Chinese Journal of Tissue Engineering Research ›› 2013, Vol. 17 ›› Issue (36): 6455-6461.doi: 10.3969/j.issn.2095-4344.2013.36.012
Previous Articles Next Articles
Hepatic arterial transplantation of autologous bone marrow mesenchymal stem cells in treatment of decompensated liver cirrhosis
Ouyang Shi1, Liu Shu-ren1, Cheng Tao1, Chen Yang-shu1, Kong Xiang-ping1, Zhou Chi-long1, Mu Liang-jing2
- 1Department of Infectious Diseases, Institute of Hepatology, the 458 Hospital of PLA, Guangzhou 510602, Guangdong Province, China; 2Health Center, No. 21 Division, Department of Joint Logistics, Guangzhou Military Command Area of PLA, Guangzhou 510063, Guangdong Province, China
-
Received:2012-12-06Revised:2012-12-25Online:2013-09-03Published:2013-09-03 -
About author:Ouyang Shi★, Master, Attending physician, Department of Infectious Diseases, Institute of Hepatology, the 458 Hospital of PLA, Guangzhou 510602, Guangdong Province, China ouyangshi2005@163.com -
Supported by:Science and Technology Project of the State Health Ministry of General Logistics Department, No. 2009-174*
CLC Number:
Cite this article
Ouyang Shi, Liu Shu-ren, Cheng Tao, Chen Yang-shu, Kong Xiang-ping, Zhou Chi-long, Mu Liang-jing. Hepatic arterial transplantation of autologous bone marrow mesenchymal stem cells in treatment of decompensated liver cirrhosis[J]. Chinese Journal of Tissue Engineering Research, 2013, 17(36): 6455-6461.
share this article
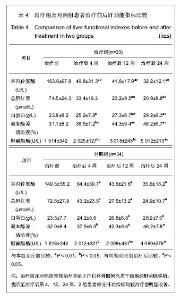
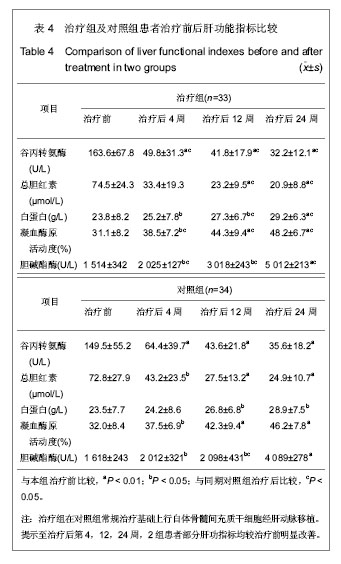
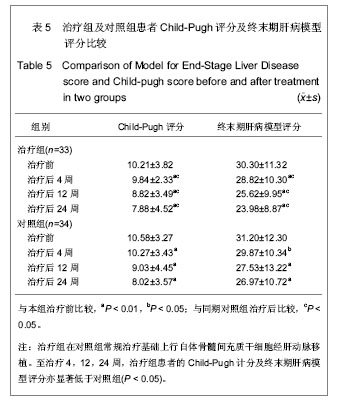
| [1] Petersen BE, Bowen WC,Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999; 284 (5417):1168-1170. [2] Theise ND, Badve S, Saxena R, et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31(1): 235-240. [3] Schwartz RE, Reyes M, Koodie L, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte like cells. J Clin Invest. 2002;109(10): 1291-1302. [4] Fang B, Shi M, Liao L,et al. Systemic infusion of FLK1(+) mesenchmal stem cells ameliorate carbon tetrachloride- induced liver fibrosis in mice. Transplantation. 2004;78(1):83-88. [5] Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147. [6] Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008; 15(2):109-116. [7] 中华医学会肝病学分会感染病学分会.慢性乙型肝炎防治指南[J].中华肝脏病杂志,2011,19(1):13-24. [8] 解放军第四五八医院.一种干细胞分离液及其用于于细胞分离的方法:中国,200610035900.5[专利]. 2007:12-19. [9] Friedenstein AJ, Gorskaja JF, Kulagina NN, et al. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5):267-274. [10] Kashofer K,Siapati EK,Bonnet D.In Vivo Formation of Unstable Heterokaryons after Liver Damage and Hematopoietic Stem Cell/Progenitor Transplantation. Stem Cells. 2006;24(4):1104-1112. [11] Oh SH,Witek RP, Bae SH,et al. Bone marrow-derived hepatic oval cells difierentiate into hepatocytes in 2-acetylaminofluorene/partial hepatectomy-induced liver regeneration. Gastroenterology. 2007;132(3):1077-1019. [12] Deng X,Chen YX,Zhang X,et al.Hepatic stellate cells modulate the differentiation of bone marrow messenchymal stem cells into hepatocyte like cells. J Cell Physiol. 2008; 217(1):128-144. [13] Okuyama H,Krishamachary B,Zhou YF, et al. Expression of vascular endothelial growth factor 1 in bone marrow-derived mesenchymal cells is dependent on hypoxia-inducible factor. J Biol Chem. 2006;281(22):15554-15563. [14] 刘彦,李昌平.骨髓干细胞向肝细胞分化方式的研究进展[J].中国组织工程研究与临床康复,2008,12(3):555-559. [15] 邢秀伟,李建生.干细胞移植治疗肝脏疾病的研究进展[J].中国组织工程研究与临床康复,2012,16(6):1115-1118. [16] Pournasr B,Mohamadnejad M,Bagheri M,et al. In vitro differentiation of human bone marrow mesenchymal stem cells into hepatocyte-like cells. Arch lran Med. 2011;14(4): 244-249. [17] Tian H, Bharadwaj S,Liu Y,et al. Differentiation of human bone marrow mesenchymal stem cells into bladder cells:potential for urological tissue engineering. Tissue Eng Part A. 2010;16 (5):1769-1779. [18] Farrell E,Both SK,Odrfer KI,et al. In vivo generation of bone via endochondral ossification by in-vitro chondrogenie priming of adult human and rat mesenchymal sterm cells. BMC Musculoskelet Disord. 2011;12:31. [19] Polisetti N,Chaitanya VG, Babu PP, et al.Isolation, characterization and differentiation potential of rat bone marrow stromal cells. Neurol India. 2010;58(2):201-208. [20] Gao J,Dennis JE, Muzic RF, et al. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169(1):12-20. [21] Kang XQ, Zang WJ, Bao LJ, et al. Fibroblast growth factor-4 and hepatoeyte growth factor induce differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatoeytes. World J Gastroenterol. 2005;11(47):7461-7465. [22] Honczarenko M, Le Y, Swierkowski M, et al. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24(4): 1030-1041. [23] Kollet O,Shivtiel S,Chen YQ,et al. HGF,SDF-1 and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112(1):160-169. [24] Oh SH,Miyazaki M,Kouchi H,et al.Hepaocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro. Biochem Biophys Res Commun. 2000;279(2):500-504. [25] Dann YY, Yeoh GC. Liver stem cells: a scientific and clinical perspective. J Gastroenterol Hepatol. 2008;23(5):687-698. [26] Zhao DC, Lei JX, Chen R, et al.Bone marrow derived mesenehymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005;11(22):3431-3440. [27] 吴理茂,李连达,刘红,等.自体骨髓干细胞移植与归元方联用治疗急慢性肝损伤的实验研究[J].中国工程科学,2004,6(7):34-42. [28] Takeda M,Yamamoto M,Isoda K,et al. Availability of bone marrow stormal cells in three-dimensional coculture with hepatocytes and transplantation into liver-damaged mice. J Biosci Bioeng. 2005;100(1):77-81. [29] Higashiyama R,Inagaki Y,Hong YY,et al. Bone marrow-derived cells express matrix metalloproteinases and contribute toregression of liver fibrosisinmice. Hepatology. 2007;45(1):213- 222. [30] 王东旭,姜海行,苏思标,等,体外共培养大鼠骨髓间充质干细胞对肝星状细胞增殖的影响:Cyclin D1与P27表达调控[J].中国组织工程研究与临床康复,2010,14(10):1764-1768. [31] Banas A,Teratani T,Yamamoto Y,et al. IFATS collection:in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells. 2008;26:2705-2712. [32] Kuo TK,Hung SP,Chuang CH,et al. Stem cell therapy for liver disease:parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008; 134:2111-2121. [33] Takeda M,Yamamoto M,Isoda K,et al. Availability of bone marrow stem cells in three-dimensional coculture with hepatocytes and transplantation into liver damaged mice. J Biosci Bioeng. 2005;100(1):77-81. [34] 施晓雷,丁义涛,仇毓东,等.小鼠骨髓干细胞体外定向诱导为肝细胞样细胞的实验研究[J].肝胆外科杂志,2006,14(4):304-308. [35] Snykers S, Vanhaecke T, Papeleu P, et al. Sequential exposure to cytokines reflecting embryogenesis:the key for in vitro differentiation of adult bone marow stem cells into functional hepatocyte-like cells. Toxicol Sci. 2006;94(2): 235-239. [36] Chen Y, Dong XJ, Zhang GR, et al. In vitro differentiation of mouse bone marrow stromal stem cells into hepatocytes induced by conditioned culture medium of hepatocytes. J Cell Biochem. 2007;102 (1):52-63. [37] Lange C,Bruns H,Kluth D,et al. Hepatocytic difierentiation of mesenchymal stem cells in cocultures with fetal liver cells. World J Gastroenterol. 2006;12(15):2394-2401. [38] Aurich I,Mueller LP, Aurich H, et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007;56(3):405-415. [39] Alison MR,Islam S,Lira S. Stem cells in liver regeneration.fibrosis and cancer:the good,the bad and the ugly. J Pathol. 2009;217:282-298. [40] Terai S, Ishikawa T, Omori K, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow ceil infusion therapy. Stem Cells. 2006;24:2292-2298. [41] Pal M,Zacharoulis D,Milicevic MN,et al. Autologous infusion of expanded mobilized adult bone marrow derived CD34 cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952-1958. [42] Kharaziha P,Hellstrim PM, Noorinayer B,et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection:a phase I- II clinical trial. Eur J Gastroenterol Hepatol. 2009;21(10):1199-1205. [43] Mohamadnejad M,Alimoghaddam K,MohyeddinBonab M,et al. Phase I human trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated cirrhosis. Arch Iran Med. 2007;10:459-466. [44] Terai S,Ishikawa T,Omori K,et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292-2298. [45] Gaia S,Smedile A,Omede P,et al. Feasibility and safety of G-CSF administration to induce bone marrow derived cells mobilization in patients with end stage liver disease. J Hepatol. 2006;45:13-19. [46] Lorenzini S,Isidori A,Catani I,et al. Stem cell mobilization and collection in patients with liver cirrhosis.Aliment Pharmacol Ther. 2008;27:932-939. [47] Oyagi S, Hirose M,Kojima M,et al.Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4 injured rats. J Hepatol. 2006;44(4):742-748. [48] 姚鹏,胡大荣,王帅,等.自体骨髓干细胞移植治疗慢性重症肝病临床研究[J].透析与人工器官,2006,17(1):18-21. [49] 李连达,吴理茂,刘红.自体骨髓干细胞移植研究进展[J].中国工程科学,2004,22(8):23-25. [50] Levicar N,Pal M,Habib NA. Long term clinical results of autologous infusion of mobilized adult bone marrow derived CD34 cells in patients with chronic liver disease. Cell Prolif. 2008;41:115-125. [51] Khan AA,Parveen N,Mahaboob VS, et al. Safety and efficacy of autologous bone marrow stem cell transplantation through hepatic artery for the treatment of chronic liver failure; a preliminary study. Transplant Proc. 2008;40:1140-1144. [52] Muraca M,Neri D,Parenti A,et al. Intraportal hepatocyte tran splantation in the pig:hcmodynamic and histopathological study. Transplantation. 2002;73(6):890-896. |
| [1] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [2] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [3] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [4] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [5] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [6] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [7] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [8] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [9] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [10] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [11] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [12] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [13] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [14] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [15] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||