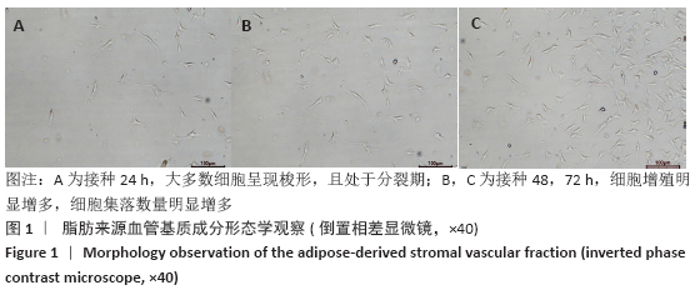
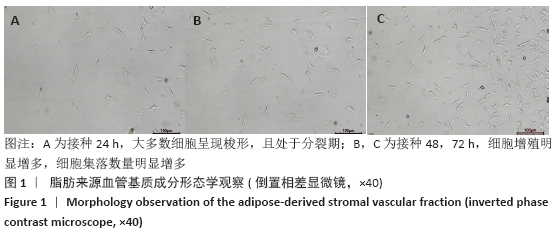
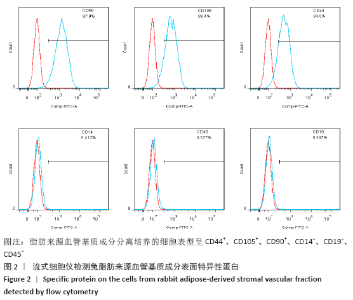
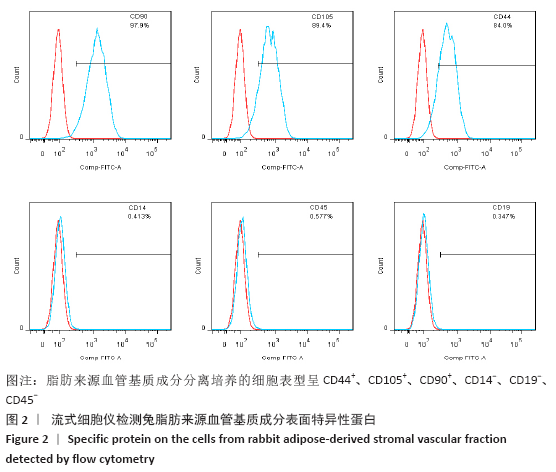
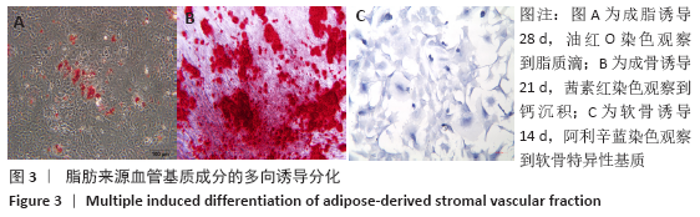
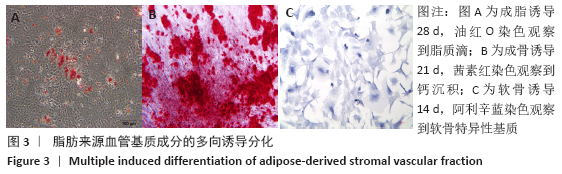
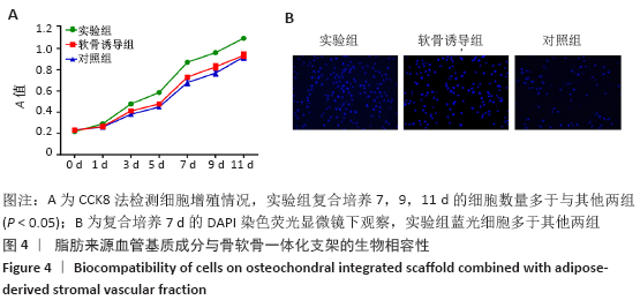
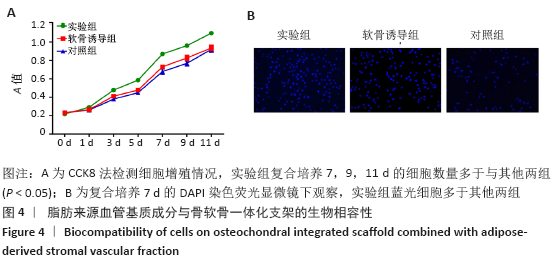
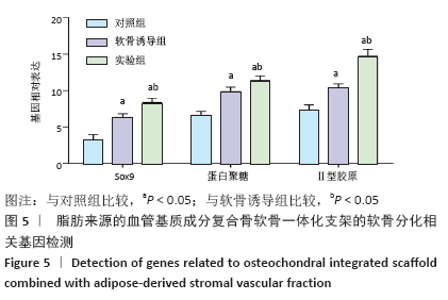
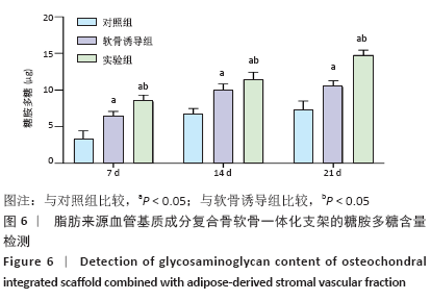
Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (22): 3487-3492.doi: 10.3969/j.issn.2095-4344.3230
Previous Articles Next Articles
In vitro evaluation of adipose-derived stromal vascular fraction combined with osteochondral integrated scaffold
Chen Lei, Zheng Rui, Jie Yongsheng, Qi Hui, Sun Lei, Shu Xiong
- Beijing Jishuitan Hospital, Beijing Institute of Traumatology and Orthopaedics, Beijing 100035, China
-
Received:2020-08-17Revised:2020-08-21Accepted:2020-10-09Online:2021-08-08Published:2021-01-19 -
Contact:Shu Xiong, Assistant Researcher, Beijing Jishuitan Hospital, Beijing Institute of Traumatology and Orthopaedics, Beijing 100035, China -
About author:Chen Lei, Technician-in-charge, Beijing Jishuitan Hospital, Beijing Institute of Traumatology and Orthopaedics, Beijing 100035, China -
Supported by:the Science and Technology Development Project of Beijing Municipal Medical Research Institute, No. PXM2018_026275_000004 (to CL); the Public Welfare Development and Reform Pilot Project of Beijing Municipal Medical Research Institutes, No. BMHC2018-4, BMHC2019-9 (to CL)
CLC Number:
Cite this article
Chen Lei, Zheng Rui, Jie Yongsheng, Qi Hui, Sun Lei, Shu Xiong. In vitro evaluation of adipose-derived stromal vascular fraction combined with osteochondral integrated scaffold[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3487-3492.
share this article
| [1] HUEY DJ, HU JC, ATHANASIOU KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917-927. [2] GUGJOO MB, SHARMA GT, AITHAL HP, et al. Cartilage tissue engineering: Role of mesenchymal stem cells along with growth factors & scaffolds. Indian J Med Res. 2016;144(3):339-347. [3] ALBRIGHT JC, DAOUD AK. Microfracture and Microfracture Plus. Clin Sports Med. 2017;36(3):501-507. [4] KRAEUTLER MJ, HOUCK DA, MCQUEEN MB, et al. A Cost-Effectiveness Analysis of Surgical Treatment Modalities for Chondral Lesions of the Knee: Microfracture, Osteochondral Autograft Transplantation, and Autologous Chondrocyte Implantation. Orthop J Sports Med. 2017; 5(5):1-8. [5] GOMOLL AH, MADRY H, KNUTSEN G, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):434-447. [6] ASIK M, CIFTCI F, SEN C, et al. The microfracture technique for the treatment of full-thickness articular cartilage lesions of the knee: midterm results. Arthroscopy. 2008;24(11):1214-1220. [7] ARMIENTO AR, STODDART MJ, ALINI M, et al. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018;65:1-20. [8] PATEL JM, WISE BC, BONNEVIE ED, et al. A Systematic Review and Guide to Mechanical Testing for Articular Cartilage Tissue Engineering. Tissue Eng Part C Methods. 2019;25(10):593-608. [9] WALTER SG, OSSENDORFF R, SCHILDBERG FA, et al. Articular cartilage regeneration and tissue engineering models: a systematic review. Arch Orthop Trauma Surg. 2019;139(3):305-316. [10] KWON H, BROWN WE, LEE CA, et al. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat Rev Rheumatol. 2019;15(9):550-570. [11] MEHRANFAR S, ABDI RAD I, MOSTAFAV E, et al. The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: an overview of clinical trials. Artif Cells Nanomed Biotechnol. 2019;47(1):882-890. [12] BANSAL H, COMELLA K, LEON J, et al. Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J Transl Med. 2017; 15(1):141. [13] 郑蕊,杰永生,陈磊,等.脱细胞真皮基质/生物矿化胶原一体化骨软骨支架的制备及其生物相容性的研究[J].中国医药生物技术, 2019,14(2):121-126. [14] 舒雄,杰永生,郑蕊,等.激活的富血小板血浆促进Pellet 培养的脂肪间充质干细胞成软骨样分化[J].中国医药生物技术,2018, 13(4):328-333. [15] ZHOU W, LIN J, ZHAO K, et al. Single-Cell Profiles and Clinically Useful Properties of Human Mesenchymal Stem Cells of Adipose and Bone Marrow Origin. Am J Sports Med. 2019;47(7):1722-1733. [16] ZHAO Z, FAN C, CHEN F, et al. Progress in Articular Cartilage Tissue Engineering: A Review on Therapeutic Cells and Macromolecular Scaffolds. Macromol Biosci. 2020;20(2):e1900278. [17] BAKHSHAYESH A, BABAIE S, NASRABADI HT, et al. An overview of various treatment strategies, especially tissue engineering for damaged articular cartilage. Artif Cells Nanomed Biotechnol. 2020;48(1):1089-1104. [18] CIPOLLARO L, CIARDULLI MC, PORTA GD, et al. Biomechanical issues of tissue-engineered constructs for articular cartilage regeneration: in vitro and in vivo approaches. Br Med Bull. 2019;132(1):53-80. [19] LEMOINE M, CASEY SM, O’BYRNE JM, et al. The development of natural polymer scaffold-based therapeutics for osteochondral repair. Biochem Soc Trans. 2020;48(4):1433-1445. [20] Dan X, Wei L, Li JJ, et al. Engineering 3D functional tissue constructs using self-assembling cell-laden microniches. Acta Biomater. 2020; 114:170-182. [21] BAPTISTA LS. Adipose stromal/stem cells in regenerative medicine: Potentials and limitations. World J Stem Cells. 2020;12(1):1-7. [22] WEN YT, DAI NT, HSU SH. Biodegradable water-based polyurethane scaffolds with a sequential release function for cell-free cartilage tissue engineering. Acta Biomater. 2019;88:301-313. [23] CARVALHO MS, SILVA JC, UDANGAWA RN, et al. Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2019;99:479-490. [24] TAKAHASHI H, OKANO T. Thermally-triggered fabrication of cell sheets for tissue engineering and regenerative medicine. Adv Drug Deliv Rev. 2019;138:276-292. [25] NEO PY, TEH TK, TAY AS, et al. Stem cell-derived cell-sheets for connective tissue engineering. Connect Tissue Res. 2016;57(6): 428-442. [26] CHOI JS, CHAE DS, RYU HA, et al. Transplantation of human adipose tissue derived-SVF enhance liver function through high anti-inflammatory property. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(12):158526. [27] KILINC MO, SANTIDRIAN A, MINEV I, et al. The ratio of ADSCs to HSC-progenitors in adipose tissue derived SVF may provide the key to predict the outcome of stem-cell therapy. Clin Transl Med. 2018;7(1):5. [28] SOLODEEV I, ORGIL M, COHEN M, et al. Cryopreservation of Stromal Vascular Fraction Cells Reduces Their Counts but Not Their Stem Cell Potency. Plast Reconstr Surg Glob Open. 201;7(7):e2321. [29] MORANDI EM, PLONER C, WOLFRAM D, et al. Risk factors and complications after body-contouring surgery and the amount of stromal vascular fraction cells found in subcutaneous tissue. Int Wound J. 2019;16(6):1545-1552. [30] ZANATA F, BOWLES A, FRAZIER T, et al. Effect of Cryopreservation on Human Adipose Tissue and Isolated Stromal Vascular Fraction Cells: In Vitro and In Vivo Analyses. Plast Reconstr Surg. 2018;141(2): 232e-243e. [31] MEHRANFAR S, RAD IA, MOSTAFAV E, et al. The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: an overview of clinical trials. Artif Cells Nanomed Biotechnol. 2019;47(1):882-890. [32] YOKOTA N, HATTORI M, OHTSURU T, et al. Comparative Clinical Outcomes After Intra-articular Injection With Adipose-Derived Cultured Stem Cells or Noncultured Stromal Vascular Fraction for the Treatment of Knee Osteoarthritis. Am J Sports Med. 2019;47(11):2577-2583. [33] FRANKLIN SP, STOKER AM, BOZYNSKI CC, et al. Comparison of Platelet-Rich Plasma, Stromal Vascular Fraction (SVF), or SVF with an Injectable PLGA Nanofiber Scaffold for the Treatment of Osteochondral Injury in Dogs. J Knee Surg. 2018;31(7):686-697. [34] DONGEN JA, HARMSEN MC, STEVENS HP, et al. Isolation of Stromal Vascular Fraction by Fractionation of Adipose Tissue. Methods Mol Biol. 2019;1993:91-103. [35] YAO Y, DONG Z, LIAO Y, et al. Adipose Extracellular Matrix/Stromal Vascular Fraction Gel: A Novel Adipose Tissue-Derived Injectable for Stem Cell Therapy. Plast Reconstr Surg. 2017;139(4):867-879. [36] SCHNEIDER MC, CHU S, RANDOLPH MA, et al. An in vitro and in vivo comparison of cartilage growth in chondrocyte-laden matrix metalloproteinase-sensitive poly(ethylene glycol) hydrogels with localized transforming growth factor β3. Acta Biomater. 2019;93: 97-110. [37] JIA ZF, WANG SJ, LIANG YJ, et al. Combination of kartogenin and transforming growth factor-β3 supports synovial fluid-derived mesenchymal stem cell-based cartilage regeneration. Am J Transl Res. 2019;11(4):2056-2069. [38] QU D, ZHU JP, CHILDS HR, et al. Nanofiber-based transforming growth factor-β3 release induces fibrochondrogenic differentiation of stem cells. Acta Biomater. 2019;93:111-122. [39] CHEN YR, ZHOU ZX, ZHANG JY, et al. Low-Molecular-Weight Heparin-Functionalized Chitosan-Chondroitin Sulfate Hydrogels for Controlled Release of TGF-β3 and in vitro Neocartilage Formation. Front Chem. 2019;7:745. [40] NAKAGAWA Y, FORTIER LA, MAO JJ, et al. Long-term Evaluation of Meniscal Tissue Formation in 3-dimensional-Printed Scaffolds With Sequential Release of Connective Tissue Growth Factor and TGF-β3 in an Ovine Model. Am J Sports Med. 2019;47(11):2596-2607. |
| [1] | Xu Feng, Kang Hui, Wei Tanjun, Xi Jintao. Biomechanical analysis of different fixation methods of pedicle screws for thoracolumbar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1313-1317. |
| [2] | Jiang Yong, Luo Yi, Ding Yongli, Zhou Yong, Min Li, Tang Fan, Zhang Wenli, Duan Hong, Tu Chongqi. Von Mises stress on the influence of pelvic stability by precise sacral resection and clinical validation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1318-1323. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Zhang Yu, Tian Shaoqi, Zeng Guobo, Hu Chuan. Risk factors for myocardial infarction following primary total joint arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1340-1345. |
| [5] | Wei Wei, Li Jian, Huang Linhai, Lan Mindong, Lu Xianwei, Huang Shaodong. Factors affecting fall fear in the first movement of elderly patients after total knee or hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1351-1355. |
| [6] | Wang Jinjun, Deng Zengfa, Liu Kang, He Zhiyong, Yu Xinping, Liang Jianji, Li Chen, Guo Zhouyang. Hemostatic effect and safety of intravenous drip of tranexamic acid combined with topical application of cocktail containing tranexamic acid in total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1356-1361. |
| [7] | Xiao Guoqing, Liu Xuanze, Yan Yuhao, Zhong Xihong. Influencing factors of knee flexion limitation after total knee arthroplasty with posterior stabilized prostheses [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1362-1367. |
| [8] | Huang Zexiao, Yang Mei, Lin Shiwei, He Heyu. Correlation between the level of serum n-3 polyunsaturated fatty acids and quadriceps weakness in the early stage after total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1375-1380. |
| [9] | Zhang Chong, Liu Zhiang, Yao Shuaihui, Gao Junsheng, Jiang Yan, Zhang Lu. Safety and effectiveness of topical application of tranexamic acid to reduce drainage of elderly femoral neck fractures after total hip arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1381-1386. |
| [10] | Wang Haiying, Lü Bing, Li Hui, Wang Shunyi. Posterior lumbar interbody fusion for degenerative lumbar spondylolisthesis: prediction of functional prognosis of patients based on spinopelvic parameters [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1393-1397. |
| [11] | Lü Zhen, Bai Jinzhu. A prospective study on the application of staged lumbar motion chain rehabilitation based on McKenzie’s technique after lumbar percutaneous transforaminal endoscopic discectomy [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1398-1403. |
| [12] | Chen Xinmin, Li Wenbiao, Xiong Kaikai, Xiong Xiaoyan, Zheng Liqin, Li Musheng, Zheng Yongze, Lin Ziling. Type A3.3 femoral intertrochanteric fracture with augmented proximal femoral nail anti-rotation in the elderly: finite element analysis of the optimal amount of bone cement [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1404-1409. |
| [13] | Du Xiupeng, Yang Zhaohui. Effect of degree of initial deformity of impacted femoral neck fractures under 65 years of age on femoral neck shortening [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1410-1416. |
| [14] | Zhang Shangpu, Ju Xiaodong, Song Hengyi, Dong Zhi, Wang Chen, Sun Guodong. Arthroscopic suture bridge technique with suture anchor in the treatment of acromioclavicular dislocation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1417-1422. |
| [15] | Liang Yan, Zhao Yongfei, Xu Shuai, Zhu Zhenqi, Wang Kaifeng, Liu Haiying, Mao Keya. Imaging evaluation of short-segment fixation and fusion for degenerative lumbar scoliosis assisted by highly selective nerve root block [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1423-1427. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||