中国组织工程研究 ›› 2025, Vol. 29 ›› Issue (3): 579-589.doi: 10.12307/2025.140
• 骨与关节综述 bone and joint review • 上一篇 下一篇
椎间盘退变中的力学信号转导蛋白
高夕林1,2,吴 思1,2,张 超1,2,朱立国3,符碧峰1,2,王 平1,2
- 1天津中医药大学第一附属医院,天津市 300193;2国家中医针灸临床医学研究中心,天津市 300193;3中国中医科学院望京医院,北京市 100102
-
收稿日期:2023-11-30接受日期:2024-03-06出版日期:2025-01-28发布日期:2024-06-04 -
通讯作者:符碧峰,博士,主治医师,天津中医药大学第一附属医院骨伤科,天津市 300193;国家中医针灸临床医学研究中心,天津市 300193 王平,博士,主任医师,博士生导师,天津中医药大学第一附属医院骨伤科,天津市300193;国家中医针灸临床医学研究中心,天津市 300193 -
作者简介:高夕林,男,1997年生,河北省唐山市人,汉族,天津中医药大学在读博士,主要从事骨与关节疾病临床研究。 -
基金资助:天津市科技计划项目(21JCQNJC01690),项目负责人:吴思;天津市名中医传承工作室建设项目(881022),项目负责人:王平;国家重点研发计划项目(2021YFC1712802),项目负责人:朱立国;天津市教委科研计划项目(2023KJ156),项目负责人:符碧峰;京津冀中医药协同发展专科联盟建设项目
Mechanotransduction proteins in intervertebral disc degeneration
Gao Xilin1, 2, Wu Si1, 2, Zhang Chao1, 2, Zhu Liguo3, Fu Bifeng1, 2, Wang Ping1, 2
- 1First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China; 2National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin 300193, China; 3Wangjing Hospital of China Academy of Chinese Medical Sciences, Beijing 100102, China
-
Received:2023-11-30Accepted:2024-03-06Online:2025-01-28Published:2024-06-04 -
Contact:Fu Bifeng, PhD, Attending physician, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China; National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin 300193, China Wang Ping, PhD, Chief physician, Doctoral supervisor, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China; National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin 300193, China -
About author:Gao Xilin, Doctoral candidate, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China; National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin 300193, China -
Supported by:Tianjin Science and Technology Plan Project, No. 21JCQNJC01690 (to WS); Tianjin Famous Traditional Chinese Medicine Inheritance Studio Construction Project, No. 881022 (to WP); National Key Research and Development Program Topic, No. 2021YFC1712802 (to ZLG); Tianjin Education Commission Research Plan Project, No. 2023KJ156 (to FBF); Construction Project of Beijing-Tianjin-Hebei Traditional Chinese Medicine Specialized Alliance for Collaborative Development
摘要:
文题释义:
力学信号转导蛋白:是一类能够感知并响应机械力,将力学信号转化为生化信号的蛋白质,这类蛋白质在机械力的作用下发生构象变化或化学改变(如磷酸化),从而激活相关信号通路,影响细胞内的生化活动和基因表达。
椎间盘退变:是指椎间盘受到机械、营养及遗传因素的影响发生病理性改变的过程。
摘要
背景:近年来的研究表明椎间盘退变与异常压力负荷密切相关,而力学信号转导蛋白在其中发挥了关键作用。
目的:探讨力学信号转导蛋白在异常机械力刺激诱导椎间盘退变的力化学信号换能过程中发挥的作用及机制,总结目前靶向力学信号延缓椎间盘退变的治疗策略。
方法:以“椎间盘,髓核,纤维环,软骨终板,细胞,力,信号转导,蛋白,生物力学”为中文检索词,以“intervertebral disc,nucleus pulposus,annulus fibrosus,cartilaginous endplate,cell,mechanical stimulation,signal transduction,protein,biomechanics”为英文检索词,检索PubMed、CNKI数据库中的相关文献,最终纳入88篇文献进行综述。
结果与结论:椎间盘细胞能通过多种力学信号转导蛋白感知外界机械刺激并将其转化为细胞内生物学反应,这些转导蛋白主要包括细胞外基质中的胶原蛋白、细胞膜表面受体(如整合素及离子通道)、细胞骨架结构蛋白等。力学信号转导蛋白调控力化学信号换能的过程主要包括多个通路的激活,如PI3K/AKT信号通路、核因子κB信号通路、Ca2+/Calpain2/Caspase3通路等。力学信号转导蛋白在椎间盘细胞机械信号换能中发挥了关键作用,这些蛋白表达异常或由此导致的细胞外基质环境改变会破坏椎间盘细胞的力学平衡,引发椎间盘退变。深入研究椎间盘细胞力学信号转导蛋白的表达及调控机制,寻找关键的病理环节和治疗靶点,对开发椎间盘退变治疗策略具有重要意义,目前靶向力学信号延缓椎间盘退变的策略主要包括对转导蛋白的调控、对细胞外基质的改良等,然而这方面研究还处于初级阶段,随着研究的不断深入,力学信号转导蛋白调控椎间盘退变有望实现新的突破。
中图分类号:
引用本文
高夕林, 吴 思, 张 超, 朱立国, 符碧峰, 王 平. 椎间盘退变中的力学信号转导蛋白[J]. 中国组织工程研究, 2025, 29(3): 579-589.
Gao Xilin, Wu Si Zhang Chao Zhu Liguo, Fu Bifeng, Wang Ping. Mechanotransduction proteins in intervertebral disc degeneration[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(3): 579-589.
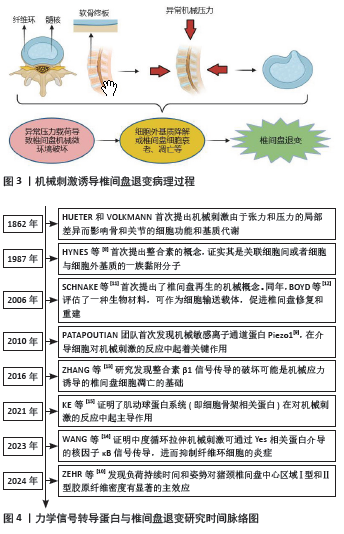
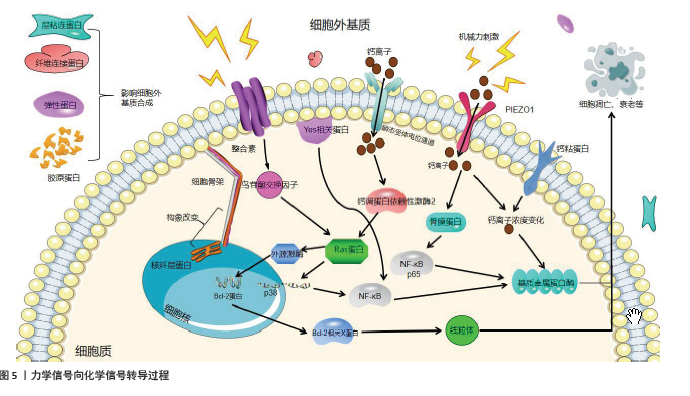
根据Hueter-Volkmann原理[7],异常机械刺激可破坏椎间盘正常力学微环境中张力和压力的平衡,使之产生局部差异,影响椎间盘细胞的功能和基质代谢,诱导椎间盘退变的发生(图3)。而力学信号转导蛋白在介导椎间盘细胞对机械刺激的反应中扮演了重要角色,从1987年HYNES等[8]首次提出整合素的概念,证实其是关联细胞间或者细胞与细胞外基质的一族黏附分子,到2010年,DAVID JULIUS和ARDEM PATAPOUTIAN首次发现机械敏感离子通道蛋白Piezo1[9],并在2021年(获得诺贝尔生理医学奖)发现温度和触觉受体可以感知热、冷和机械力并引发神经冲动,再到ZEHR等[10]研究发现负荷持续时间和姿势对椎间盘中心区域Ⅰ型和Ⅱ型胶原纤维密度有显著的主效应,提示临床腰伤预防和负荷管理工作应考虑在施加机械力刺激治疗的同时进行腰部姿势管理及矫正。关于力学信号转导蛋白在机械力刺激影响椎间盘退变的过程中发挥作用的研究经历了漫长过程,具体研究进展见图4[8-15]。
2.2 细胞外基质中的力学信号转导蛋白 细胞外基质是分布在细胞膜表面和细胞之间的复杂大分子网络结构,不仅为细胞提供机械支持,还参与细胞的生长、分化等一系列免疫应答过程[16]。参与组成细胞外基质的蛋白质可分为胶原蛋白、非胶原蛋白(如层粘连蛋白、纤维连接蛋白、弹性蛋白)及糖胺聚糖和蛋白聚糖3大类[17],这些蛋白均与力学信号转导过程及椎间盘退变关系密切。在椎间盘细胞中,Ⅱ型胶原分泌减少等因素会导致细胞外基质的合成代谢和分解代谢紊乱,破坏椎间盘的力学微环境,诱发椎间盘退变[18]。
2.2.1 胶原蛋白 胶原蛋白是细胞外基质的主要组成成分,通过三螺旋区域与细胞表面受体结合,然后进行力学信号的传导[19]。正常的椎间盘细胞中发挥主要作用的胶原蛋白有Ⅰ、Ⅱ及Ⅵ型胶原蛋白。髓核细胞的细胞外基质中主要成分为Ⅱ型胶原蛋白,具有一定的形变能力,可保持水分;而纤维环细胞的细胞外基质中Ⅰ型胶原蛋白含量要多于Ⅱ型胶原蛋白,Ⅰ型胶原蛋白的抗牵张能力及修复能力强,是椎间盘承受压力的主要功能胶原[20]。有研究发现,当椎间盘发生退变时,髓核及纤维环细胞中Ⅱ型胶原蛋白的含量减少、Ⅰ型胶原蛋白的含量相对增加,从而增加了椎间盘组织的硬度,不能将压力均匀分散,当压力反复作用于纤维环某一处时该处纤维环容易破裂,髓核变性突出[21-22]。Ⅵ型胶原作为一种短链胶原可在基质和细胞膜之间形成联系桥梁进行信号传导,还能围绕在细胞周围形成囊状结构以应对不同载荷[23]。ZEHR等[10]经过实验研究证明了循环负荷可诱导胶原蛋白含量的变化,导致细胞外基质代谢状态改变和微观结构损伤,说明胶原蛋白可作为延缓椎间盘退变的相关靶点进行深入
研究。
2.2.2 非胶原蛋白 层粘连蛋白可与锚定在基底膜附近细胞膜上的受体相互作用,进行信号传导并调节细胞活动[24]。作为连接细胞外基质和细胞骨架的关键元素,层粘连蛋白能感知并响应细胞外基质的物理属性,将其转化为化学信号。
纤维连接蛋白在受到外界机械力刺激后会发生构象改变,影响其与细胞膜表面受体的结合,进而激活络氨酸激酶等磷酸激酶,并激活下游信号通路。据NAHA等[25]的研究,在椎间盘退变过程中纤维连接蛋白含量增加,促进了核因子κB的激活,刺激椎间盘细胞产生基质金属蛋白酶,从而抑制细胞外基质合成。
弹性蛋白通过相互连接形成弹性网状结构参与构成椎间盘框架。研究表明,在长期异常应力载荷作用下构成弹性蛋白的原纤维会变得松散,弹性蛋白纤维密度较正常成人椎间盘明显降低[26],说明弹性蛋白与椎间盘退变具有相关性。MERCURI等[27]根据椎间盘退变早期髓核细胞细胞外基质破坏的病理机制,试图构建化学稳定的弹性蛋白-糖胺聚糖-胶原蛋白复合物水凝胶来重建髓核的弹性和亲水性,有望成为延缓椎间盘退变的新材料。
2.3 细胞膜上的力学信号转导蛋白 细胞膜力学感受器主要包括黏附受体及机械力敏感离子通道两大类,这两种感受器相互配合,一方面感知并响应细胞外的力学刺激,另一方面激活细胞内的信号转导通路,使细胞适应生长环境中的力学变化。
2.3.1 整合素 整合素是由α和β亚基以非共价键结合组成的异源二聚体,分布于细胞表面,连接了细胞外基质和细胞内骨架,属于跨膜糖蛋白受体[28]。在力学信号转导过程中,胞膜外区整合素的氨基端结构域通过改变其折叠内旋的结构识别精氨酸-氨基乙酸-门冬氨酸序列多肽位点,与细胞外特异配体相结合;胞内区除与细胞骨架等结构蛋白结合外,还可与黏着斑激酶、蛋白激酶C等结合,将力学信号转变为化学信号。另外,整合素还能将细胞受到的机械刺激直接传递到细胞骨架,细胞骨架的形变引起细胞内相关通路的激活[29]。在椎间盘细胞膜上表达的整合素主要有整合素α1、α5、αv、β1、β3,如α1β1亚单位是胶原蛋白的膜受体,α5β1亚单位是纤维连接蛋白的膜受体[30]。
整合素对椎间盘细胞的生物学行为有重要意义,参与了力学信号转导、细胞外基质降解、细胞凋亡等多个过程,其异常表达也是椎间盘退变的重要病理机制。GAO等[31]对大鼠髓核细胞提供压力为0-0.3 MPa、频率为0-
1 Hz的周期性静水压力,结果显示,周期性机械应力显著上调了整合素α1 mRNA表达并促进了磷脂酶C-γ1的磷酸化,但抑制了整合素α5和αv的表达水平,说明整合素可通过促进磷脂酶C-γ1的磷酸化,使软骨面积扩大、维持椎间盘稳定性。KANDA等[32]将40只大鼠的80个椎间盘模型分为在有或无精氨酰-甘氨酰-天冬氨酸肽干预的前提下予以1.3 MPa、1.0 Hz动态循环压缩压力和不予以此压力的4组,通过免疫组织化学方法在髓核细胞和纤维环细胞中都发现了整合素α5β1表达上调,并且在动态循环载荷的条件下,整合素α5β1和基质降解酶的上调降低了髓核细胞的活性;而在精氨酰-甘氨酰-天冬氨酸肽干预下,整合素α5β1表达受到抑制,延迟了髓核细胞的表型变化并减少了细胞凋亡,说明抑制整合素α5β1可能是预防早期椎间盘退变的治疗靶点。ZHENG
等[33]对不同月龄的小鼠髓核细胞进行体外应力加载,发现机械应力可通过促进甲状旁腺激素Ⅰ型受体向纤毛的转运来激活甲状旁腺激素信号通路,促进整合素αvβ6的合成,进而激活转化生长因子β-结缔组织生长因子-基质蛋白信号级联,有助于维持椎间盘代谢平衡,是治疗椎间盘退变的潜在作用靶点。XU等[34]观察大鼠髓核细胞在高氧张力作用下的变化情况,结果表明,高氧环境下整合素α6随着髓核细胞中PI3K/AKT信号通路被激活而上调;同时高氧环境抑制了缺氧诱导因子1α的生成,增加了整合素α6的表达,整合素α6的表达同时抑制了缺氧诱导因子1α的表达,表明整合素α6可能是椎间盘退变发病机制的保护因子。
2.3.2 钙黏蛋白 钙黏蛋白是黏附连接中一种钙离子依赖的黏附蛋白,可以分为钙黏蛋白Ⅰ型与Ⅱ型,由细胞外结构域、单程跨膜区域和包含2个主要结合区域的短细胞质尾组成。细胞外区域通过与邻近细胞上其他钙黏蛋白的外域结合建立黏合键负责黏附识别,细胞质尾与p120-连环蛋白和β-连环蛋白结合将钙黏蛋白受体连接至细胞骨架,此种黏合连接通过抵抗内源性收缩力或流体剪切应力、压缩力等外源力从本质上将机械信息传达给细胞[35]。N-钙黏蛋白作为典型的Ⅰ型钙黏蛋白与整合素β1一起被认为是椎间盘细胞中公认的机械敏感因素[36]。
LI等[37]将猪椎间盘予以8 h/d、0.4 MPa、1.0 Hz压力刺激,研究发现长时间机械压力显著降低了N-钙黏蛋白的表达,增加了髓核细胞凋亡率,诱导了椎间盘退变。ZHOU等[38]研究也发现静态压力作用下N-钙黏蛋白表达下调,可使髓核细胞特异性表型丢失,细胞外基质合成减少。KE等[39]认为N-钙黏蛋白过表达可通过形成N-钙黏蛋白/β-连环蛋白/Yes相关蛋白复合物抑制Yes相关蛋白核易位,从而逆转椎间盘退变过程中髓核细胞基质刚度增加引起的ASCL4表达增加,从而抑制铁死亡,延缓椎间盘退变,这为在小分子层面延缓椎间盘退变提供了参考。
2.3.3 机械力敏感离子通道蛋白 机械力敏感离子通道蛋白嵌于细胞膜上,在机械力的刺激下允许离子进行跨膜运输,改变膜电位,激活下游的信号转导通路,可在毫秒内将机械刺激(例如细胞膜的张力和卷曲力)通过自身的分子构象变化转化为电信号或生化信号,从而引发对细胞的调节,使细胞产生适应性反应。因此,机械敏感通道蛋白介导的机械力传导过程是迄今已知生物体内最快速的传导体系。目前,被发现的存在于椎间盘细胞内并对椎间盘细胞进行调节,可能与椎间盘退变相关的机械敏感通道包括瞬态受体电位通道及最新发现的Piezo蛋白等。
(1) Piezo蛋白:是由COSTE等于2010年在小鼠神经母细胞瘤里发现的新型人类重组蛋白,主要包括2个结构与基因相似的蛋白——Piezo1和Piezo2[9]。肖百龙课题组提出了Piezo在信号转导过程中的双重调控机制[40]:机械力使细胞膜发生形变,膜张力使Piezo相关结构曲率半径增大,导致Piezo在细胞膜上的投影面积增大,调控“门塞-闩锁”模型开放通道;中央跨膜内侧螺旋区在微丝的牵引下,按照“丝张力”模型开放离子通道[41]。Piezo1在髓核细胞、纤维环细胞、终板软骨细胞中均有不同程度的表达,并且与椎间盘退变有关。而Piezo2对于疼痛和触觉以及本体感觉至关重要,目前仅发现在神经细胞中高度表达,仅有少部分存在于纤维环细胞和终板软骨细胞中,其与椎间盘退变之间的关系及作用机制还需要进一步研究[42]。
Piezo1对机械应力诱导椎间盘退变有重要意义。SHI等[43]将髓核细胞暴露于1.0 Hz、15%压缩强度下,发现Piezo1在过度压缩下上调,并且可以促进分泌促炎细胞因子NOD样受体热蛋白结构域相关蛋白3,通过诱导线粒体功能障碍、抑制自噬引发髓核细胞的凋亡和衰老,减少细胞外基质中Ⅱ型胶原蛋白和蛋白聚糖的合成,加速椎间盘退变。WU等[44]通过Piezo1特异性激动剂Yoda1注射诱导大鼠尾部椎间盘退变,发现Piezo1的表达以时间依赖性方式显著升高,然后对椎间盘模型施加机械应力,在此过程中骨膜蛋白的表达显著上调并激活了核因子κB p65,加速了髓核细胞的衰老及骨膜蛋白的分泌,形成正反馈调节,说明Piezo1可通过骨膜蛋白加速椎间盘退变进程,Piezo1和骨膜蛋白中和抗体可能是延缓椎间盘退变进程的新靶点。
Piezo1在纤维环细胞中的异常表达可能是椎间盘退变的病理机制之一。LIU等[45]首先建立大鼠腰椎不稳模型,然后通过Flexcell系统建立循环机械拉伸力刺激下的大鼠纤维环细胞凋亡模型,在此过程中发现Piezo1高度表达且促进了钙离子内流,Ca2+/Calpain2/Caspase3通路被激活,增加了纤维环细胞凋亡,说明异常机械负荷可以通过激活Piezo1和下又Calpain2/Bax/Caspase3途径诱导纤维环细胞细胞凋亡,加速椎间盘退变。
Piezo1在终板软骨细胞中可响应机械张力诱导终板软骨退变。DING等[46]研究首次揭示了Yes相关蛋白1是机械张力介导椎间盘退变的关键调节因子,随着机械刺激强度和时间的增加,终板软骨细胞中Yes相关蛋白1的表达显著降低,而Piezo1的表达显著上升。研究认为Piezo1可能通过激活Hippo信号通路调节Yes相关蛋白1的表达,从而延缓椎间盘退变,这可能是治疗由软骨终板退变导致椎间盘退变的宝贵策略。
(2)瞬态受体电位通道蛋白:是由4个瞬态受体电位亚基组成同源或异源四聚体,其参与细胞对机械刺激响应过程的分子机制有两种可能:一是作为信号接收器直接感受机械刺激,将力学信号转化为化学信号;二是作为信号调制器,将上一级传递而来的钙离子、磷脂酶C等信号进行接收并放大[47]。目前已知的28个瞬态受体电位通道中有26个在mRNA水平上在椎间盘退变过程中表达,而其中发挥主要作用的是瞬态受体电位4[48]。
EASSON等[49]研究结果表明,瞬态受体电位4对椎间盘的影响取决于重复表达次数、渗透压及压力载荷大小,瞬态受体电位4的瞬时、单次激活增加了钙离子通量、核因子κB信号转导的级联反应以及白细胞介素6炎症因子的数量,但瞬态受体电位4的反复激活改善了细胞外基质刚度,增加了髓核细胞中的糖胺聚糖含量,提高了水合作用;在持续性机械压力载荷作用下,白细胞介素6和血管内皮生长因子A的表达增加,而低渗透条件下瞬态受体电位4表达增加,抑制了白细胞介素6和血管内皮生长因子A的表达,延缓了椎间盘退变,但是对于机械载荷导致的直接损伤(如纤维环胶原蛋白的紊乱等)并无作用。CAMBRIA小组[50]发现,瞬态受体电位4可以将超生理机械负荷转导为增加的环氧化酶2基因表达,增加纤维环细胞中前列腺素E2的释放,并且动态压缩期间的瞬态受体电位4抑制激活了ERK/MAPK通路,降低了椎间盘退变程度。所以,在治疗椎间盘退变过程中注射特异性拮抗剂或诱导基因编辑可能是治疗椎间盘退变的新策略。
2.4 细胞骨架 作用于细胞核上的机械力主要来源于细胞骨架,细胞骨架由肌球蛋白微丝、微管和中间丝构成,而椎间盘细胞中的细胞骨架蛋白主要包括肌动蛋白、微管蛋白和波形蛋白,这几种蛋白以及YAP/TAZ信号转导通路可能参与了力学信号的转导并影响了椎间盘退变过程[51]。
2.4.1 细胞骨架蛋白 肌动蛋白以单体(G-肌动蛋白)和多聚体(F-肌动蛋白)的形式广泛存在于真核细胞中,可帮助维持细胞刚性和形状。关于肌动蛋白在信号转导过程中的研究,PRITCHARD等[52]研究认为脊柱的压力载荷会改变椎间盘的渗透环境,在高渗环境下椎间盘细胞体积减小,机械刺激所引起的肌动蛋白结构改变可以使钙离子通道开放,增加钙离子通量,提示肌动蛋白可能在机械信号刺激和钙离子介导的生物学反应中起到桥梁作用。YU等[53]研究表明在循环静水压力下,整合素α5的表达降低,当信号通过整合素α5传递到细胞中时肌动蛋白受到影响,这可能是椎间盘细胞中机械信号转导的重要机制之一。
微管蛋白主要参与维持细胞中多种细胞器的分布以及蛋白质的分泌和运输等细胞代谢相关活动。有研究表明,牵拉力可以改变微管蛋白在椎间盘细胞中的表达水平,从而抑制蛋白聚糖的合成和分泌[54]。ZHANG等[55]研究结果表明,滑膜间充质干细胞中的微管稳定促进了髓核细胞中Ⅱ型胶原蛋白的表达,同时激活了 Hippo信号通路,抑制Yes相关蛋白的表达和活性,减轻大鼠椎间盘退变。此研究首次将微管确定为椎间盘退变的一个有前途的治疗靶点,靶向微管乙酰化可能是治疗椎间盘退变的有效策略。
关于波形蛋白,LORETO等[56]研究结果显示正常椎间盘中存在波形蛋白阳性细胞,而退变椎间盘中波形蛋白含量较少,说明波形蛋白的表达与椎间盘退变程度具有相关性,但其在椎间盘细胞机械信号转导中的具体作用机制尚未明确,目前仅有TSURUTA等[57]研究推测波形蛋白的磷酸化可能参与了椎间盘细胞的机械转导过程。
2.4.2 Yes相关蛋白 Yes相关蛋白是细胞外基质刚度和细胞结构变化的核效应因子,主要通过被上游Hippo通路信号激活以响应力学刺激[58]。生物力学信号、配体等上游激活因素通过引起STE20样蛋白激酶1/2、蛋白萨尔瓦多同源物1、MOB激酶激活因子1A/B磷酸化后形成复合体,激活丝氨酸/苏氨酸蛋白激酶1/2并与磷酸化的MOB激酶激活因子1A/B结合形成蛋白聚合物,从而导致Yes相关蛋白磷酸化,最后被蛋白酶体降解[59]。若Hippo信号通路被抑制,上游激活因素也可通过肌动蛋白张力传导改变核孔渗透性调控Yes相关蛋白的活性[60],说明Yes相关蛋白在力学信号转导中发挥着重要作用。
Hippo-Yes相关蛋白信号通路参与了椎间盘细胞的增殖、衰老、凋亡以及维持细胞外基质稳态等多个过程,在椎间盘退变进展中起着至关重要的作用。ZHANG等[61]研究发现,与正常髓核细胞相比,衰老大鼠髓核细胞中Hippo信号激活和Yes相关蛋白磷酸化更明显,并且Yes相关蛋白的活化和去磷酸化随着年龄的增长逐渐降低,而Yes相关蛋白下调可通过上调P53和P21的表达来显著增加髓核细胞的衰老。研究表明Hippo-Yes相关蛋白的信号转导与静水压力引起的细胞凋亡有明显相关性,如
在≥1.0 MPa的静水压力下,髓核细胞和纤维环细胞出现大量凋亡,此时细胞核中Yes相关蛋白表达升高[62]。总体而言,专注于Hippo信号级联和恢复Yes相关蛋白活性可以被视为探索椎间盘退变治疗的策略之一。此外,Hippo-Yes相关蛋白还被证明与软骨稳态相关,一项研究报道称Yes相关蛋白可通过与SRY-box转录因子6相互作用促进软骨细胞增殖[63];同时,Yes相关蛋白激活或抑制STE20样蛋白激酶1/2的上调有助于维持关节软骨的完整性[64],说明Hippo-Yes相关蛋白信号通路可能是治疗因终板软骨退变而导致椎间盘退变的新线索。
2.5 细胞核骨架蛋白 细胞核骨架蛋白包括跨核膜的核骨架-细胞骨架连接体复合物、位于核浆内的致密网状核纤层及相关蛋白(如核纤层蛋白等)。核骨架-细胞骨架连接体复合物由定位于外核膜的突触核膜蛋白通过KASH结构域与位于内核膜上的SUN蛋白结合,形成物理连接。相关研究证实,核骨架-细胞骨架连接体复合物不仅平衡了部分细胞骨架施加在核外膜的拉力,还可通过改变细胞核刚度而抵抗施加在细胞核上的压力,其中核纤层蛋白是核刚度改变的主要决定因素[65]。核纤层蛋白是一种位于核膜内表面的结构蛋白,主要包括A型和B型,可以通过改变其分子组成与构象来调节细胞核力学性质,并对张力做出动态响应[66]。现已有研究证实,椎间盘退变的发生与核纤层蛋白A的异常表达有关,核纤层蛋白A可通过破坏线粒体的形态而加速髓核细胞衰老[67]。WU等[68]在手术期间取得退变的人体椎间盘组织,通过免疫染色首次报道了椎间盘组织中核纤层蛋白A/C的存在,认为其表达可能与椎间盘退变相关。在此之前,欧定强等[69]发现与健康人和腰椎间盘突出患者相比,腰椎管狭窄患者椎间盘组织中核纤层蛋白A表达最高,认为核纤层蛋白A的表达可能随着椎间盘压力负荷的增加而上调,并且椎间盘组织的抗压能力随之下降,加速纤维环破裂,但这种假设并未得到证实。
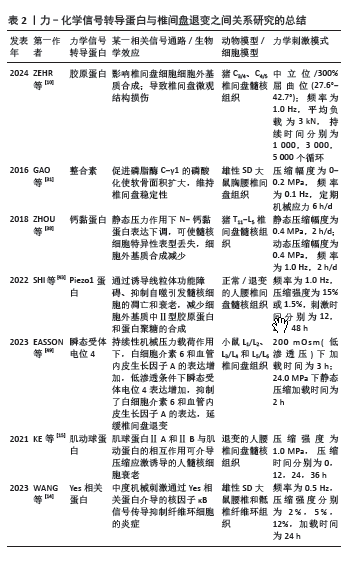
2.6 小结 异常的机械负荷作为导致椎间盘退变的重要因素之一,可以直接或间接地影响到椎间盘细胞的生理和代谢活动(如诱导髓核细胞凋亡),从而加速椎间盘退变
(图5)。前文对力-化学信号转导蛋白进行了相关研究总结(表1),并且对力学信号转导蛋白与椎间盘退变之间的关系进行了归纳和思考(表2)。

| [1] BAKHSHANDEH B, SORBONI SG, RANJBAR N, et al. Mechanotransduction in tissue engineering: Insights into the interaction of stem cells with biomechanical cues. Exp Cell Res. 2023; 431(2):113766. [2] 罗卓荆,杨柳,王迪.我国椎间盘退变的生物学研究成就及展望[J].空军军医大学学报,2023,44(6):481-485+489. [3] CHEN S, FU P, WU H, et al. Meniscus, articular cartilage and nucleus pulposus: a comparative review of cartilage-like tissues in anatomy, development and function. Cell Tissue Res. 2017;370(1):53-70. [4] 房晓阳,唐田,王楠,等.椎间盘全层纤维环修复与再生治疗[J].中国组织工程研究,2022,26(10):1582-1587. [5] 孙尚,赵振达,蒋嫒,等.力学刺激在椎体软骨终板退变中的作用及机制[J].医用生物力学,2021,36(4):652-657. [6] DESMOULIN GT, PRADHAN V, MILNER TE. Mechanical Aspects of Intervertebral Disc Injury and Implications on Biomechanics. Spine (Phila Pa 1976). 2020;45(8):E457-E464. [7] ZHU L, ZHANG C, PENG L, et al. A case report on digital preoperative design, clinical application and finite element analysis for a patient with ankylosing spondylitis kyphosis. Front Bioeng Biotechnol. 2023; 11:1220102. [8] HYNES RO, RUOSLAHTI E, SPRINGER TA. Reflections on Integrins-Past, Present, and Future: The Albert Lasker Basic Medical Research Award. JAMA. 2022;328(13):1291-1292. [9] COSTE B, MATHUR J, SCHMIDT M, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55-60. [10] ZEHR JD, QUADRILATERO J, CALLAGHAN JP. Initiation and accumulation of loading induced changes to native collagen content and microstructural damage in the cartilaginous endplate. Spine J. 2024;24(1):161-171. [11] SCHNAKE KJ, PUTZIER M, HAAS NP, et al. Mechanical concepts for disc regeneration. Eur Spine J. 2006;15 Suppl 3(Suppl 3):S354-360. [12] BOYD LM, CARTER AJ. Injectable biomaterials and vertebral endplate treatment for repair and regeneration of the intervertebral disc. Eur Spine J. 2006;15 Suppl 3(Suppl 3):S414-421. [13] ZHANG K, DING W, SUN W, et al. Beta1 integrin inhibits apoptosis induced by cyclic stretch in annulus fibrosus cells via ERK1/2 MAPK pathway. Apoptosis. 2016;21(1):13-24. [14] WANG H, ZHANG W, CAI Y, et al. Moderate mechanical stimulation antagonizes inflammation of annulus fibrosus cells through YAP-mediated suppression of NF-κB signaling. J Orthop Res. 2023;41(12): 2667-2684. [15] KE W, WANG B, HUA W, et al. The distinct roles of myosin IIA and IIB under compression stress in nucleus pulposus cells. Cell Prolif. 2021; 54(2):e12987. [16] SARASWATHIBHATLA A, INDANA D, CHAUDHURI O. Cell-extracellular matrix mechanotransduction in 3D. Nat Rev Mol Cell Biol. 2023;24(7): 495-516. [17] THEOCHARIS AD, SKANDALIS SS, GIALELI C, et al. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4-27. [18] ZHANG S, LIU W, CHEN S, et al. Extracellular matrix in intervertebral disc: basic and translational implications. Cell Tissue Res. 2022;390(1):1-22. [19] LEITINGER B. Transmembrane collagen receptors. Annu Rev Cell Dev Biol. 2011;27:265-290. [20] XU H, ZHANG Y, ZHANG Y, et al. A novel rat model of annulus fibrosus injury for intervertebral disc degeneration. Spine J. 2024;24(2): 373-386. [21] AHSAN R, TAJIMA N, CHOSA E, et al. Biochemical and morphological changes in herniated human intervertebral disc. J Orthop Sci. 2001; 6(6):510-518. [22] ZHOU X, TAO Y, CHEN E, et al. Genipin-cross-linked type II collagen scaffold promotes the differentiation of adipose-derived stem cells into nucleus pulposus-like cells. J Biomed Mater Res A. 2018;106(5):1258-1268. [23] BASHEY RI, MARTINEZ-HERNANDEZ A, JIMENEZ SA. Isolation, characterization, and localization of cardiac collagen type VI. Associations with other extracellular matrix components. Circ Res. 1992;70(5):1006-1017. [24] AUMAILLEY M. The laminin family. Cell Adh Migr. 2013;7(1):48-55. [25] NAHA A, DRISCOLL TP. Fibronectin sensitizes activation of contractility, YAP, and NF-κB in nucleus pulposus cells. J Orthop Res. 2024;42(2): 434-442. [26] CYRIL D, GIUGNI A, BANGAR SS, et al. Elastic Fibers in the Intervertebral Disc: From Form to Function and toward Regeneration. Int J Mol Sci. 2022;23(16):8931. [27] MERCURI J, ADDINGTON C, PASCAL R 3RD, et al. Development and initial characterization of a chemically stabilized elastin-glycosaminoglycan-collagen composite shape-memory hydrogel for nucleus pulposus regeneration. J Biomed Mater Res A. 2014;102(12):4380-4393. [28] CAMPBELL ID, HUMPHRIES MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3(3):a004994. [29] KANCHANAWONG P, CALDERWOOD DA. Organization, dynamics and mechanoregulation of integrin-mediated cell-ECM adhesions. Nat Rev Mol Cell Biol. 2023;24(2):142-161. [30] XIA M, ZHU Y. Expression of integrin subunits in the herniated intervertebral disc. Connect Tissue Res. 2008;49(6):464-469. [31] GAO G, HE J, NONG L, et al. Periodic mechanical stress induces the extracellular matrix expression and migration of rat nucleus pulposus cells by upregulating the expression of intergrin α1 and phosphorylation of downstream phospholipase Cγ1. Mol Med Rep. 2016;14(3):2457-2464. [32] KANDA Y, YURUBE T, MORITA Y, et al. Delayed notochordal cell disappearance through integrin α5β1 mechanotransduction during ex-vivo dynamic loading-induced intervertebral disc degeneration. J Orthop Res. 2021;39(9):1933-1944. [33] ZHENG L, CAO Y, NI S, et al. Ciliary parathyroid hormone signaling activates transforming growth factor-β to maintain intervertebral disc homeostasis during aging. Bone Res. 2018;6:21. [34] XU Z, ZHENG J, ZHANG Y, et al. Increased Expression of Integrin Alpha 6 in Nucleus Pulposus Cells in Response to High Oxygen Tension Protects against Intervertebral Disc Degeneration. Oxid Med Cell Longev. 2021; 2021:8632823. [35] YULIS M, KUSTERS DHM, NUSRAT A. Cadherins: cellular adhesive molecules serving as signalling mediators. J Physiol. 2018;596(17): 3883-3898. [36] HWANG PY, JING L, MICHAEL KW, et al. N-Cadherin-Mediated Signaling Regulates Cell Phenotype for Nucleus Pulposus Cells of the Intervertebral Disc. Cell Mol Bioeng. 2015;8(1):51-62. [37] LI P, ZHANG R, WANG L, et al. Long-term load duration induces N-cadherin down-regulation and loss of cell phenotype of nucleus pulposus cells in a disc bioreactor culture. Biosci Rep. 2017;37(2): BSR20160582. [38] ZHOU H, SHI J, ZHANG C, et al. Static compression down-regulates N-cadherin expression and facilitates loss of cell phenotype of nucleus pulposus cells in a disc perfusion culture. Biosci Rep. 2018; 38(1):BSR20171551. [39] KE W, LIAO Z, LIANG H, et al. Stiff Substrate Induces Nucleus Pulposus Cell Ferroptosis via YAP and N-Cadherin Mediated Mechanotransduction. Adv Healthc Mater. 2023;12(23):e2300458. [40] WANG J, JIANG J, YANG X, et al. Tethering Piezo channels to the actin cytoskeleton for mechanogating via the cadherin-β-catenin mechanotransduction complex. Cell Rep. 2022;38(6):110342. [41] 张珂诚,李聪,陈知行.力信号转导的基本元件:机械力敏感离子通道的研究进展[J].生命科学,2021,33(2):205-222. [42] SZCZOT M, NICKOLLS AR, LAM RM, et al. The Form and Function of PIEZO2. Annu Rev Biochem. 2021;90:507-534. [43] SHI S, KANG XJ, ZHOU Z, et al. Excessive mechanical stress-induced intervertebral disc degeneration is related to Piezo1 overexpression triggering the imbalance of autophagy/apoptosis in human nucleus pulpous. Arthritis Res Ther. 2022;24(1):119. [44] WU J, CHEN Y, LIAO Z, et al. Self-amplifying loop of NF-κB and periostin initiated by PIEZO1 accelerates mechano-induced senescence of nucleus pulposus cells and intervertebral disc degeneration. Mol Ther. 2022;30(10):3241-3256. [45] LIU C, GAO X, LOU J, et al. Aberrant mechanical loading induces annulus fibrosus cells apoptosis in intervertebral disc degeneration via mechanosensitive ion channel Piezo1. Arthritis Res Ther. 2023; 25(1):117. [46] DING B, XIAO L, XU H. YAP1 controls degeneration of human cartilage chondrocytes in response to mechanical tension. Cell Biol Int. 2022; 46(10):1637-1648. [47] 邹文娟,黄桂芳,康利军.TRP通道在生物体对机械性刺激响应中的功能及作用机制[J].浙江大学学报(医学版),2012,41(2):222-228. [48] KRUPKOVA O, ZVICK J, WUERTZ-KOZAK K. The role of transient receptor potential channels in joint diseases. Eur Cell Mater. 2017; 34:180-201. [49] EASSON GWD, SAVADIPOUR A, ANANDARAJAH A, et al. Modulation of TRPV4 protects against degeneration induced by sustained loading and promotes matrix synthesis in the intervertebral disc. FASEB J. 2023;37(2):e22714. [50] CAMBRIA E, HEUSSER S, SCHEUREN AC, et al. TRPV4 mediates cell damage induced by hyperphysiological compression and regulates COX2/PGE2 in intervertebral discs. JOR Spine. 2021;4(3):e1149. [51] 薛荣亮,李思远.细胞骨架与机械信号传导:椎间盘突出机制研究的新靶点[J].中国疼痛医学杂志,2016,22(5):326-328. [52] PRITCHARD S, ERICKSON GR, GUILAK F. Hyperosmotically induced volume change and calcium signaling in intervertebral disk cells: the role of the actin cytoskeleton. Biophys J. 2002;83(5):2502-2510. [53] YU SJ, QIU GX, BURTON Y, et al. [Expression of integrin alpha5 and actin in the cells of intervertebral disc under cyclic hydrostatic pressure in vitro]. Zhonghua Wai Ke Za Zhi. 2005;43(24):1605-1608. [54] LI S, JIA X, DUANCE VC, et al. The effects of cyclic tensile strain on the organisation and expression of cytoskeletal elements in bovine intervertebral disc cells: an in vitro study. Eur Cell Mater. 2011;21: 508-522. [55] ZHANG X, SHU S, FENG Z, et al. Microtubule stabilization promotes the synthesis of type 2 collagen in nucleus pulposus cell by activating hippo-yap pathway. Front Pharmacol. 2023;14:1102318. [56] LORETO C, MUSUMECI G, CASTORINA A, et al. Degenerative disc disease of herniated intervertebral discs is associated with extracellular matrix remodeling, vimentin-positive cells and cell death. Ann Anat. 2011;193(2):156-162. [57] TSURUTA D, JONES JC. The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J Cell Sci. 2003;116(Pt 24):4977-4984. [58] CHAKRABORTY S, NJAH K, POBBATI AV, et al. Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway. Cell Rep. 2017;18(10):2464-2479. [59] 詹永通,吴桂豪,范旭红,等.Hippo-YAP信号通路调控细胞衰老的分子机制[J].中国病理生理杂志,2022,38(5):920-931. [60] 鲍远,聂铭博,施佳,等.YAP介导的力学信号转导在血管生成中的研究进展[J].医学综述,2020,26(3):438-442. [61] ZHANG C, WANG F, XIE Z, et al. Dysregulation of YAP by the Hippo pathway is involved in intervertebral disc degeneration, cell contact inhibition, and cell senescence. Oncotarget. 2017;9(2):2175-2192. [62] WANG Y, BAI B, HU Y, et al. Hydrostatic Pressure Modulates Intervertebral Disc Cell Survival and Extracellular Matrix Homeostasis via Regulating Hippo-YAP/TAZ Pathway. Stem Cells Int. 2021;2021: 5626487. [63] DENG Y, WU A, LI P, et al. Yap1 Regulates Multiple Steps of Chondrocyte Differentiation during Skeletal Development and Bone Repair. Cell Rep. 2016;14(9):2224-2237. [64] DENG Y, LU J, LI W, et al. Reciprocal inhibition of YAP/TAZ and NF-κB regulates osteoarthritic cartilage degradation. Nat Commun. 2018; 9(1):4564. [65] 齐颖新.细胞核与应力信号转导[J].医用生物力学,2022,37(3): 385-388. [66] GUILLUY C, OSBORNE LD, VAN LANDEGHEM L, et al. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol. 2014;16(4):376-381. [67] XU X, WANG D, ZHENG C, et al. Progerin accumulation in nucleus pulposus cells impairs mitochondrial function and induces intervertebral disc degeneration and therapeutic effects of sulforaphane. Theranostics. 2019;9(8):2252-2267. [68] WU CZ, OU DQ, RONG LM, et al. Expression of lamin A/C protein in degenerated human intervertebral disc. Eur Rev Med Pharmacol Sci. 2018;22(22):7607-7613. [69] 欧定强,吴承志,刘钰瑜,等.A型核纤层蛋白在腰椎退行性疾病中的表达及其意义[J].新医学,2015,46(11):724-727. [70] 张波.基于MAPK/ERK/mTOR通路探讨牵张力调节自噬延缓椎间盘退变的作用机制[D].济南:山东中医药大学,2023. [71] LI S, JIA X, DUANCE VC, et al. The effects of cyclic tensile strain on the organisation and expression of cytoskeletal elements in bovine intervertebral disc cells: an in vitro study. Eur Cell Mater. 2011;21: 508-522. [72] LAZARO-PACHECO D, MOHSENI M, RUDD S, et al. The role of biomechanical factors in models of intervertebral disc degeneration across multiple length scales. APL Bioeng. 2023;7(2):021501. [73] SAGGESE T, THAMBYAH A, WADE K, et al. Differential Response of Bovine Mature Nucleus Pulposus and Notochordal Cells to Hydrostatic Pressure and Glucose Restriction. Cartilage. 2020;11(2):221-233. [74] ALINI M, DIWAN AD, ERWIN WM, et al. An update on animal models of intervertebral disc degeneration and low back pain: Exploring the potential of artificial intelligence to improve research analysis and development of prospective therapeutics. JOR Spine. 2023;6(1):e1230. [75] DAI J, XING Y, XIAO L, et al. Microfluidic Disc-on-a-Chip Device for Mouse Intervertebral Disc-Pitching a Next-Generation Research Platform To Study Disc Degeneration. ACS Biomater Sci Eng. 2019;5(4): 2041-2051. [76] GROWNEY KALAF EA, SELL SA, BLEDSOE JG. Developing a mechanical and chemical model of degeneration in young bovine lumbar intervertebral disks and reversing loss in mechanical function. J Spinal Disord Tech. 2014;27(5):E168-175. [77] BEATTY AM, BOWDEN AE, BRIDGEWATER LC. Functional Validation of a Complex Loading Whole Spinal Segment Bioreactor Design. J Biomech Eng. 2016;138(6):064501. [78] XU HG, ZHENG Q, SONG JX, et al. Intermittent cyclic mechanical tension promotes endplate cartilage degeneration via canonical Wnt signaling pathway and E-cadherin/β-catenin complex cross-talk. Osteoarthritis Cartilage. 2016;24(1):158-168. [79] DU X, WANG X, CUI K, et al. Tanshinone IIA and Astragaloside IV Inhibit miR-223/JAK2/STAT1 Signalling Pathway to Alleviate Lipopolysaccharide-Induced Damage in Nucleus Pulposus Cells. Dis Markers. 2021;2021:6554480. [80] ZHAO R, YANG L, HE S, et al. Nucleus pulposus cell senescence is regulated by substrate stiffness and is alleviated by LOX possibly through the integrin β1-p38 MAPK signaling pathway. Exp Cell Res. 2022;417(2):113230. [81] ZHOU X, WANG J, FANG W, et al. Genipin cross-linked type II collagen/chondroitin sulfate composite hydrogel-like cell delivery system induces differentiation of adipose-derived stem cells and regenerates degenerated nucleus pulposus. Acta Biomater. 2018;71:496-509. [82] YANG JJ, LIN YY, CHAO KH, et al. Gelatin-Poly (γ-Glutamic Acid) Hydrogel as a Potential Adhesive for Repair of Intervertebral Disc Annulus Fibrosus: Evaluation of Cytocompatibility and Degradability. Spine (Phila Pa 1976). 2021;46(4):E243-E249. [83] DEWLE A, RAKSHASMARE P, SRIVASTAVA A. A Polycaprolactone (PCL)-Supported Electrocompacted Aligned Collagen Type-I Patch for Annulus Fibrosus Repair and Regeneration. ACS Appl Bio Mater. 2021;4(2): 1238-1251. [84] YANG XX, YIP CH, ZHAO S, et al. A bio-inspired nano-material recapitulating the composition, ultra-structure, and function of the glycosaminoglycan-rich extracellular matrix of nucleus pulposus. Biomaterials. 2023;293:121991. [85] OKORO PD, FRAYSSINET A, DE OLIVEIRA S, et al. Combining biomimetic collagen/hyaluronan hydrogels with discogenic growth factors promotes mesenchymal stroma cell differentiation into Nucleus Pulposus like cells. Biomater Sci. 2023;11(24):7768-7783. [86] LUO H, WANG Z, HE Z, et al. Injectable chondroitin sulfate-grafted self-antioxidant hydrogels ameliorate nucleus pulposus degeneration against overactive inflammation. Biomater Sci. 2023;11(10):3629-3644. [87] CHEN J, ZHU H, ZHU Y, et al. Injectable self-healing hydrogel with siRNA delivery property for sustained STING silencing and enhanced therapy of intervertebral disc degeneration. Bioact Mater. 2021;9:29-43. [88] BAO J, GAO W, ZHANG W, et al. Fibrin glue delivery system containing rhein ameliorates intervertebral disc degeneration by anti-inflammatory efficacy. J Orthop Surg Res. 2023;18(1):485. |
| [1] | 孙晓君, 王华明, 张德宏, 宋学文, 黄 晋, 张 辰, 裴生太. 有限元法在儿童发育性髋关节发育不良及治疗中的作用[J]. 中国组织工程研究, 2025, 29(9): 1897-1904. |
| [2] | 余 帅, 刘家伟, 朱 彬, 潘 檀, 李兴龙, 孙广峰, 于海洋, 丁 亚, 王宏亮. 小分子药物治疗骨关节炎的热点问题及应用前景[J]. 中国组织工程研究, 2025, 29(9): 1913-1922. |
| [3] | 李良奎, 黄永灿, 王鹏, 于滨生. 颈椎前路椎体骨化物可控前移融合对后纵韧带骨化物和内植物影响的有限元分析[J]. 中国组织工程研究, 2025, 29(9): 1761-1767. |
| [4] | 徐 彪, 路 坦, 姜亚琼, 阴玉娇. 有限元分析不同程度冈上肌断裂对肩关节应力的影响[J]. 中国组织工程研究, 2025, 29(9): 1768-1774. |
| [5] | 周金海, 李江伟, 王序全, 庄 颖, 赵 瑛, 杨渝勇, 王嘉嘉, 杨 阳, 周仕炼. 不同骨强度下全膝置换过程中发生股骨前皮质切迹的三维有限元分析[J]. 中国组织工程研究, 2025, 29(9): 1775-1782. |
| [6] | 付恩洪, 杨 行, 梁 成, 张小刚, 张亚丽, 靳忠民. 基于OpenSim预测青少年跖屈肌无力的下肢生物力学行为[J]. 中国组织工程研究, 2025, 29(9): 1789-1795. |
| [7] | 赵嘉诚, 任诗齐, 祝 秦, 刘佳佳, 朱 翔, 杨 洋. 原发性骨质疏松潜在生物标志物的生物信息学分析[J]. 中国组织工程研究, 2025, 29(8): 1741-1750. |
| [8] | 芦劼明, 李亚静, 杜培洁, 徐冬青.
人工和天然草地对青年女性跳跃落地时下肢生物力学表现的影响
[J]. 中国组织工程研究, 2025, 29(6): 1101-1107. |
| [9] | 张 帅, 李子春, 徐亦豪, 谢晓峰, 郭忠圣, 赵清扬. 经颅磁声电刺激强度对小鼠前额叶皮质网络可塑性的影响[J]. 中国组织工程研究, 2025, 29(6): 1108-1117. |
| [10] | 钱 琨, 李子卿, 孙 水. 内质网应激与常见退行性骨骼疾病的发生与发展[J]. 中国组织工程研究, 2025, 29(6): 1285-1295. |
| [11] | 项 攀, 车艳军, 罗宗平. 压应力激活SOST/Wnt/β-catenin通路诱导软骨终板细胞退变[J]. 中国组织工程研究, 2025, 29(5): 951-957. |
| [12] | 丁至立, 黄 杰, 蒋 强, 李土胜, 刘 江, 丁 宇. X射线透视引导下不同方式建立兔椎间盘退变模型的结果对比[J]. 中国组织工程研究, 2025, 29(5): 995-1002. |
| [13] | 张艺璇, 李东娜, 刘春艳. 牙周炎的病理过程、炎症反应及相关生物标志物:多组学分析[J]. 中国组织工程研究, 2025, 29(35): 7601-7610. |
| [14] | 杨祎铖, 郑智臻, 梁霜雪, 吴成亮, 杜芸芸. 篮球鞋功能参数对人体运动生物力学的影响与启示[J]. 中国组织工程研究, 2025, 29(35): 7620-7628. |
| [15] | 苏永昆, 孙 红, 刘 淼, 杨 华, 李青松. 开发纳米水凝胶系统搭载新型抗氧化剂与抗氧化剂联合治疗椎间盘退变[J]. 中国组织工程研究, 2025, 29(34): 7376-7384. |
椎间盘退变是退行性脊柱疾病的关键病理基础,常导致腰椎间盘突出、腰椎椎管狭窄等疾病,给患者和社会带来负担。在椎间盘退变的各类非遗致病因素中异常机械力刺激占据了主导地位,包括拉伸力、流体剪切力等。研究表明,这些异常的机械力刺激可通过力学信号转导蛋白诱导椎间盘细胞变性、细胞外基质降解以及髓核细胞的衰老、凋亡,导致椎间盘退行性变[2]。该文对参与机械力刺激下信号转导过程的几种蛋白进行了系统性总结,并且详细阐述了它们在椎间盘生物力学信号传递及椎间盘退变过程中发挥的关键作用,同时对其在生物医学领域中的潜在应用进行了展望,也对靶向力学信号转导蛋白延缓椎间盘退变的治疗策略进行了相关论述。
中国组织工程研究杂志出版内容重点:人工关节;骨植入物;脊柱;骨折;内固定;数字化骨科;组织工程
1.1.1 检索人及检索时间 由第一作者于2023年11月进行检索。
1.1.2 检索文献时限 各数据库建库时间至2024年1月。
1.1.3 检索数据库 在中国知网和PubMed数据库中进行检索。
1.1.4 检索词 中文检索词包括“椎间盘,髓核,纤维环,软骨终板,细胞,力,信号转导,蛋白,生物力学”,英文检索词包括“intervertebral disc,nucleus pulposus,annulus fibrosus,cartilaginous endplate,cell,mechanical stimulation,signal transduction,protein,biomechanics”。
1.1.5 检索文献类型 实验研究、临床研究和综述。
1.1.6 手工检索情况 无。
1.1.7 检索策略 中英文数据库检索策略,见图1。
1.2.1 纳入标准 ①研究主题为力学信号转导过程;②研究主题与椎间盘退变或椎间盘细胞衰老、凋亡相关;③研究主题为机械力刺激诱导椎间盘退变的机制;④研究内容为力-化学信号转导过程中的某一特定蛋白或蛋白相关通路。
1.2.2 排除标准 重复性文献及与此次研究不相关的文献。
1.3 文献质量评价 由2名独立研究人员按照设定的纳入和排除标准分别对检索出来的文献设立数据库,阅读题目、摘要和全文对文献进行筛选,如果筛选过程中遇见分歧,2人进行讨论,并参考第3者意见获得最终决定。
根据纳入及排除标准,对88篇符合标准的文献进行综述,文献筛选流程图见图2。
较少。
3.2 作者综述区别于他人他篇的特点 该文以细胞结构为基础,根据力化学信号转导过程,依次从细胞外基质中的力学信号转导蛋白、细胞膜力学感受器、细胞骨架等不同层面,全面、深入地探讨了椎间盘细胞对机械刺激的反应机制,对力学信号转导蛋白在椎间盘细胞的各个结构层面、力化学信号换能的各个过程中发挥的作用进行了系统性总结和梳理。对椎间盘退变发生机制提出了新的理解,即认为细胞膜力学感受器、机械敏感通道、细胞骨架等作为生物力学信号的传导途径,通过调节椎间盘细胞的增殖、凋亡、衰老、细胞外基质合成和降解等生物学过程影响椎间盘退变的进程。同时,该文也对靶向力学信号延缓椎间盘退变的治疗策略进行了系统性总结。
3.3 综述的局限性 该文对于力学信号转导蛋白在椎间盘细胞对机械刺激反应中的作用做了详细介绍,但对于其他可能影响蛋白作用的因素,如基因、环境因素等并未进行详细探讨;力化学信号换能过程极为复杂,其相关蛋白在不同的椎间盘细胞中发挥作用不同,该文并未对各蛋白之间的调节反馈机制进行研究,此机制或可影响椎间盘退变进程。
3.4 综述的重要意义 椎间盘退变是一种由多种因素导致椎间盘细胞衰老、凋亡最终引发一系列椎间盘退行性疾病的病理过程,由椎间盘退变诱发的腰椎间盘突出症、腰椎椎管狭窄症等疾病具有较高的发病率和复发率,严重影响患者的生活质量,给患者和社会带来较大负担,因此,对椎间盘退变进行早期诊断和有效干预具有重要意义。作者所在团队前期通过大量临床和基础研究发现,手法机械刺激可以改变椎间盘的应力分布,在此过程中力学信号转导蛋白发挥了重要作用。该文以椎间盘细胞的结构和功能为基础,根据力化学信号换能过程,分别从细胞外基质、细胞膜、细胞骨架等多个层面对参与力化学信号转导过程的相关蛋白进行归纳总结,论述了各蛋白的实际意义及其在椎间盘退变过程中的具体作用,在一定程度上为手法治疗腰椎退行性疾病提供了理论依据,为寻找延缓椎间盘退变的新治疗靶点提供参考。
3.5 课题专家组对未来研究的建议 ①对力学信号转导蛋白及力学信号转导机制的深入研究、寻找新的治疗靶点是椎间盘退变诊疗的重要方向,例如:通过调控髓核细胞骨架的组成和功能改变髓核细胞的力学性质,进而改善椎间盘细胞的力学环境;或是通过调控力学信号转导蛋白的活性和表达改变椎间盘细胞对力学刺激的响应能力。目前这些研究都还处于初级阶段,希望未来能发现更多的潜在信号转导机制,为椎间盘退变的治疗提供新的思路和方法。②力学信号转导蛋白的研究主要依赖于体外实验室环境中的观察,而在体内环境中这些蛋白的行为可能与实验室环境中的行为有所不同。因此,需要开发更加精确和高效的实验方法和技术,以便在体内环境中研究这些蛋白。③针对细胞力化学信号转导蛋白进行药物治疗以延缓甚至逆转椎间盘退变进程,是未来研究的重要方向,需要广大研究人员进行更深层次的研究。
中国组织工程研究杂志出版内容重点:人工关节;骨植入物;脊柱;骨折;内固定;数字化骨科;组织工程
文题释义:#br# 力学信号转导蛋白:是一类能够感知并响应机械力,将力学信号转化为生化信号的蛋白质,这类蛋白质在机械力的作用下发生构象变化或化学改变(如磷酸化),从而激活相关信号通路,影响细胞内的生化活动和基因表达。#br# 椎间盘退变:是指椎间盘受到机械、营养及遗传因素的影响发生病理性改变的过程。#br# #br# 中国组织工程研究杂志出版内容重点:人工关节;骨植入物;脊柱;骨折;内固定;数字化骨科;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||