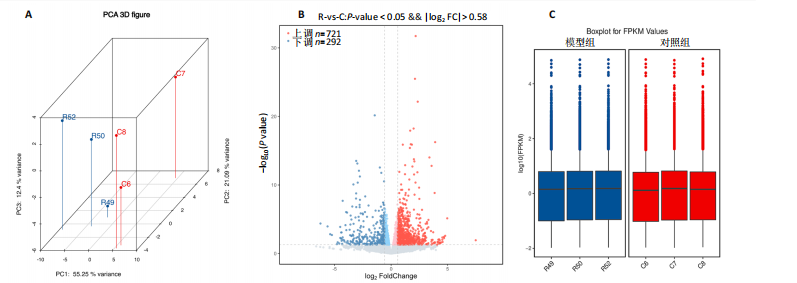
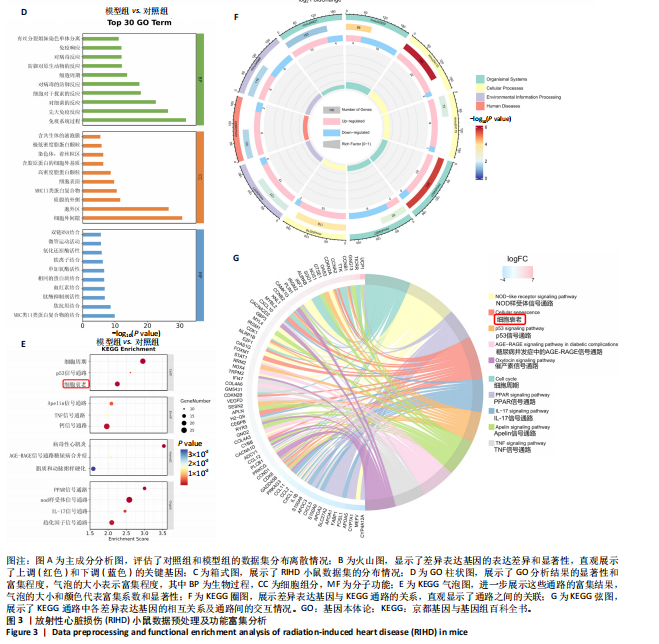
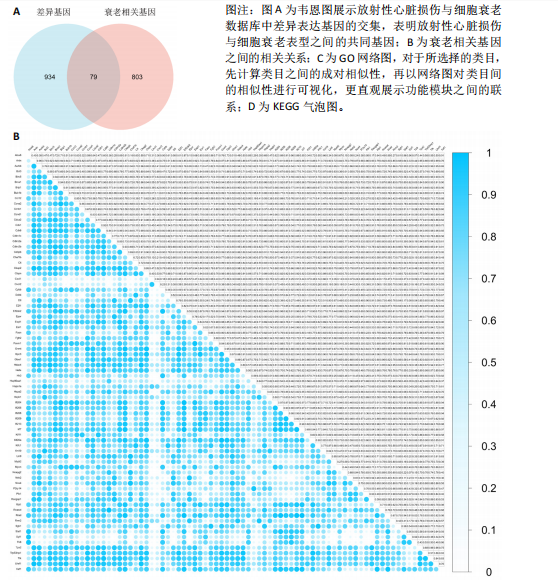
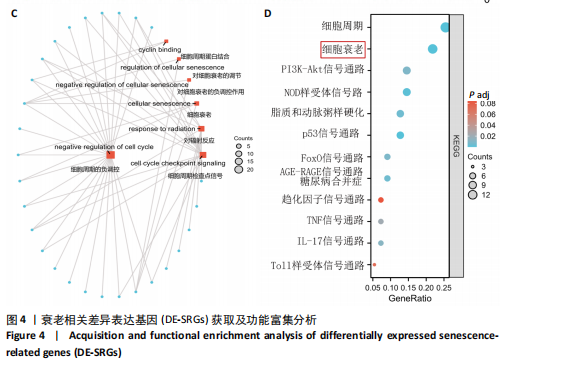
[1] JAHNG JWS, LITTLE MP, NO HJ, et al. Consequences of ionizing radiation exposure to the cardiovascular system. Nat Rev Cardiol. 2024;21(12):880-898.
[2] BEDI R, AHMAD A, HORBAL P, et al. Radiation-associated Arrhythmias: Putative Pathophysiological Mechanisms, Prevalence, Screening and Management Strategies. Arrhythm Electrophysiol Rev. 2023;12:e24.
[3] KIM L, LOCCOH EC, SANCHEZ R, et al. Contemporary Understandings of Cardiovascular Disease After Cancer Radiotherapy: a Focus on Ischemic Heart Disease. Curr Cardiol Rep. 2020;22(11):151.
[4] DONOVAN EK, POND GR, SEOW H, et al. Cardiac Morbidity Following Chemoradiation in Stage III Non-small Cell Lung Cancer Patients: A Population-Based Cohort Study. Clin Oncol (R Coll Radiol). 2023; 35(2):e182-e188.
[5] DARBY SC, EWERTZ M, MCGALE P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987-998.
[6] BASELET B, SONVEAUX P, BAATOUT S, et al. Pathological effects of ionizing radiation: endothelial activation and dysfunction. Cell Mol Life Sci. 2019;76(4):699-728.
[7] PHILIPP J, AZIMZADEH O, SUBRAMANIAN V, et al. Radiation-Induced Endothelial Inflammation Is Transferred via the Secretome to Recipient Cells in a STAT-Mediated Process. J Proteome Res. 2017;16(10):3903-3916.
[8] LEE CL, MODING EJ, CUNEO KC, et al. p53 functions in endothelial cells to prevent radiation-induced myocardial injury in mice. Sci Signal. 2012;5(234):ra52.
[9] ZAGAR TM, CARDINALE DM, MARKS LB. Breast cancer therapy-associated cardiovascular disease. Nat Rev Clin Oncol. 2016;13(3):172-184.
[10] KORPELA E, LIU SK. Endothelial perturbations and therapeutic strategies in normal tissue radiation damage. Radiat Oncol. 2014;9:266.
[11] BLOOM SI, ISLAM MT, LESNIEWSKI LA, et al. Mechanisms and consequences of endothelial cell senescence. Nat Rev Cardiol. 2023; 20(1):38-51.
[12] HAN Y, KIM SY. Endothelial senescence in vascular diseases: current understanding and future opportunities in senotherapeutics. Exp Mol Med. 2023;55(1):1-12.
[13] HARTMAN RJG, OWSIANY K, MA L, et al. Sex-Stratified Gene Regulatory Networks Reveal Female Key Driver Genes of Atherosclerosis Involved in Smooth Muscle Cell Phenotype Switching. Circulation. 2021;143(7):713-726.
[14] MOCCI G, SUKHAVASI K, ÖRD T, et al. Single-Cell Gene-Regulatory Networks of Advanced Symptomatic Atherosclerosis. Circ Res. 2024; 134(11):1405-1423.
[15] LEWIS GD, FARACH A. Cardiovascular Toxicities of Radiation Therapy. Methodist Debakey Cardiovasc J. 2019;15(4):274-281.
[16] DA SILVA RMFL. Effects of Radiotherapy in Coronary Artery Disease. Curr Atheroscler Rep. 2019;21(12):50.
[17] DUNN AN, DONNELLAN E, JOHNSTON DR, et al. Long-Term Outcomes of Patients With Mediastinal Radiation-Associated Coronary Artery Disease Undergoing Coronary Revascularization With Percutaneous Coronary Intervention and Coronary Artery Bypass Grafting. Circulation. 2020;142(14):1399-1401.
[18] WU W, MASRI A, POPOVIC ZB, et al. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation. 2013;127(14):1476-1485.
[19] DEO RC. Machine Learning in Medicine. Circulation. 2015;132(20): 1920-1930.
[20] HAMPE N, WOLTERINK JM, VAN VELZEN SGM, et al. Machine Learning for Assessment of Coronary Artery Disease in Cardiac CT: A Survey. Front Cardiovasc Med. 2019;6:172.
[21] HUANG AA, HUANG SY. Computation of the distribution of model accuracy statistics in machine learning: Comparison between analytically derived distributions and simulation-based methods. Health Sci Rep. 2023;6(4):e1214.
[22] WENGROFSKY P, FELDMAN S. The Role of Multimodality Cardiac Imaging in Patients Undergoing Cancer Treatment. Curr Cardiol Rep. 2023;25(1):1-8.
[23] CHEN X, MUMME RP, CORRIGAN KL, et al. Deep learning-based automatic segmentation of cardiac substructures for lung cancers. Radiother Oncol. 2024;191:110061.
[24] MORRIS ED, GHANEM AI, DONG M, et al. Cardiac substructure segmentation with deep learning for improved cardiac sparing. Med Phys. 2020;47(2):576-586.
[25] PETRICCIUOLO S, DELLE DONNE MG, AIMO A, et al. Pre-treatment high-sensitivity troponin T for the short-term prediction of cardiac outcomes in patients on immune checkpoint inhibitors. Eur J Clin Invest. 2021;51(4):e13400.
[26] SU X, HUO HH, ZENG Y, et al. Effective Echocardiography Parameters to Identify Radiation-Induced Heart Disease in the Mouse Model. Int J Radiat Oncol Biol Phys. 2022;114(3, Supplement):e516.
[27] WU T, HU E, XU S, et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb). 2021;2(3):100141.
[28] LIU Y, WANG Y, ZHANG J. New machine learning algorithm: Random forest//Information Computing and Applications: Third International Conference, ICICA 2012, Chengde, China, September 14-16, 2012. Proceedings 3. Springer Berlin Heidelberg, 2012:246-252.
[29] LANGFELDER P, HORVATH S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559.
[30] FINOTELLO F, MAYER C, PLATTNER C, et al. Molecular and pharmacological modulators of the tumor immune contexture revealed by deconvolution of RNA-seq data. Genome Med. 2019;11(1):34.
[31] ISHWARAN H, KOGALUR UB, BLACKSTONE EH, et al. Random survival forests. Ann Appl Stat. 2008;2(3):841-860.
[32] LIU T, CHEN Y, HOU L, et al. Immune cell-mediated features of atherosclerosis. Front Cardiovasc Med. 2024;11:1450737.
[33] SCHLAAK RA, FREI A, FISH BL, et al. Acquired Immunity Is Not Essential for Radiation-Induced Heart Dysfunction but Exerts a Complex Impact on Injury. Cancers (Basel). 2020;12(4):983.
[34] TAHA MY, MOHAMED NO, ALHAJ LG, et al. CCND1 as a Prognostic and Diagnostic Biomarker and the Impact of Its Epigenetic Alterations on Cancer Survival. Cureus. 2024;16(7):e65504.
[35] JIANG D, SONG Q, ZHANG F, et al. Prognostic significance of CCND1 amplification/overexpression in smoking patients with esophageal squamous cell carcinoma. Cancer Genet. 2023;278-279:1-8.
[36] BELZILE-DUGAS E, EISENBERG MJ. Radiation-Induced Cardiovascular Disease: Review of an Underrecognized Pathology. J Am Heart Assoc. 2021;10(18):e021686.
[37] KRUG P, GEETS X, BERLIÈRE M, et al. Cardiac structure, function, and coronary anatomy 10 years after isolated contemporary adjuvant radiotherapy in breast cancer patients with low cardiovascular baseline risk. Eur Heart J Cardiovasc Imaging. 2024;25(5):645-656.
[38] SANTRA MK, WAJAPEYEE N, GREEN MR. F-box protein FBXO31 mediates cyclin D1 degradation to induce G1 arrest after DNA damage. Nature. 2009;459(7247):722-725.
[39] LEONTIEVA OV, DEMIDENKO ZN, BLAGOSKLONNY MV. MEK drives cyclin D1 hyperelevation during geroconversion. Cell Death Differ. 2013;20(9):1241-1249.
[40] BURTON DG, SHEERIN AN, OSTLER EL, et al. Cyclin D1 overexpression permits the reproducible detection of senescent human vascular smooth muscle cells. Ann N Y Acad Sci. 2007;1119:20-31.
[41] LEONTIEVA OV, LENZO F, DEMIDENKO ZN, et al. Hyper-mitogenic drive coexists with mitotic incompetence in senescent cells. Cell Cycle. 2012;11(24):4642-4649.
[42] CAMPISI J, D’ADDA DI FAGAGNA F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729-740.
[43] DU C, ZHAO S, SHAN T, et al. Cellular nucleic acid binding protein facilitates cardiac repair after myocardial infarction by activating β-catenin signaling. J Mol Cell Cardiol. 2024;189:66-82.
[44] VALENZUELA C, BROWN NE. Cyclin D1, metabolism, and the autophagy-senescence balance//HINDS PW, BROWN NE. D-type Cyclins and Cancer. Cham: Springer International Publishing, 2018:111-131.
[45] JIA G, AROOR AR, JIA C, et al. Endothelial cell senescence in aging-related vascular dysfunction. Biochim Biophys Acta Mol Basis Dis. 2019;1865(7):1802-1809.
[46] LIU L, LI J, WANG R, et al. MicroRNA-298 Exacerbates Myocardial Ischemic Injury via Targeting Cyclin D1. Pharmazie. 2019;74(6):369-373.
[47] SMITH TA, KIRKPATRICK DR, SMITH S, et al. Radioprotective agents to prevent cellular damage due to ionizing radiation. J Transl Med. 2017;15(1):232.
[48] JEONG K, KIM JH, MURPHY JM, et al. Nuclear Focal Adhesion Kinase Controls Vascular Smooth Muscle Cell Proliferation and Neointimal Hyperplasia Through GATA4-Mediated Cyclin D1 Transcription. Circ Res. 2019;125(2):152-166.
[49] QI Y, ZHANG Y, GUAN S, et al. Common ground on immune infiltration landscape and diagnostic biomarkers in diabetes-complicated atherosclerosis: an integrated bioinformatics analysis. Front Endocrinol (Lausanne). 2024;15:1381229.
[50] WITARTO BS, VISUDDHO V, ALDIAN FM, et al. Blood-based circulating microRNAs as diagnostic biomarkers for subclinical carotid atherosclerosis: A systematic review and meta-analysis with bioinformatics analysis. Diabetes Metab Syndr. 2023;17(10):102860.
[51] SILVESTRE-ROIG C, BRASTER Q, ORTEGA-GOMEZ A, et al. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol. 2020; 17(6):327-340.
[52] WANG B, HAN J, ELISSEEFF JH, et al. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat Rev Mol Cell Biol. 2024;25(12):958-978.
[53] FASANARO P, MAGENTA A, ZACCAGNINI G, et al. Cyclin D1 degradation enhances endothelial cell survival upon oxidative stress. FASEB J. 2006;20(8):1242-1244.
[54] GENG X, WANG DW, LI H. The pivotal role of neutrophil extracellular traps in cardiovascular diseases: Mechanisms and therapeutic implications. Biomed Pharmacother. 2024;179:117289.
[55] KHOURY MK, YANG H, LIU B. Macrophage Biology in Cardiovascular Diseases. Arterioscler Thromb Vasc Biol. 2021;41(2):e77-e81.
[56] CHEN R, ZHANG H, TANG B, et al. Macrophages in cardiovascular diseases: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. 2024;9(1):130.
[57] ZHANG H, ZHANG X, ZHANG Y, et al. WTAP/CCND1 axis accelerates esophageal squamous cell carcinoma progression by MAPK signaling pathway. Neoplasma. 2024;71(4):359-373.
[58] WANG JZ, WANG Y, SHAO Q, et al. Dynamic changes in cardiac biomarkers in radiotherapy for oesophageal cancer and their correlations with cardiac radiation dosimetry. Clin Transl Radiat Oncol. 2024;45:100750.
[59] UNGER K, LI Y, YEH C, et al. Plasma metabolite biomarkers predictive of radiation induced cardiotoxicity. Radiother Oncol. 2020;152:133-145.
[60] AZIMZADEH O, MERL-PHAM J, SUBRAMANIAN V, et al. Late Effects of Chronic Low Dose Rate Total Body Irradiation on the Heart Proteome of ApoE-/- Mice Resemble Premature Cardiac Ageing. Cancers (Basel). 2023;15(13):3417.
[61] LIU Z, WANG K, JIANG C, et al. Morusin Alleviates Aortic Valve Calcification by Inhibiting Valve Interstitial Cell Senescence Through Ccnd1/Trim25/Nrf2 Axis. Adv Sci (Weinh). 2024;11(20):e2307319.
[62] CAI W, SHU LZ, LIU DJ, et al. Targeting cyclin D1 as a therapeutic approach for papillary thyroid carcinoma. Front Oncol. 2023;13: 1145082.
|