogenesis, and chemotaxis as relevant processes for wound healing. Transfusion. 2019;59(11): 3492-3500. [56] GIUDICE A, ESPOSITO M, BENNARDO F, et al. Dental extractions for patients on oral antiplatelet: a within-person randomised controlled trial comparing haemostatic plugs, advanced-platelet-rich fibrin (A-PRF+) plugs, leukocyte- and platelet-rich fibrin (L-PRF) plugs and suturing alone. Int J Oral Implantol (Berl). 2019;12(1):77-87. [57] ERIKSSON O, MOHLIN C, NILSSON B, et al. The Human Platelet as an Innate Immune Cell: Interactions Between Activated Platelets and the Complement System. Front Immunol. 2019;10:1590. [58] SCHOLZ J, FINNERUP NB, ATTAL N, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019;160(1): 53-59. [59] COLLOCA L, LUDMAN T, BOUHASSIRA D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017; 3:17002. [60] SHI T, YU Z, CHEN Z, et al. The impact of time from injury to surgery on the risk of neuropathic pain after traumatic spinal cord injury. J Orthop Surg Res. 2023;18(1):857. [61] FINNERUP NB, ATTAL N, HAROUTOUNIAN S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. [62] BUTT AM. ATP: a ubiquitous gliotransmitter integrating neuron-glial networks. Semin Cell Dev Biol. 2011;22(2):205-213. [63] KANNO H, OZAWA H, SEKIGUCHI A, et al. The role of mTOR signaling pathway in spinal cord injury. Cell Cycle. 2012;11(17):3175-3179. [64] 阚厚铭,范利君,陈学泰,等.富血小板血浆在神经病理性疼痛中的应用[J].中国组织工程研究,2022,26(8):1286-1292. [65] ALSOUSOU J, ALI A, WILLETT K, et al. The role of platelet-rich plasma in tissue regeneration. Platelets. 2013;24(3):173-182. [66] XIE X, ZHANG C, TUAN RS. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res Ther. 2014;16(1):204. [67] 苏辉,谭国庆,徐展望.富血小板血浆在脊髓损伤神经再生微环境治疗机制研究进展[J].河北医学,2021,27(9):1578-1581. [68] FARID MF, YASIN NAE, AL-MOKADDEM AK, et al. Combined laser-activated SVF and PRP remodeled spinal sclerosis via activation of Olig-2, MBP, and neurotrophic factors and inhibition of BAX and GFAP. Sci Rep. 2024;14(1):3096. [69] SALARINIA R, SADEGHNIA HR, ALAMDARI DH, et al. Platelet rich plasma: Effective treatment for repairing of spinal cord injury in rat. Acta Orthop Traumatol Turc. 2017;51(3):254-257. [70] SUN X, LIU H, TAN Z, et al. Remodeling Microenvironment for Endogenous Repair through Precise Modulation of Chondroitin Sulfate Proteoglycans Following Spinal Cord Injury. Small. 2023;19(6): e2205012. [71] ZHANG ZJ, ZHANG XL, WANG CG, et al. Enhancement of motor functional recovery using immunomodulatory extracellular vesicles-loaded injectable thermosensitive hydrogel post spinal cord injury. Chem Eng J. 2022;433:15. [72] WANG R, WU X, TIAN Z, et al. Sustained release of hydrogen sulfide from anisotropic ferrofluid hydrogel for the repair of spinal cord injury. Bioact Mater. 2023;23:118-128. [73] WANG Y, LV HQ, CHAO X, et al. Multimodal therapy strategies based on hydrogels for the repair of spinal cord injury. Mil Med Res. 2022;9(1):16. [74] BASU S, PACELLI S, FENG Y, et al. Harnessing the Noncovalent Interactions of DNA Backbone with 2D Silicate Nanodisks To Fabricate Injectable Therapeutic Hydrogels. ACS Nano. 2018;12(10):9866-9880. [75] BASU S, PACELLI S, PAUL A. Self-healing DNA-based injectable hydrogels with reversible covalent linkages for controlled drug delivery. Acta Biomater. 2020;105:159-169. [76] ZHANG J, GUO Y, PAN G, et al. Injectable Drug-Conjugated DNA Hydrogel for Local Chemotherapy to Prevent Tumor Recurrence. ACS Appl Mater Interfaces. 2020;12(19):21441-21449. [77] WANG X, DING Z, WANG C, et al. Bioactive Silk Hydrogels with Tunable Mechanical Properties. J Mater Chem B. 2018;6(18):2739-2746. [78] BUITRAG JO, PATEL KD, EL-FIQI A, et al. Silk fibroin/collagen protein hybrid cell-encapsulating hydrogels with tunable gelation and improved physical and biological properties. Acta Biomater. 2018;69:218-233. [79] XU R, MA S, LIN P, et al. High Strength Astringent Hydrogels Using Protein as the Building Block for Physically Cross-linked Multi-Network. ACS Appl Mater Interfaces. 2018;10(9):7593-7601. [80] XIANG Y, MAO H, TONG SC, et al. A Facile and Versatile Approach to Construct Photoactivated Peptide Hydrogels by Regulating Electrostatic Repulsion. ACS Nano. 2023;17(6):5536-5547. [81] DUTTA SD, HEXIU J, PATEL DK, et al. 3D-printed bioactive and biodegradable hydrogel scaffolds of alginate/gelatin/cellulose nanocrystals for tissue engineering. Int J Biol Macromol. 2021;167: 644-658. [82] FIORATI A, LINCIANO C, GALANTE C, et al. Bioactive Hydrogels: Design and Characterization of Cellulose-Derived Injectable Composites. Materials (Basel). 2021;14(16):4511-4511. [83] SHAH SA, SOHAIL M, KARPERIEN M, et al. Chitosan and carboxymethyl cellulose-based 3D multifunctional bioactive hydrogels loaded with nano-curcumin for synergistic diabetic wound repair. Int J Biol Macromol. 2023;227:1203-1220. [84] RAKHSHAEI R, NAMAZI H. A potential bioactive wound dressing based on carboxymethyl cellulose/ZnO impregnated MCM-41 nanocomposite hydrogel. Mater Sci Eng C Mater Biol Appl. 2017; 73:456-464. [85] XU Q, CHANG M, ZHANG Y, et al. PDA/Cu Bioactive Hydrogel with “Hot Ions Effect” for Inhibition of Drug-Resistant Bacteria and Enhancement of Infectious Skin Wound Healing. ACS Appl Mater Interfaces. 2020; 12(28):31255-31269. [86] LIU N, CHEN J, ZHUANG J, et al. Fabrication of engineered nanoparticles on biological macromolecular (PEGylated chitosan) composite for bio-active hydrogel system in cardiac repair applications. Int J Biol Macromol. 2018;117:553-558. [87] LI S, DONG Q, PENG X, et al. Self-Healing Hyaluronic Acid Nanocomposite Hydrogels with Platelet-Rich Plasma Impregnated for Skin Regeneration. ACS Nano. 2022;16(7):11346-11359. [88] HAN L, XU J, LU X, et al. Biohybrid methacrylated gelatin/polyacrylamide hydrogels for cartilage repair. J Mater Chem B. 2017;5(4):731-741. [89] 邓博文,蒋昇源,刘港,等.携川芎嗪导电水凝胶促进脊髓损伤后血管新生和神经保护的实验研究[J].中国修复重建外科杂志,2024, 38(2):189-197. [90] LIU Y, ZHANG Z, ZHANG Y, et al. Construction of adhesive and bioactive silk fibroin hydrogel for treatment of spinal cord injury. Acta Biomater. 2023;158:178-189. [91] LI Y, CHENG S, WEN H, et al. Coaxial 3D printing of hierarchical structured hydrogel scaffolds for on-demand repair of spinal cord injury. Acta Biomater. 2023;168:400-415. [92] WANG S, WANG R, CHEN J, et al. Controlled extracellular vesicles release from aminoguanidine nanoparticle-loaded polylysine hydrogel for synergistic treatment of spinal cord injury. J Control Release. 2023; 363:27-42. [93] WANG H, TANG Q, LU Y, et al. Berberine-loaded MSC-derived sEVs encapsulated in injectable GelMA hydrogel for spinal cord injury repair. Int J Pharm. 2023;643:123283. [94] BOIDO M, GHIBAUDI M, GENTILE P, et al. Chitosan-based hydrogel to support the paracrine activity of mesenchymal stem cells in spinal cord injury treatment. Sci Rep. 2019;9(1):6402. [95] YAO S, HE F, CAO Z, et al. Mesenchymal Stem Cell-Laden Hydrogel Microfibers for Promoting Nerve Fiber Regeneration in Long-Distance Spinal Cord Transection Injury. ACS Biomater Sci Eng. 2020;6(2): 1165-1175. [96] AN H, LI Q, WEN J. Bone marrow mesenchymal stem cells encapsulated thermal-responsive hydrogel network bridges combined photo-plasmonic nanoparticulate system for the treatment of urinary bladder dysfunction after spinal cord injury. J Photochem Photobiol B. 2020;203:111741. [97] YAO M, LI J, ZHANG J, et al. Dual-enzymatically cross-linked gelatin hydrogel enhances neural differentiation of human umbilical cord mesenchymal stem cells and functional recovery in experimental murine spinal cord injury. J Mater Chem B. 2021;9(2):440-452. [98] JALALI MONFARED M, NASIRINEZHAD F, EBRAHIMI-BAROUGH S, et al. Transplantation of miR-219 overexpressed human endometrial stem cells encapsulated in fibrin hydrogel in spinal cord injury. J Cell Physiol. 2019;234(10):18887-18896. [99] LIU D, LU G, SHI B, et al. ROS-Scavenging Hydrogels Synergize with Neural Stem Cells to Enhance Spinal Cord Injury Repair via Regulating Microenvironment and Facilitating Nerve Regeneration. Adv Healthc Mater. 2023;12(18):e2300123. [100] RUZICKA J, ROMANYUK N, JIRAKOVA K, et al. The Effect of iPS-Derived Neural Progenitors Seeded on Laminin-Coated pHEMA-MOETACl Hydrogel with Dual Porosity in a Rat Model of Chronic Spinal Cord Injury. Cell Transplant. 2019;28(4):400-412. [101] ALBASHARI A, HE Y, ZHANG Y, et al. Thermosensitive bFGF-Modified Hydrogel with Dental Pulp Stem Cells on Neuroinflammation of Spinal Cord Injury. ACS Omega. 2020;5(26):16064-16075. [102] GOMES ED, GHOSH B, LIMA R, et al. Combination of a Gellan Gum-Based Hydrogel With Cell Therapy for the Treatment of Cervical Spinal Cord Injury. Front Bioeng Biotechnol. 2020;8:984. [103] SUN Y, LIU Q, QIN Y, et al. Exosomes derived from CD271(+)CD56(+) bone marrow mesenchymal stem cell subpopoulation identified by single-cell RNA sequencing promote axon regeneration after spinal cord injury. Theranostics. 2024;14(2):510-527. [104] LIU D, SHEN H, ZHANG K, et al. Functional Hydrogel Co-Remolding Migration and Differentiation Microenvironment for Severe Spinal Cord Injury Repair. Adv Healthc Mater. 2024; 13(3):e2301662. [105] RYBACHUK O, NESTERENKO Y, PINET É, et al. Neuronal differentiation and inhibition of glial differentiation of murine neural stem cells by pHPMA hydrogel for the repair of injured spinal cord. Exp Neurol. 2023;368:114497. [106] XIAO L, XIE P, MA J, et al. A Bioinspired Injectable, Adhesive, and Self-Healing Hydrogel with Dual Hybrid Network for Neural Regeneration after Spinal Cord Injury. Adv Mater. 2023; 35(41):e2304896. [107] SALARINIA R, HOSSEINI M, MOHAMADI Y, et al. Combined use of platelet-rich plasma and adipose tissue-derived mesenchymal stem cells shows a synergistic effect in experimental spinal cord injury. J Chem Neuroanat. 2020;110:101870. [108] YOSHIDA T, TASHIRO S, NAGOSHI N, et al. Chronic Spinal Cord Injury Regeneration with Combined Therapy Comprising Neural Stem/Progenitor Cell Transplantation, Rehabilitation, and Semaphorin 3A Inhibitor. eNeuro. 2024;11(2):0378-23. [109] TAN C, YANG C, LIU H, et al. Effect of Schwann cell transplantation combined with electroacupuncture on axonal regeneration and remyelination in rats with spinal cord injury. Anat Rec (Hoboken). 2021;304(11):2506-2520. [110] GUAN P, FAN L, ZHU Z, et al. M2 microglia-derived exosome-loaded electroconductive hydrogel for enhancing neurological recovery after spinal cord injury. J Nanobiotechnology. 2024;22(1):8. [111] AI A, HASANZADEH E, SAFSHEKAN F, et al. Enhanced spinal cord regeneration by gelatin/alginate hydrogel scaffolds containing human endometrial stem cells and curcumin-loaded PLGA nanoparticles in rat. Life Sci. 2023;330:122035. [112] ZHANG L, DONG Y, LIU Y, et al. Multifunctional hydrogel/platelet-rich fibrin/nanofibers scaffolds with cell barrier and osteogenesis for guided tissue regeneration/guided bone regeneration applications. Int J Biol Macromol. 2023;253(Pt 4):126960. [113] MU Z, CHEN K, YUAN S, et al. Gelatin Nanoparticle-Injectable Platelet-Rich Fibrin Double Network Hydrogels with Local Adaptability and Bioactivity for Enhanced Osteogenesis. Adv Healthc Mater. 2020;9(5) :e1901469. [114] GAN S, ZHENG Z, ZHANG M, et al. Lyophilized Platelet-Rich Fibrin Exudate-Loaded Carboxymethyl Chitosan/GelMA Hydrogel for Efficient Bone Defect Repair. ACS Appl Mater Interfaces. 2023;15(22): 26349-26362. [115] DONG Q, YANG X, LIANG X, et al. Composite Hydrogel Conduit Incorporated with Platelet-Rich Plasma Improved the Regenerative Microenvironment for Peripheral Nerve Repair. ACS Appl Mater Interfaces. 2023;15(20):24120-24133. [116] CAI C, ZHU H, CHEN Y, et al. Platelet-Rich Plasma Composite Organohydrogel with Water-Locking and Anti-Freezing to Accelerate Wound Healing. Adv Healthc Mater. 2023;12(28):e2301477. [117] BAI L, ZHANG X, LI X, et al. Impact of a Novel Hydrogel with Injectable Platelet-Rich Fibrin in Diabetic Wound Healing. J Diabetes Res. 2023; 2023:7532637. [118] ODA H, KAIZAWA Y, FRANKLIN A, et al. Assessment of a Synergistic Effect of Platelet-Rich Plasma and Stem Cell-Seeded Hydrogel for Healing of Rat Chronic Rotator Cuff Injuries. Cell Transplant. 2023;32: 9636897231190174. [119] XU K, DENG S, ZHU Y, et al. Platelet Rich Plasma Loaded Multifunctional Hydrogel Accelerates Diabetic Wound Healing via Regulating the Continuously Abnormal Microenvironments. Adv Healthc Mater. 2023; 12(28):e2301370. [120] HE Z, ZANG H, ZHU L, et al. An anti-inflammatory peptide and brain-derived neurotrophic factor-modified hyaluronan-methylcellulose hydrogel promotes nerve regeneration in rats with spinal cord injury. Int J Nanomedicine. 2019;14:721-732. [121] HU X, LI R, WU Y, et al. Thermosensitive heparin-poloxamer hydrogel encapsulated bFGF and NGF to treat spinal cord injury. J Cell Mol Med. 2020;24(14):8166-8178. [122] YAMANE K, MAZAKI T, SHIOZAKI Y, et al. Collagen-Binding Hepatocyte Growth Factor (HGF) alone or with a Gelatin- furfurylamine Hydrogel Enhances Functional Recovery in Mice after Spinal Cord Injury. Sci Rep. 2018;8(1):917. [123] KIM WK, KANG BJ. Transplantation of Heat-Shock Preconditioned Neural Stem/Progenitor Cells Combined with RGD-Functionalised Hydrogel Promotes Spinal Cord Functional Recovery in a Rat Hemi-Transection Model. Stem Cell Rev Rep. 2024;20(1):283-300. [124] SEBLANI M, ERTLEN C, COYLE T, et al. Combined effect of trifluoperazine and sodium cromoglycate on reducing acute edema and limiting lasting functional impairments after spinal cord injury in rats. Exp Neurol. 2024;372:114612. [125] SILVA D, SCHIRMER L, PINHO TS, et al. Sustained Release of Human Adipose Tissue Stem Cell Secretome from Star-Shaped Poly(ethylene glycol) Glycosaminoglycan Hydrogels Promotes Motor Improvements after Complete Transection in Spinal Cord Injury Rat Model. Adv Healthc Mater. 2023; 12(17):e2202803. [126] PATIL N, KORENFELD O, SCALF RN, et al. Electrical stimulation affects the differentiation of transplanted regionally specific human spinal neural progenitor cells (sNPCs) after chronic spinal cord injury. Stem Cell Res Ther. 2023;14(1):378. [127] SHEN K, LI X, HUANG G, et al. High rapamycin-loaded hollow mesoporous Prussian blue nanozyme targets lesion area of spinal cord injury to recover locomotor function. Biomaterials, 2023;303:122358. [128] SOROURI F, HOSSEINI P, SHARIFZADEH M, et al. In Situ Cross-Linkable Hyaluronic-Ferulic Acid Conjugate Containing Bucladesine Nanoparticles Promotes Neural Regeneration after Spinal Cord Injury. ACS Appl Mater Interfaces. 2023;15(36):42251-42270. |
| [1] | 赖鹏宇, 梁 冉, 沈 山. 组织工程技术修复颞下颌关节:问题与挑战[J]. 中国组织工程研究, 2025, 29(在线): 1-9. |
| [2] | 王自林, 牟秋菊, 刘宏杰, 申玉雪, 祝丽丽. 载富血小板血浆水凝胶对L929细胞氧化损伤的保护作用[J]. 中国组织工程研究, 2025, 29(4): 771-779. |
| [3] | 赵红霞, 孙政伟, 韩 阳, 吴学超, 韩 静. 富血小板纤维蛋白复合甲基丙烯酰化明胶水凝胶的促成骨性能[J]. 中国组织工程研究, 2025, 29(4): 809-817. |
| [4] | 张 煜, 徐睿安, 方 蕾, 历龙飞, 刘姝妍, 丁凌雪, 王悦熹, 郭子琰, 田 丰, 薛佳佳. 梯度人工骨修复支架调控骨骼系统组织的修复与再生[J]. 中国组织工程研究, 2025, 29(4): 846-855. |
| [5] | 赵增波, 李晨曦, 窦晨雷, 马 娜, 周冠军. 壳聚糖/甘油磷酸钠/海藻酸钠/益母草碱水凝胶的抗炎与促成骨作用[J]. 中国组织工程研究, 2025, 29(4): 678-685. |
| [6] | 董美林, 都海宇, 刘 源. 负载槲皮素的羧甲基壳聚糖水凝胶促进糖尿病大鼠创面愈合[J]. 中国组织工程研究, 2025, 29(4): 692-699. |
| [7] | 张 博, 张 振, 江 东. 单宁酸改性互穿网络水凝胶促进断裂跟腱术后的组织重塑[J]. 中国组织工程研究, 2025, 29(4): 721-729. |
| [8] | 杨 彬, 陶广义, 杨 顺, 许俊杰, 黄俊卿. 人工智能在脊髓神经损伤与修复领域研究热点的可视化分析[J]. 中国组织工程研究, 2025, 29(4): 761-770. |
| [9] | 徐振华, 李彦杰, 秦合伟, 刘昊源, 朱博超, 王煜普. 中药单体治疗脊髓损伤后神经炎症:核转录因子κB信号通路的作用[J]. 中国组织工程研究, 2025, 29(3): 590-598. |
| [10] | 李永航, 李文铭, 严才平, 王星宽, 向 超, 张 袁, 蒋 科, 陈 路. 抗纤维化与促“H”型血管核壳结构生物支架修复临界骨缺损[J]. 中国组织工程研究, 2025, 29(16): 3420-3431. |
| [11] | 何 蕊, 李重一, 王瑞瑶, 曾 丹, 范代娣. MXene基水凝胶在创面修复领域的应用[J]. 中国组织工程研究, 2025, 29(16): 3486-3493. |
| [12] | 刘忠钰, 李文娅, 范永鸿, 吕 双, 裴 娟, 陈娅琴, 刘倍余, 孙红玉. 甲基丙烯酰化改性真皮细胞外基质水凝胶促进腹壁缺损修复[J]. 中国组织工程研究, 2025, 29(10): 2074-2082. |
| [13] | 闻哲嘉, 吕 芳. 基于微流控芯片评估富血小板血浆促进子宫内膜细胞的增殖[J]. 中国组织工程研究, 2025, 29(10): 2091-2096. |
目前已有较多文献提及了富血小板血浆、水凝胶治疗脊髓损伤的作用及其机制,但较少有文章归纳总结它们治疗脊髓损伤的策略。该文不仅归纳总结了富血小板血浆、水凝胶单独应用于脊髓损伤修复性治疗的策略,还介绍了二者联合应用治疗脊髓损伤的效果及现状,为改善脊髓损伤的治疗效果提供理论依据,最终为制定最佳的脊髓损伤治疗方案打下坚实的基础。
1.1.1 检索人及检索时间 由第一作者在2024年1-2月进行检索。
1.1.2 检索文献时限 各数据库建库至2024年3月之前发表的文献。
1.1.3 检索数据库 中国知网 和PubMed数据库。
1.1.4 检索词 中文检索词为“脊髓损伤,富血小板血浆,水凝胶”,英文检索词为“spinal cord injury,spinal cord,Platelet-rich plasma,hydrogel,angiogenesis,neuralgia,combination therapy”。
1.1.5 检索文献类型 研究原著及综述。
1.1.6 检索策略 以PubMed数据库检索策略为例,见图1。
1.1.7 检索文献量 初步检索到1 489篇英文文献,78篇中文文献。
1.2 入选标准
1.2.1 纳入标准 ①与富血小板血浆和脊髓损伤密切相关的文献;②与水凝胶和脊髓损伤密切相关的文献;③与富血小板血浆和水凝胶联合治疗脊髓损伤密切相关的文献;④与脊髓损伤病理进程相关的文献;⑤内容真实可靠的文献;⑥选择发表在权威杂志的相关文献。
1.2.2 排除标准 ①研究重复;②内容久远的文献;③结论不明确的文献;④相关性差或无关的文献。
1.3 文献质量评估及数据提取 通过计算机初步检索到1 567篇文献(英文文献1 489篇,中文文献78篇),通过查看期刊年份,阅读文献题目、摘要、结果、讨论部分,初步排除1 331篇相关文献(英文文献1 275篇,中文文献56篇),通过阅读全文后再次排除与研究目的有所偏差的、质量不高的文献108篇,最终纳入128篇符合标准的文献(英文文献125篇,中文文献3篇)进行综述,见图2。
生物产品(比如富血小板血浆)在脊髓损伤治疗的基础实验中已显示出较好效果,比如促进神经轴突再生、促进血管生成、治疗神经性疼痛等,但富血小板血浆也需要长时间多次给药才能维持治疗效果,故富血小板血浆的可控化释放是解决该问题的一大挑战。目前最新研究的水凝胶支架在溶胀率、孔隙率、机械强度、导电性、结构特征和生化特性等特性方面已经可以密切模仿天然脊髓组织。可注射水凝胶可以通过为受损脊髓组织提供用于递送细胞、药物和生物活性分子的基质来填补不规则损伤的间隙,并改善损伤部位的微环境。但目前材料学研究仍是一大挑战,寻找合适材料构成稳定的递送系统并兼有良好的生物学性能是一项难题。
单一的治疗方法难以解决脊髓损伤的修复治疗问题,研究人员近年来进行了大量联合治疗的尝试,并取得了丰硕的成果,包括但不限于细胞移植联合药物、细胞移植联合材料、细胞移植联合生物产品、生物产品联合材料、
药物联合材料等,与单一用药相比,许多联合治疗方法表现出在神经轴突再生、运动和感觉功能恢复等方面的协同作用,但仍有一的局限,不能完全修复脊髓损伤。在生物产品和材料的联合应用方面,富血小板血浆和水凝胶联合应用是一项有潜力的治疗方法,可利用水凝胶的可控性释放药物等特点实现精准、长期的富血小板血浆治疗作用。
3.2 展望
3.2.1 应加快脊髓损伤临床试验验证进程 目前脊髓损伤的治疗十分有限,临床上大多数仅仅是以早期干预为主。富血小板血浆种类繁多,水凝胶的材料合成也在不断地更新换代,发展迅速,但基础研究大多数用的是啮齿动物模型,与人类存在着偏差,不能代替临床,真正的治疗效果、安全性的检验还需要进行进一步的临床试验。
3.2.2 实现富血小板血浆的精准化、可控化释放 已有研究利用水凝胶理化性质来进行一定的药物精准、可控释放。目前富血小板血浆在使用上存在作用时间较短的问题,这限制了其作用的发挥,从而不利于脊髓损伤的恢复。若是可以将水凝胶与富血小板血浆联合实现富血小板血浆的精准释放,一定会显著提高富血小板血浆的作用效率。实现富血小板血浆精准化、可控化释放的障碍主要有以下几点:①寻找一种安全可靠、同时兼具良好生物降解性的材料是一大障碍;②研发可控化释放水凝胶涉及材料领域的问题,需要跨学科交流;③富血小板血浆与水凝胶的兼容性问题;④基础研究中的模型与人类偏差;⑤在生物研究领域,对脊髓损伤、富血小板血浆的认识有限,限制了脊髓损伤修复中全面的疗效评估和创新策略设计。对于富血小板血浆与水凝胶联合应用实现精准化释放,应该注意避免生物产品和材料到达一定部位时可能的不良反应,应根据不同的运动神经元及各种中间神经元分化、维持等特性定制化地应用不同材料的水凝胶及不同分类的富血小板血浆,这将为不同部位脊髓损伤的神经恢复提供理论依据。
3.3 该综述区别于他人他篇的特点 对比国内外的相关综述,该综述首先介绍了脊髓损伤的病理过程、分类等信息,然后介绍了富血小板血浆的分类以及在脊髓损伤治疗中发挥作用的有效成分,并对其前景及局限提出评价;其次,该综述还介绍了用于脊髓损伤的水凝胶的分类与作用,并对其前景与局限展开评价;同时,该综述将富血小板血浆的不足和水凝胶的优势结合起来,提出富血小板血浆与水凝胶联合应用,综述了联合应用的进展,并对未来可控化释放富血小板血浆提出展望。
3.4 该综述的局限性 首先,阐述脊髓损伤详细的病理进程对于该疾病的治疗具有重要意义,该综述对脊髓损伤的病理进程仅做了简明扼要的介绍并未深入展开其病理机制;其次,富血小板血浆成分复杂、作用众多,该综述仅做主要整理,还有好多未完全研究透彻的作用和成分未包含其中;此外,目前研发的水凝胶种类繁多,该文只选择主要种类进行简单举例说明,并未将全部用于脊髓损伤的水凝胶一一列举。
3.5 该综述的重要意义 目前还没有文章对富血小板血浆和水凝胶修复脊髓损伤的策略进行总结和归纳,该文先介绍脊髓损伤的病理过程,然后介绍了富血小板血浆、水凝胶在脊髓损伤治疗中的作用,将二者的特点结合起来,提出联合治疗来实现富血小板血浆精准化、可控化释放等优化方向,这将为脊髓损伤的治疗提供新思路。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||