Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (14): 2169-2176.doi: 10.3969/j.issn.2095-4344.1669
Previous Articles Next Articles
Type I collagen combined titanium dioxide nanotube composite coating modified titanium surface improves osteoblast adhesion and osseointegration
Li Ying1, You Yapeng1, Li Baoe2, Song Yunjia1, Ma Aobo1, Chen Bo1, Han Wen1, Li Changyi1
- 1Hospital of Stomatology, Tianjin Medical University, Tianjin 300070, China; 2School of Materials Science and Engineering, Hebei University of Technology, Tianjin 300130, China
-
Received:2018-12-26 -
Contact:Li Changyi, Professor, Doctoral supervisor, Chief physician, Hospital of Stomatology, Tianjin Medical University, Tianjin 300070, China -
About author:Li Ying, MD, Associate chief physician, Master’s supervisor, Hospital of Stomatology, Tianjin Medical University, Tianjin 300070, China -
Supported by:the National Natural Science Foundation of China, No. 81870809, 31470920 (to LCY), and 81500886 (to LY); the Natural Science Foundation of Tianjin, No. 16JCYBJC28700 (to LY); the Natural Science Foundation of Hebei Province, No. E2017202032 (to LY)
CLC Number:
Cite this article
Li Ying, You Yapeng, Li Baoe, Song Yunjia, Ma Aobo, Chen Bo, Han Wen, Li Changyi. Type I collagen combined titanium dioxide nanotube composite coating modified titanium surface improves osteoblast adhesion and osseointegration[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(14): 2169-2176.
share this article
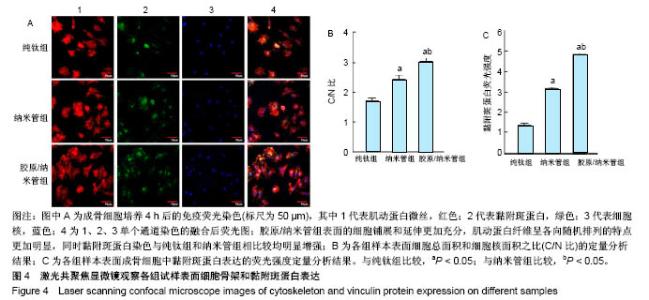
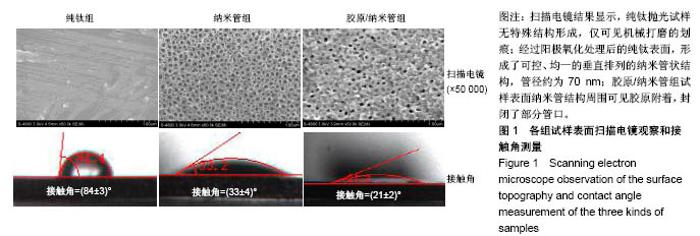
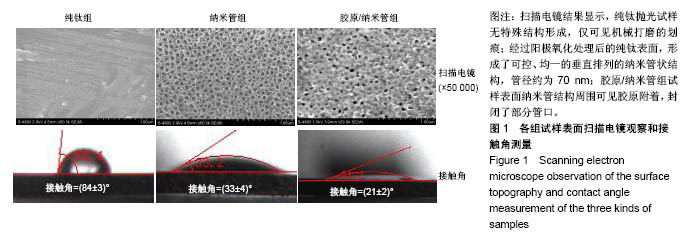
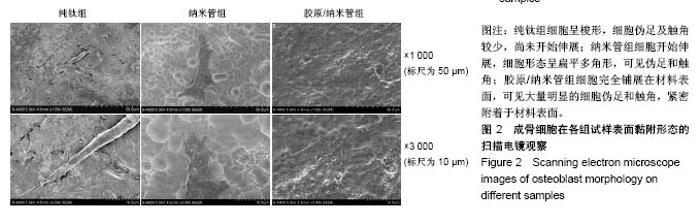
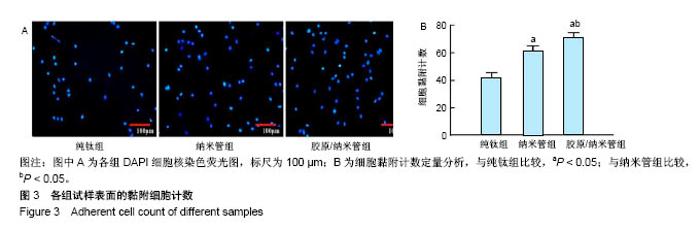
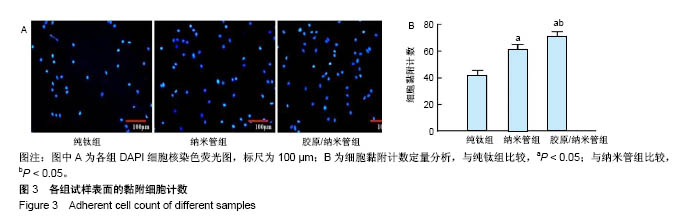
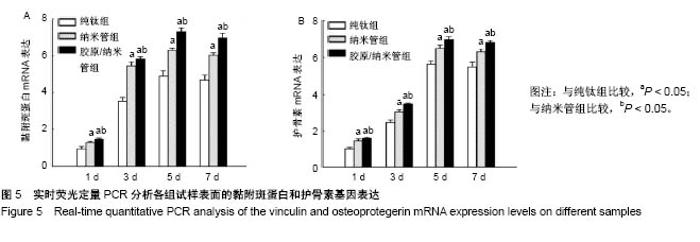
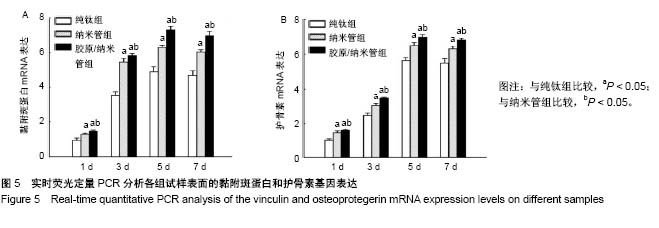
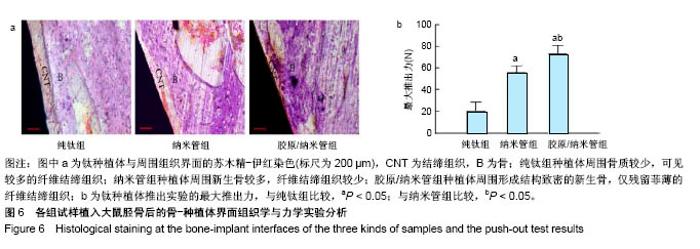
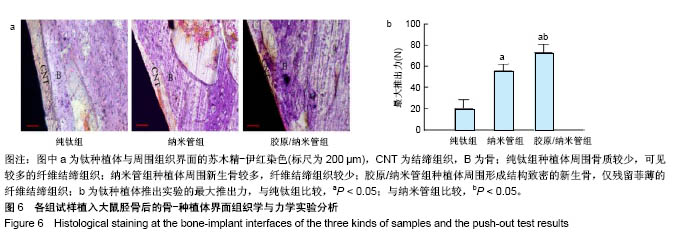
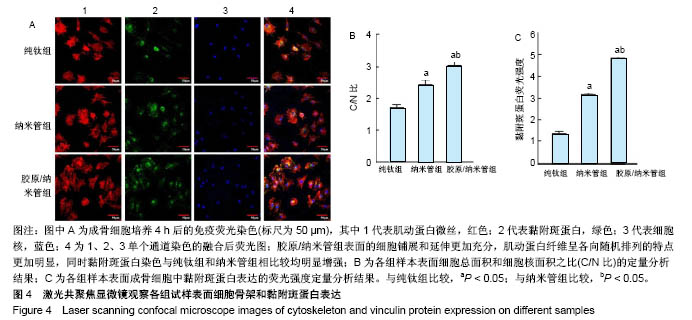
2.4 各组细胞骨架和黏附斑蛋白染色结果 图4展示的是成骨细胞在各组试样表面的骨架铺展形态和黏附斑蛋白表达情况。由图4A可见,细胞在纯钛表面沿打磨划痕方向伸展,该现象可能是由接触诱导引起;成骨细胞在纳米管表面的铺展明显优于纯钛组,呈现出各个方向伸展的骨架形态,黏附斑蛋白染色较之纯钛组明显;值得注意的是,胶原/纳米管组表面的细胞铺展和延伸更加充分,肌动蛋白纤维呈各向随机排列的特点更加明显,同时黏附斑蛋白染色与纯钛组和纳米管组相比较均明显增强。图4B显示的是细胞总面积和细胞核面积之比(C/N比)的定量分析结果,胶原/纳米管组、纳米管组的C/N比分别是纯钛组的1.7倍和1.4倍。图4C进一步分析了各组试样表面细胞黏附斑染色荧光强度,胶原/纳米管组、纳米管组的黏附斑蛋白染色荧光强度分别为纯钛组强度值的3.5倍和2.3倍。"
| [1] Bagno A,Di Bello C. Surface treatments and roughness properties of ti-based biomaterials. J Mater Sci Mater Med. 2004;15(9):935-949.[2] Shi Q,Qian Z,Liu D,et al.Surface modification of dental titanium implant by layer-by-layer electrostatic self-assembly. Front Physiol.2017;8:574.[3] Decuzzi P,Ferrari M.Modulating cellular adhesion through nanotopography. Biomaterials. 2010;31(1):173-179.[4] Meswania IM,Bousdras VA,Ahir SP,et al. A novel closed-loop electromechanical stimulator to enhance osseointegration with immediate loading of dental implant restorations. Proc Inst Mech Eng H. 2010;224(10):1221-1232.[5] 李莺,李长义.钛种植体表面改性策略及对骨整合的影响[J].中国组织工程研究,2013,17(29):5395-5402.[6] Mendonca G,Mendonca DB,Aragao FJ,et al. Advancing dental implant surface technology--from micron- to nanotopography. Biomaterials. 2008;29(28):3822-3835.[7] Li B,Li Y,Li J,et al.Influence of nanostructures on the biological properties of ti implants after anodic oxidation.J Mater Sci Mater Med.2014;25(1):199-205.[8] Minagar S,Wang J,Berndt CC,et al.Cell response of anodized nanotubes on titanium and titanium alloys. J Biomed Mater Res A.2013;101(9):2726-2739.[9] Li Y,Li B,Fu X,et al.Anodic oxidation modification improve bioactivity and biocompatibility of titanium implant surface.J Hard Tissue Biol.2013;22(3):351-358.[10] Avila G,Misch K,Galindo-Moreno P,et al. Implant surface treatment using biomimetic agents. Implant Dent. 2009;18(1): 17-26.[11] 川叶,马敏先,张弢,等.Ⅰ型胶原修饰纯钛片促进人脂肪间充质干细胞增殖[J].中国组织工程研究, 2014,18(25):4032-4037.[12] Ricard-Blum S,Ruggiero F.The collagen superfamily: From the extracellular matrix to the cell membrane. Pathol Biol (Paris).2005;53(7):430-442.[13] 李赛娜,康跻耀,高建萍,等.胶原涂层对3d 打印种植体表面生物相容性的影响[J].中国组织工程研究, 2017,21(10):1558-1564.[14] Morra M,Cassinelli C,Meda L,et al. Surface analysis and effects on interfacial bone microhardness of collagen-coated titanium implants: A rabbit model. Int J Oral Maxillofac Implants. 2005;20(1):23-30.[15] Sartori M,Giavaresi G,Parrilli A,et al. Collagen type i coating stimulates bone regeneration and osteointegration of titanium implants in the osteopenic rat.Int Orthop. 2015;39(10): 2041-2052.[16] Liu P,Hao Y,Zhao Y,et al.Surface modification of titanium substrates for enhanced osteogenetic and antibacterial properties. Colloids Surf B Biointerfaces.2017;160:110-116.[17] Tang H,Li Y,Ma J,et al.Improvement of biological and mechanical properties of titanium surface by anodic oxidation. Biomed Mater Eng.2016;27(5):485-494.[18] Song Y,Ma A,Ning J,et al. Loading icariin on titanium surfaces by phase-transited lysozyme priming and layer-by-layer self-assembly of hyaluronic acid/chitosan to improve surface osteogenesis ability. Int J Nanomedicine.2018;13 6751-6767.[19] Geissler U,Hempel U,Wolf C,et al.Collagen type i-coating of ti6al4v promotes adhesion of osteoblasts. J Biomed Mater Res.2000;51(4):752-760.[20] Roehlecke C,Witt M,Kasper M,et al. Synergistic effect of titanium alloy and collagen type i on cell adhesion, proliferation and differentiation of osteoblast-like cells. Cells Tissues Organs. 2001;168(3):178-187.[21] Caiazza S,Colangelo P,Bedini R,et al.Evaluation of guided bone regeneration in rabbit femur using collagen membranes. Implant Dent. 2000;9(3):219-225.[22] Itoh S,Kikuchi M,Takakuda K,et al.The biocompatibility and osteoconductive activity of a novel hydroxyapatite/collagen composite biomaterial, and its function as a carrier of rhbmp-2. J Biomed Mater Res. 2001;54(3):445-453.[23] Andres JL,DeFalcis D,Noda M,et al.Binding of two growth factor families to separate domains of the proteoglycan betaglycan. J Biol Chem. 1992;267(9):5927-5930.[24] Brighton CT,Albelda SM.Identification of integrin cell-substratum adhesion receptors on cultured rat bone cells. J Orthop Res.1992;10(6):766-773.[25] Ruoslahti E,Pierschbacher MD.New perspectives in cell adhesion: Rgd and integrins. Science. 1987;238(4826): 491-497.[26] Salasznyk RM,Williams WA,Boskey A,et al. Adhesion to vitronectin and collagen i promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol. 2004;2004(1):24-34.[27] Rammelt S,Schulze E,Bernhardt R,et al. Coating of titanium implants with type-i collagen. J Orthop Res. 2004;22(5): 1025-1034.[28] Rammelt S,Schulze E,Witt M,et al. Collagen type i increases bone remodelling around hydroxyapatite implants in the rat tibia. Cells Tissues Organs. 2004;178(3):146-157.[29] Mathews S,Bhonde R,Gupta PK,et al. A novel tripolymer coating demonstrating the synergistic effect of chitosan, collagen type 1 and hyaluronic acid on osteogenic differentiation of human bone marrow derived mesenchymal stem cells. Biochem Biophys Res Commun. 2011;414(1): 270-276.[30] Chen S,Guo Y,Liu R,et al. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf B Biointerfaces. 2018;164: 58-69.[31] Bays JL,DeMali KA.Vinculin in cell-cell and cell-matrix adhesions.Cell Mol Life Sci. 2017;74(16):2999-3009.[32] Zhao J,Watanabe T,Bhawal UK,et al.Transcriptome analysis of beta-tcp implanted in dog mandible. Bone. 2011;48(4): 864-877.[33] Zhan X,Zhang C,Dissanayaka WL,et al.Storage media enhance osteoclastogenic potential of human periodontal ligament cells via rankl-independent signaling. Dent Traumatol.2013;29(1):59-65.[34] Sul YT.Electrochemical growth behavior, surface properties, and enhanced in vivo bone response of tio2 nanotubes on microstructured surfaces of blasted, screw-shaped titanium implants. Int J Nanomedicine.2010;5:87-100.[35] von Wilmowsky C,Bauer S,Roedl S,et al. The diameter of anodic tio2 nanotubes affects bone formation and correlates with the bone morphogenetic protein-2 expression in vivo.Clin Oral Implants Res. 2012;23(3):359-366.[36] Popat KC,Leoni L,Grimes CA,et al.Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials. 2007; 28(21):3188-3197. |
| [1] | Shen Jinbo, Zhang Lin. Micro-injury of the Achilles tendon caused by acute exhaustive exercise in rats: ultrastructural changes and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1190-1195. |
| [2] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [3] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [4] | Li Xingping, Xiao Dongqin, Zhao Qiao, Chen Shuo, Bai Yiguang, Liu Kang, Feng Gang, Duan Ke. Preparation and properties of copper-loaded antibacterial functional film on titanium surface [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 553-557. |
| [5] | Shi Xiaoxiu, Mao Shilong, Liu Yang, Ma Xingshuang, Luo Yanfeng. Comparison of tantalum and titanium (alloy) as orthopedic materials: physical and chemical indexes, antibacterial and osteogenic ability [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 593-599. |
| [6] | Liu Liu, Zhou Qingzhu, Gong Zhuo, Liu Boyan, Yang Bin, Zhao Xian. Characteristics and manufacturing techniques of collagen/inorganic materials for constructing tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 607-613. |
| [7] | Chen Yang, Huang Denggao, Gao Yuanhui, Wang Shunlan, Cao Hui, Zheng Linlin, He Haowei, Luo Siqin, Xiao Jingchuan, Zhang Yingai, Zhang Shufang. Low-intensity pulsed ultrasound promotes the proliferation and adhesion of human adipose-derived mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3949-3955. |
| [8] | Xu Xiaoming, Chen Yan, Song Qian, Yuan Lu, Gu Jiaming, Zhang Lijuan, Geng Jie, Dong Jian. Human placenta derived mesenchymal stem cell gel promotes the healing of radiation skin damage in SD rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(25): 3976-3980. |
| [9] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [10] | Chen Lei, Zheng Rui, Jie Yongsheng, Qi Hui, Sun Lei, Shu Xiong. In vitro evaluation of adipose-derived stromal vascular fraction combined with osteochondral integrated scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3487-3492. |
| [11] | Huo Hua, Cheng Yuting, Zhou Qian, Qi Yuhan, Wu Chao, Shi Qianhui, Yang Tongjing, Liao Jian, Hong Wei. Effects of drug coating on implant surface on the osseointegration [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3558-3564. |
| [12] | Yang Li, Li Xueli, Song Jinghui, Yu Huiqian, Wang Weixia. Effect of cryptotanshinone on hypertrophic scar of rabbit ear and its related mechanism [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(20): 3150-3155. |
| [13] | Liu Zhendong, Wang Rui, Li Xiaolei, Wang Jingcheng. Review of interferon alpha-2b inhibiting scar formation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(2): 317-321. |
| [14] | Wei Qin, Zhang Xue, Ma Lei, Li Zhiqiang, Shou Xi, Duan Mingjun, Wu Shuo, Jia Qiyu, Ma Chuang. Platelet-derived growth factor-BB induces the differentiation of rat bone marrow mesenchymal stem cells into osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2953-2957. |
| [15] | Guo Zhibin, Wu Chunfang, Liu Zihong, Zhang Yuying, Chi Bojing, Wang Bao, Ma Chao, Zhang Guobin, Tian Faming. Simvastatin stimulates osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2963-2968. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||