Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (1): 248-259.doi: 10.12307/2025.565
Previous Articles Next Articles
Targeting diverse chimeric antigen receptor T cell-related targets in treatment of B-cell hematological malignancies: a review of long-term follow-up data
Xu Fanping, Li Qinchun, Tang Dongfang
- Hunan University of Science and Engineering, Yongzhou 425199, Hunan Province, China
-
Received:2024-09-07Accepted:2024-11-22Online:2026-01-08Published:2025-07-02 -
Contact:Tang Dongfang, PhD, Lecturer, Hunan University of Science and Engineering, Yongzhou 425199, Hunan Province, China -
About author:Xu Fanping, MS, Assistant experimentalist, Hunan University of Science and Engineering, Yongzhou 425199, Hunan Province, China -
Supported by:National Natural Science Foundation of China, No. 32101022 (to TDF); Hunan Natural Science Foundation, No. 2022JJ40158 (to TDF); Scientific Research Project of Hunan Provincial Education Department, No. 22A0572 (to TDF); Hunan Province Applied Characteristic Discipline Construction Project
CLC Number:
Cite this article
Xu Fanping, Li Qinchun, Tang Dongfang. Targeting diverse chimeric antigen receptor T cell-related targets in treatment of B-cell hematological malignancies: a review of long-term follow-up data[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 248-259.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
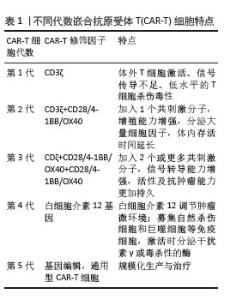
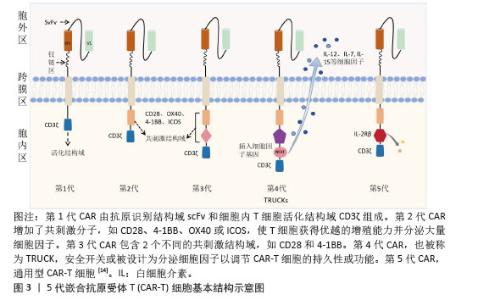
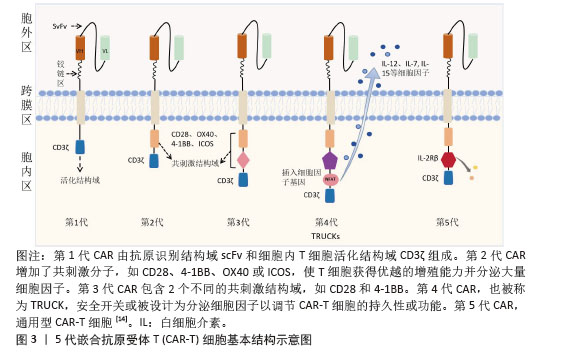
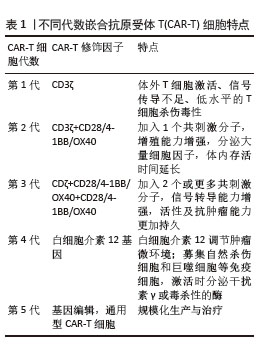
2.1 CAR-T细胞疗法 2.1.1 CAR-T细胞的组成结构及作用机制 CAR是一种跨膜嵌合蛋白,它结合T细胞形成可特异性识别和消除特定肿瘤细胞的CAR-T细胞,是CAR-T细胞的核心部件,通过反转录病毒或慢病毒转导,含胞外区、铰链区、跨膜区及胞内区4个结构域[2-3]。 各结构域具有特定的功能:①胞外区可由轻链和重链连接而成的单链可变片段(single chain fragment variable,scFv),能够特异性识别肿瘤相关抗原(tumor-associated antigens,TAAs)的单克隆抗体[4]。②铰链区是针对不同肿瘤相关抗原设计铰链区长度,以便结合靶抗原。③跨膜区选取CD3、CD8或CD28等跨膜受体蛋白作为细胞膜的锚点连接细胞内外,影响胞内区的激活信号传递,因此在活化T细胞中起重要作用。④胞内区是受体的功能端,由CD3ζ活化信号(包含免疫受体酪氨酸活化基序ITAM)和1个或1个以上的共刺激结构域[如CD28、CD134(OX40)、CD137(4-1BB)、ICOS]组成细胞内信号转导区域[2]。其中,CD28跨膜结构域作为最稳定的受体在临床中广泛应用。正常T细胞依赖T细胞受体识别抗原,T细胞受体与主要组织相容性复合体(major histocompatibility complex,MHC)分子提呈的肿瘤抗原短肽结合,通过信号通路产生级联反应活化为细胞毒性T淋巴细胞,特异性结合有相同MHC抗原肽的肿瘤细胞进而发挥细胞毒性作用[5]。而CAR-T细胞可以针对肿瘤细胞抗原构建特定的单克隆抗体,通过scFv特异性识别肿瘤特异性抗原直接将信号传到T细胞内,使T细胞活化并释放穿孔素/颗粒酶等细胞因子从而破坏肿瘤细胞[6],同时形成免疫记忆T细胞,发挥长效抗肿瘤作用。CAR-T细胞不受肿瘤MHC表达下调的限制性和抗原呈递细胞的局限,能用于治疗下调MHC Ⅰ类分子导致的免疫逃逸引起的肿瘤疾病,且CAR靶点范围较广,能识别包括蛋白质、脂类和糖类等多种物质[7-8]。 2.1.2 CAR-T发展历程及分类 按照使用的细胞种类,细胞治疗分为免疫细胞治疗和干细胞治疗。其中,过继性细胞免疫疗法是免疫细胞治疗的主要方向。目前经历3种形式发展,包括肿瘤浸润淋巴细胞(tumor infiltrating lymphocyte,TIL)疗法、T细胞受体基因工程改造的T(TCR-T)细胞疗法、CAR-T细胞疗法。TIL疗法需要从切除肿瘤中分离肿瘤浸润淋巴细胞,经体外进行扩增和培养后,再回输到体内[9]。TCR-T细胞疗法需要从外周血中分离出T细胞进行分子生物学改造,将能识别肿瘤特异性抗原的T细胞受体转导到T细胞中[10]。2022年1月美国食品药品监督管理局(Food and Drug Administration,FDA)批准全球首款治疗实体瘤(无法切除或转移性葡萄膜黑色素瘤)的TCR-T疗法产品Kimmtrak[11]。通过改造T细胞受体结构可以获得CAR-T细胞,CAR-T细胞具有靶向抗原精准识别的特点,表现出高特异性和强抗肿瘤疗效。CAR结构差异会影响T细胞活性、增殖和抗肿瘤持久性[12]。不同的CAR-T细胞共刺激结构域或胞内信号区域,代表着不同的CAR-T代数[13],目前已经发展到第5代,见表1。 "
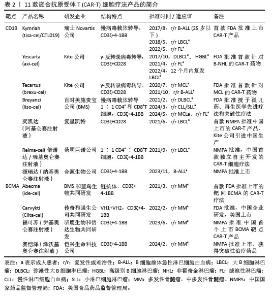
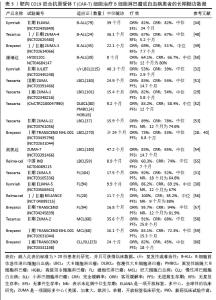
①第1代:CAR-T仅有1个激活结构域CD3ζ组成。由于缺少共刺激信号,CAR-T细胞在患者体内无法持续存在,对肿瘤无效。随着研究深入发现共刺激信号对于T细胞的活化至关重要,因而在胞内区增加1个或1个以上共刺激信号形成了第2代CAR-T细胞[15]。②第2代:CAR-T细胞具有1个激活结构域和1个共刺激结构域[16]。CAR-T细胞增强信号传递并产生大量的细胞因子,提高了效应T细胞增殖能力和抗肿瘤作用[17]。目前,临床普遍使用第2代CAR-T细胞,并取得了较好的临床疗效。为进一步提高T细胞的细胞活性和毒性,对T细胞的抗肿瘤信号通路深入研究,开发了第3代和第4代CAR-T细胞。③第3代:CAR-T细胞包含1个激活结构域和2个或更多的共刺激结构域的分子,进一步提升活化T细胞产生更多的细胞因子,发挥更持久的活性和抗肿瘤效力[18]。但研究发现,第3代CAR-T细胞的临床表现并未优于甚至劣于第2代CAR-T细胞。④第4代:第4代CAR-T细胞也称为TRUCK细胞,是在第2代CAR-T的基础上插入特定细胞因子的基因,如白细胞介素基因或表达自杀的基因:白细胞介素7、白细胞介素12、白细胞介素15、白细胞介素21和iCaspase-9等[19-21]。第4代CAR-T细胞可活化T细胞核因子的转录相应元件[22]。通过对致癌靶点的特异性识别来刺激活化T细胞的最小启动子,在肿瘤区域分泌相应的细胞因子白细胞介素12,进而调节肿瘤微环境,募集并活化巨噬细胞和自然杀伤细胞(NK细胞)等免疫细胞到肿瘤部位,通过激活内源性免疫反应更有效地消除肿瘤细胞,同时减少化疗等预处理[23]。到目前为止,第3代和第4代CAR-T细胞尚未有产品获批。⑤第5代CAR-T细胞目前处于早期阶段[24]。在第2代基础上构建通用型CAR-T细胞,通常采用BBIR CAR或SUPRA CAR设计以提高CAR靶向识别的灵活性[25]。也有通过敲除内源性T细胞受体和人类白细胞抗原Ⅰ类分子的设计思路,以降低异体移植的免疫排斥反应,避免异体T细胞对宿主器官的免疫攻击,从而增加CAR-T细胞的可控性。显然,CAR-T的改进是当下一个备受关注的领域,研究人员希望开发出更有效、更安全的免疫疗法。 2.1.3 CAR-T细胞上市产品 随着第2代CD19靶向CAR-T细胞国际临床试验的成功进行,免疫细胞治疗已成为癌症治疗的前沿领域。2017年,FDA批准了全球首款CAR-T细胞疗法产品——诺华的Kymriah上市[26-27]。截至目前,全球已经上市11款CAR-T细胞疗法产品(表2),其中经美国FDA共批准的有6款,经中国国家药品监督管理局(National Medical Products Administration,NMPA)批准上市的有5款。这些获批的产品均为2代自体CAR-T细胞,结构成熟、临床效果较突出。根据嵌合抗原受体的胞外区结构分为2类:①以CD19靶点的分别为Kymriah、Yescarta、Tecartus、Breyanzi、奕凯达、Relma-cel和源瑞达,其中5种用于治疗复发性或难治性大B细胞淋巴瘤(large B cell lymphoma,LBCL),4款用于治疗复发或难治性滤泡性淋巴瘤(follicular lymphoma,FL),2款用于治疗复发/难治性套细胞淋巴瘤(mantle cell lymphoma,MCL),2款用于治疗B细胞急性淋巴细胞白血病(B cell acute lymphoblastic leukaemia,B-ALL),1款用于治疗复发/难治性慢性淋巴细胞白血病/小淋巴细胞淋巴瘤(chronic lymphocytic leukemia/small lymphocytic leukemia,CLL/SLL),1款用于治疗B细胞前体急性淋巴细胞白血病的青少或儿童[28-32];②以BCMA靶点的分别为Abecma、Carvykti、福可苏和赛恺泽,用于治疗多发性骨髓瘤(multiple myeloma,MM)患者。 "
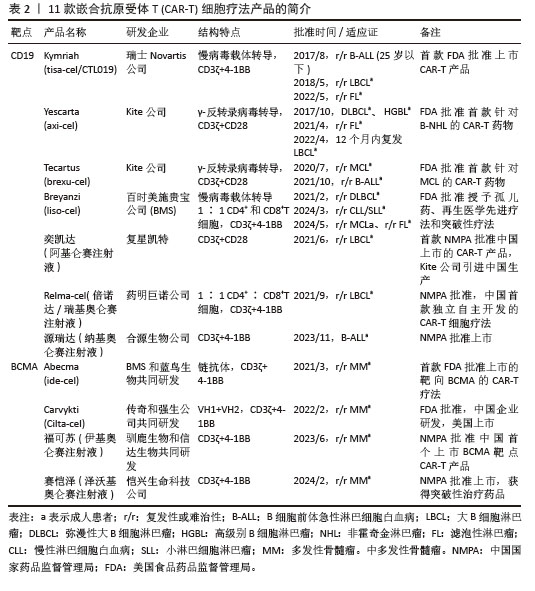
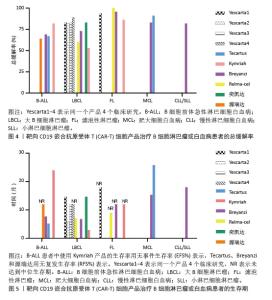
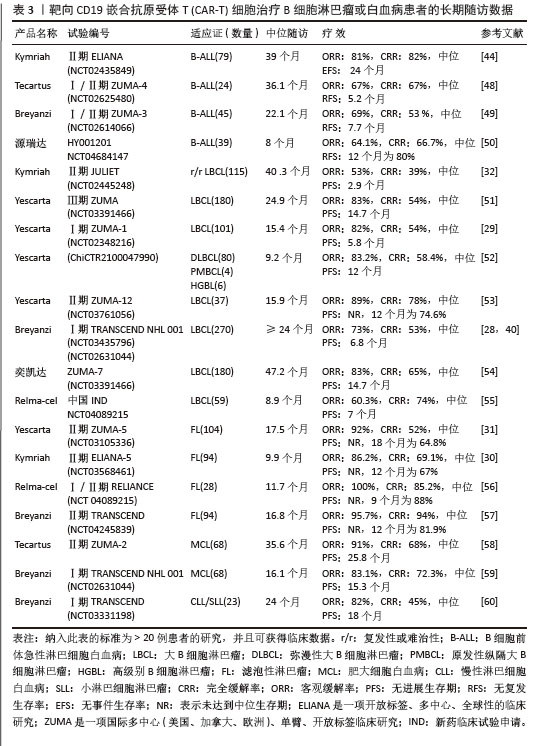
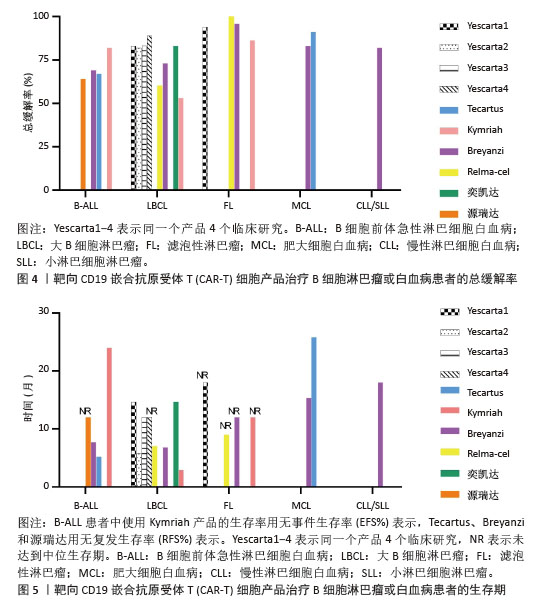
值得一提的是,除源瑞达CD19的CAR使用的是自主研发的scFv(HI19a)单链抗体外,其他产品CD19的CAR结构胞外域均使用鼠源FMC63单链抗体。 Kymriah用4-1BB作为共刺激结构域而不是CD28,其相关临床试验数据最多,可治疗疾病范围也最广。Kymriah和Yescarta使用慢病毒载体转导自体T细胞;而Yescarta和Tecartus的CAR基因使用γ-反转录病毒传递,因此针对的适应证有所不同。此外,Tecartus采用了XLP制造工艺进行T细胞筛选和淋巴细胞富集,去除了恶性细胞,其结构和Yescarta非常相像。与其他产品不同的是,Breyanzi和Relma-cel以1∶1 CD4+∶CD8+ T细胞组成递送。Abecma的CAR胞外结构域为鼠源11D5-3组成抗BCMA单链抗体。而Carvykti的CAR包含两种来源的单域抗体,这两种靶向BCMA产品的嵌合抗原受体基因都是通过慢病毒传递的。CAR-T细胞产品治疗血液恶性肿瘤具有得天独厚优势、发展迅速,临床上取得了显著的效果。国内众多企业都在积极参与,推动技术的创新和应用,为免疫细胞治疗的发展提供了强大的动力,也为患者带来了更多的治疗选择。 2.2 CAR-T细胞治疗血液系统恶性肿瘤的相关靶点 2.2.1 靶向CD19的CAR-T细胞疗法 CD19是Ⅰ型跨膜糖蛋白,属于Ig超家族,在B细胞表面发挥特异性信号转导的受体。CD19在多种B细胞恶性肿瘤表面表达,而不在其他正常组织上表达。由于CD19的表达程度高于CD20、CD22,因此CD19被认为是CAR-T治疗B细胞恶性肿瘤的首选靶点,也是血液系统恶性肿瘤相关抗原中研究最彻底的靶点[33]。目前,抗CD19 CAR-T的获批适应证已覆盖B-ALL(儿童、成人)和NHL当中的LBCL、FL、MCL,并正在积极探索其他亚型的适应证。靶向CD19的CAR-T细胞治疗的r/r B-ALL患者中可诱导83%-93%的应答率[34]。国际骨髓移植研究中心(Center for International Blood and Marrow Transplant Research,CIBMTR)分析了255例接受CAR-T治疗的B-ALL儿童患者,完全缓解率为85.5%,12个月无事件生存期(event-free survival,EFS)为52.4%[35]。BRUDNO等[36]在Ⅰ期临床试验中,20例B细胞淋巴瘤患者首次使用抗CD19-CAR(Hu19-CD828Z)T细胞,完全缓解率为55%,出现严重的神经毒性的患者仅有5%。WANG等[37]对23例r/r B-ALL患者在CAR-T治疗14 d后进行评估,缓解率为82%。MYERS等[38]在一项临床试验中,72例1-29岁r/r B-ALL患者接受人源化的抗CD19 CAR-T(huCART19)细胞治疗,73%的患者治疗后6个月仍检测到CAR-T细胞,12个月和24个月的无复发生存率分别为84%和74%,表现出了长期的疗效。目前的研究显示抗CD19 CAR-T细胞治疗r/r NHL患者效果良好,在一项涉及43例r/r B细胞淋巴瘤或CLL患者的研究中,58%的治疗患者获得了完全缓解,这部分完全缓解患者中有76%保持长期缓解,持续时间为43-113个月[39]。ABRAMSON等[40]评估了Breyanzi在269例r/r LBCL患者中的疗效,客观缓解率为73%,细胞因子释放综合征(cytokine release syndrome,CRS)≥3级为2%,患者得到长期的缓解率。而在ZUMA-1研究中,患者获得客观缓解率和完全缓解分别82%,54%[29]。2022年的一项研究中,抗CD19 CAR-T细胞治疗r/r FL的94例患者进行中位随访16.59个月,客观缓解率为86.2%,完全缓解率为69.1%,CRS为48.5%(等级≥3,0%),免疫效应细胞相关神经毒性综合征(immune effector cell-associated neurotoxicity syndrome,ICANS)为4.1%(等级≥3,1%),无治疗相关死亡事件[30],CAR-T细胞表现出高反应性和可控毒性,但CD19的下调或突变容易导致复发,仍然是该疗法面临的重大挑战[41-42]。2021年国际血液和骨髓移植研究中心数据显示,接受CAR-T治疗的451例患有r/r B-ALL的儿童/年轻人,中位随访21.5个月,客观缓解率为86.8%,12个月无复发生存率为62.5%,CRS≥3级为17.8%和发生ICANS为10%[43]。然而,在临床治疗中,需要更长时间的随访以充分了解药物的持续性。 目前,已知的接受靶向CD19的CAR-T细胞产品治疗的B细胞淋巴瘤或白血病患者长期预后的数据,包括17项研究提供已上市靶向CD19的CAR-T细胞产品的随访数据。在ELIANA、ZUMA、JULIET等国际研究中[39,44-46],均显示CD19 CAR-T细胞治疗B-ALL患者保持长期缓解,最长有5年之久。结合图4和图5可以看到,整体数据显示客观缓解率为53%-100%,并保持长期缓解。Yescarta及奕凯达治疗LBCL患者总缓解率较高,客观缓解率最高达到89%。治疗B-ALL的4款产品中Kymriah的缓解率最好,中位无事件生存期长达2年之久。治疗FL的4款产品均有较高缓解率,均达到86%以上,其中Relma-cel在3个月时的客观缓解率达到了100%,但随着时间推移会有所降低。治疗MCL以及CLL/SLL的产品缓解率均达到80%以上。半数以上的MCL患者在接受Tecartus产品治疗后2年仍处于缓解期,这表明了抗CD19 CAR-T细胞在B细胞恶性肿瘤治疗中具有长期有效的活性[47]。总的来说,这些结果表明大部分接受抗CD19 CAR-T细胞治疗的患者获得了较好缓解率,不需要进一步干预就能治愈疾病,见表3[28-32,40,44,48-60]。然而,抗原丢失或下调是肿瘤耐药性的常见原因,复发仍不可避免。因此,迫切需要开发更多的靶点和治疗模式以提高临床疗效。 2.2.2 靶向BCMA CAR-T细胞疗法 BCMA也称为CD269,是一种跨膜糖蛋白,是肿瘤坏死因子受体超家族的成员,表达于成熟的B淋巴细胞,较少表达于造血干细胞和正常组织中。BCMA的过度表达和激活与MM的进展相关[61],这使得BCMA成为MM重要治疗靶点。近5年来,抗BCMA CAR-T细胞的临床试验数量稳步增长。RAJE等[62]对33例r/r MM患者输注bb2121细胞,完全缓解率和客观缓解率为85%,45%,23例患者出现CRS(≥3级,6%)。16例有副反应的患者的微小残留病变评估均为阴性,证实了抗BCMA的CAR-T细胞治疗MM的抗肿瘤活性。另一项研究中128例接受Abecma治疗,客观缓解率为97%,完全缓解率为67%,仅有5%的患者发生≥3级的CRS[63],因此Abecma治疗MM得到较高的缓解率。针对成熟浆细胞上缺乏BCMA可能会限制疗效和复发的问题,KANG等[64]设计了新型tan-CAR,胞外区由scFv-BCMA和scFv-CD19串联,可同时表达靶向BCMA和CD19。双靶CAR-T细胞在体内外对CD19和 BCMA抗原阳性肿瘤细胞均有显著的抗肿瘤作用。不同靶向抗原的联合应用有助于降低复发率,为CAR-T细胞治疗提供新的策略。 "
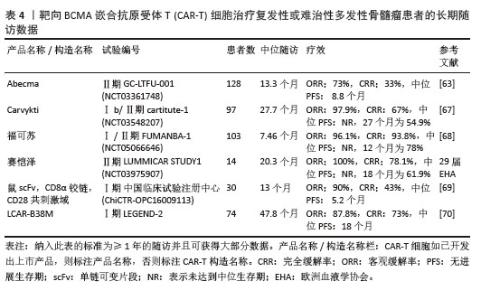
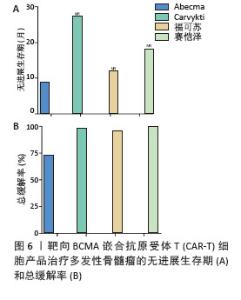
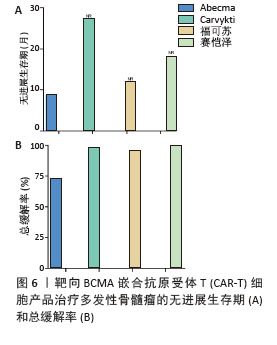
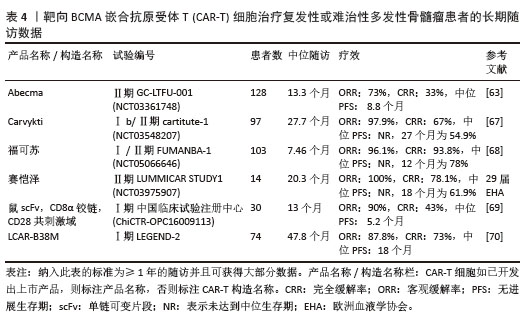
2023年,根据美国临床肿瘤学术年会(American Society of Clinical Oncology,ASCO)报告,伊基奥仑赛注射液治疗r/r MM患者增加至103例的Ⅰ/Ⅱ期临床试验研究,客观缓解率为96.1%,完全缓解率为74.3%。12个月的无进展生存率为78.8%,仅出现1例≥3级CRS,无≥3级ICANS[65]。这些患者发生的毒副作用得到了较好的处理,获得良好的长期疗效。与CD19靶向CAR-T细胞相比,由于这些结构的发展较晚,接受BCMA靶向CAR-T细胞治疗r/r MM患者的长期效果相关数据较少。目前共有6项研究,见表4[63,67-70],患者的客观缓解率从73%-100%不等。结合图6,可以看到上市产品中Abecma、Carvykti、福可苏的总缓解较高。Carvykti的中位无进展生存期达到27个月,半数以上的患者得到了长期的疗效。大部分患者达到完全缓解或更好,随着时间的推移无进展生存期有下降的趋势。这些数据表明抗BCMA CAR-T细胞治疗对r/r MM表现出持久的反应[66],但治疗后BCMA表达下调、复发以及耐药的机制仍然不明确,需进一步研究。"
| [1] PORTER DL, LEVINE BL, KALOS M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725-733. [2] LIU J, ZHONG JF, ZHANG X, et al. Allogeneic CD19-CAR-T cell infusion after allogeneic hematopoietic stem cell transplantation in B cell malignancies. J Hematol Oncol. 2017;10(1):35. [3] TIMMERS M, ROEX G, WANG Y, et al. Chimeric Antigen Receptor-Modified T Cell Therapy in Multiple Myeloma: Beyond B Cell Maturation Antigen. Front Immunol. 2019;10:1613. [4] YOON S, EOM GH. Chimeric Antigen Receptor T Cell Therapy: A Novel Modality for Immune Modulation. Chonnam Med J. 2020;56(1):6-11. [5] TI D, NIU Y, WU Z, et al. Genetic engineering of T cells with chimeric antigen receptors for hematological malignancy immunotherapy. Sci China Life Sci. 2018;61(11):1320-1332. [6] ZHANG C, LIU J, ZHONG JF, et al. Engineering CAR-T cells. Biomark Res. 2017;5:22. [7] GELDRES C, SAVOLDO B, DOTTI G. Chimeric antigen receptor-redirected T cells return to the bench. Semin Immunol. 2016;28(1):3-9. [8] HOLZINGER A, ABKEN H. CAR T Cells: A Snapshot on the Growing Options to Design a CAR. Hemasphere. 2019;3(1):e172. [9] MATSUEDA S, CHEN L, LI H, et al. Recent clinical researches and technological development in TIL therapy. Cancer Immunol Immunother. 2024;73(11):232. [10] MORGAN RA, DUDLEY ME, WUNDERLICH JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126-129. [11] NATHAN P, HASSEL JC, RUTKOWSKI P, et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N Engl J Med. 2021; 385(13):1196-1206. [12] SALVARIS R, FEDELE PL. Targeted Therapy in Acute Lymphoblastic Leukaemia. J Pers Med. 2021;11(8):715. [13] ZHAO Z, CHEN Y, FRANCISCO NM, et al. The application of CAR-T cell therapy in hematological malignancies: advantages and challenges. Acta Pharm Sin B. 2018;8(4): 539-551. [14] ZHANG X, ZHU L, ZHANG H, et al. CAR-T Cell Therapy in Hematological Malignancies: Current Opportunities and Challenges. Front Immunol. 2022;13:927153. [15] BAKER DJ, ARANY Z, BAUR JA, et al. CAR T therapy beyond cancer: the evolution of a living drug. Nature. 2023;619(7971):707-715. [16] QU C, ZHANG H, CAO H, et al. Tumor buster - where will the CAR-T cell therapy ‘missile’ go? Mol Cancer. 2022;21(1):201. [17] HU Y, SUN J, WU Z, et al. Predominant cerebral cytokine release syndrome in CD19-directed chimeric antigen receptor-modified T cell therapy. J Hematol Oncol. 2016;9(1):70. [18] ROSELLI E, BOUCHER JC, LI G, et al. 4-1BB and optimized CD28 co-stimulation enhances function of human mono-specific and bi-specific third-generation CAR T cells. J Immunother Cancer. 2021;9(10):e003354. [19] LANITIS E, ROTA G, KOSTI P, et al. Optimized gene engineering of murine CAR-T cells reveals the beneficial effects of IL-15 coexpression. J Exp Med. 2021;218(2):e20192203. [20] DUAN D, WANG K, WEI C, et al. The BCMA-Targeted Fourth-Generation CAR-T Cells Secreting IL-7 and CCL19 for Therapy of Refractory/Recurrent Multiple Myeloma. Front Immunol. 2021;12:609421. [21] HUANG R, LI X, HE Y, et al. Recent advances in CAR-T cell engineering. J Hematol Oncol. 2020; 13(1):86. [22] ROSELLI E, FRIELING JS, THORNER K, et al. CAR-T Engineering: Optimizing Signal Transduction and Effector Mechanisms. BioDrugs. 2019;33(6):647-659. [23] LIU E, MARIN D, BANERJEE P, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med. 2020;382(6): 545-553. [24] TOKAREW N, OGONEK J, ENDRES S, et al. Teaching an old dog new tricks: next-generation CAR T cells. Br J Cancer. 2019;120(1):26-37. [25] CHO JH, COLLINS JJ, WONG WW. Universal Chimeric Antigen Receptors for Multiplexed and Logical Control of T Cell Responses. Cell. 2018;173(6):1426-1438.e11. [26] BRENTJENS RJ, DAVILA ML, RIVIERE I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. [27] MULLARD A. FDA approves first CAR T therapy. Nat Rev Drug Discov. 2017;16(10):669. [28] ABRAMSON JS, PALOMBA ML, GORDON LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852. [29] NEELAPU SS, LOCKE FL, BARTLETT NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531-2544. [30] FOWLER NH, DICKINSON M, DREYLING M, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28(2):325-332. [31] JACOBSON CA, CHAVEZ JC, SEHGAL AR, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(1):91-103. [32] SCHUSTER SJ, TAM CS, BORCHMANN P, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(10): 1403-1415. [33] YAMADA S, KANEKO MK, SAYAMA Y, et al. Development of Novel Mouse Monoclonal Antibodies Against Human CD19. Monoclon Antib Immunodiagn Immunother. 2020;39(2): 45-50. [34] TU S, HUANG R, GUO Z, et al. Shortening the ex vivo culture of CD19-specific CAR T-cells retains potent efficacy against acute lymphoblastic leukemia without CAR T-cell-related encephalopathy syndrome or severe cytokine release syndrome. Am J Hematol. 2019;94(12): E322-E325. [35] PASQUINI MC, HU ZH, CURRAN K, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020;4(21):5414-5424. [36] BRUDNO JN, LAM N, VANASSE D, et al. Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat Med. 2020;26(2): 270-280. [37] WANG J, MOU N, YANG Z, et al. Efficacy and safety of humanized anti-CD19-CAR-T therapy following intensive lymphodepleting chemotherapy for refractory/relapsed B acute lymphoblastic leukaemia. Br J Haematol. 2020;191(2):212-222. [38] MYERS RM, LI Y, BARZ LEAHY A, et al. Humanized CD19-Targeted Chimeric Antigen Receptor (CAR) T Cells in CAR-Naive and CAR-Exposed Children and Young Adults With Relapsed or Refractory Acute Lymphoblastic Leukemia. J Clin Oncol. 2021;39(27): 3044-3055. [39] CAPPELL KM, SHERRY RM, YANG JC, et al. Long-Term Follow-Up of Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy. J Clin Oncol. 2020;38(32): 3805-3815. [40] ABRAMSON JS, PALOMBA ML, GORDON LI, et al. Two-Year Follow-up of Transcend NHL 001, a Multicenter Phase 1 Study of Lisocabtagene Maraleucel (liso-cel) in Relapsed or Refractory (R/R) Large B-Cell Lymphomas (LBCL). Blood. 2021; 138(Supplement 1):2840-2843. [41] MAJZNER RG, MACKALL CL. Tumor Antigen Escape from CAR T-cell Therapy. Cancer Discov. 2018;8(10):1219-1226. [42] PARK JH, RIVIÈRE I, GONEN M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018; 378(5):449-459. [43] JOHN S, PULSIPHER MA, MOSKOP A, et al. Real-World Outcomes for Pediatric and Young Adult Patients with Relapsed or Refractory (R/R) B-Cell Acute Lymphoblastic Leukemia (ALL) Treated with Tisagenlecleucel: Update from the Center for International Blood and Marrow Transplant Research (CIBMTR) Registry. Blood. 2021;138(Supplement 1):428. [44] LAETSCH TW, MAUDE SL, RIVES S, et al. Three-Year Update of Tisagenlecleucel in Pediatric and Young Adult Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia in the ELIANA Trial. J Clin Oncol. 2023;41(9):1664-1669. [45] SHAH SD, GHOBADI A, OLUWOLE OO, et al. Two-year follow-up of KTE-X19, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, in adult patients (Pts) with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL) in ZUMA-3. J Hematol Oncol. 2022; 40(16):256-257. [46] CHONG EA, RUELLA M, SCHUSTER SJ, et al. Five-Year Outcomes for Refractory B-Cell Lymphomas with CAR T-Cell Therapy. N Engl J Med. 2021; 384(7):673-674. [47] LOCKE FL, GHOBADI A, JACOBSON CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31-42. [48] WAYNE AS, HUYNH V, HIJIYA N, et al. Three-year results from phase I of ZUMA-4: KTE-X19 in pediatric relapsed/refractory acute lymphoblastic leukemia. Haematologica. 2023;108(3):747-760. [49] SHAH BD, GHOBADI A, OLUWOLE OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021; 398(10299):491-502. [50] 中国临床肿瘤学会(CSCO)白血病专家委员会,中国医师协会血液科医师分会,中华医学会血液学分会. 纳基奥仑赛注射液临床应用指导原则(2023年版)[J].白血病·淋巴瘤,2024,33(1):1-11. [51] LOCKE FL, MIKLOS DB, JACOBSON CA, et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N Engl J Med. 2022; 386(7):640-654. [52] ZHAO WL, LI YH, ZOU DH, et al. Efficacy and safety of Axicabtagene ciloleucel (Axi‐cel) for the treatment of relapse/refractory non‐Hodgkin lymphoma: First real‐world data in Chinese population. Hematol Oncol. 2023;41(S2):457-458. [53] NEELAPU SS, DICKINSON M, MUNOZ J, et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat Med. 2022;28(4):735-742. [54] WESTIN JR, OLUWOLE OO, KERSTEN MJ, et al. Survival with Axicabtagene Ciloleucel in Large B-Cell Lymphoma. N Engl J Med. 2023;389(2):148-157. [55] YING Z, YANG H, GUO Y, et al. Relmacabtagene autoleucel (relma-cel) CD19 CAR-T therapy for adults with heavily pretreated relapsed/refractory large B-cell lymphoma in China. Cancer Med. 2021;10(3):999-1011. [56] YING Z, ZOU D, YANG H, et al. Preliminary efficacy and safety of Relmacabtagene autoleucel (Carteyva) in adults with relapsed/refractory follicular lymphoma in China: A phase I/II clinical trial. Am J Hematol. 2022;97(12):E436-E438. [57] UNITED STATES FOOD AND DRUG ADMINISTRATION. FDA approves lisocabtagene maraleucel for relapsed or refractory mantle cell lymphoma.(2024-05-15) https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lisocabtagene-maraleucel-relapsed-or-refractory-mantle-cell-lymphoma. [58] WANG M, MUNOZ J, GOY A, et al. Three-Year Follow-Up of KTE-X19 in Patients With Relapsed/Refractory Mantle Cell Lymphoma, Including High-Risk Subgroups, in the ZUMA-2 Study. J Clin Oncol. 2023;41(3):555-567. [59] PHILLIPS TJ, MARTIN A, LEE AD, et al. Estimating the health care costs associated with receipt of lisocabtagene maraleucel: Insights from adults with mantle cell lymphoma (TRANSCEND NHL 001). J Clin Oncol. 2024; 42(16_suppl):7028-7028. [60] SIDDIQI T, SOUMERAI JD, DORRITIE KA, et al. Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood. 2022;139(12):1794-1806. [61] SHAH N, CHARI A, SCOTT E, et al. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia. 2020;34(4):985-1005. [62] RAJE N, BERDEJA J, LIN Y, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2019;380(18):1726-1737. [63] MUNSHI NC, ANDERSON LD JR, SHAH N, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N Engl J Med. 2021;384(8):705-716. [64] KANG L, ZHANG J, LI M, et al. Characterization of novel dual tandem CD19/BCMA chimeric antigen receptor T cells to potentially treat multiple myeloma. Biomark Res. 2020;8:14. [65] LI C, WANG D, SONG Y, et al. A Phase 1/2 Study of a Novel Fully Human B-Cell Maturation Antigen-Specific CAR T Cells (CT103A) in Patients with Relapsed and/or Refractory Multiple Myeloma. Blood. 2021; 138(Supplement 1):547. [66] SAMUR MK, FULCINITI M, AKTAS SAMUR A, et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat Commun. 2021; 12(1):868. [67] MARTIN T, USMANI SZ, BERDEJA JG, et al. Ciltacabtagene Autoleucel, an Anti-B-cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up. J Clin Oncol. 2023;41(6): 1265-1274. [68] 中国国家药品监督管理局.国家药监局附条件批准伊基奥仑赛注射液上市[EB/OL]. (2023-06-30) https://www.nmpa.gov.cn/directory/web/nmpa/zhuanti/ypqxgg/gggzjzh/20230630195006116.html. [69] LI C, CAO W, QUE Y, et al. A phase I study of anti-BCMA CAR T cell therapy in relapsed/refractory multiple myeloma and plasma cell leukemia. Clin Transl Med. 2021;11(3):e346. [70] ZHAO WH, WANG BY, CHEN LJ, et al. Four-year follow-up of LCAR-B38M in relapsed or refractory multiple myeloma: a phase 1, single-arm, open-label, multicenter study in China (LEGEND-2). J Hematol Oncol. 2022; 15(1):86. [71] RUELLA M, MAUS MV. Catch me if you can: Leukemia Escape after CD19-Directed T Cell Immunotherapies. Comput Struct Biotechnol J. 2016;14:357-362. [72] PIERCE JM, MEHTA A. Diagnostic, prognostic and therapeutic role of CD30 in lymphoma. Expert Rev Hematol. 2017;10(1):29-37. [73] CUI W, ZHANG X, DAI H, et al. Tandem CD19/CD22 Dual Targets CAR T-Cells Bridging Hematopoietic Stem Cells Transplantation Acquires Robust Remission for Relapsed and Refractory B Acute Lymphoblastic Leukemia Patients. Blood. 2021;138(Supplement 1): 1753. [74] CUI W, ZHANG X, DAI H, et al. Tandem CD19/CD22 Dual Targets CAR-T Cells Therapy Acquires Superior CR Rate Than CD19 CAR-T Cells: A Case Controlled Study. Blood. 2020;136(Supplement 1):44. [75] ZHANG WY, LIU Y, WANG Y, et al. Long-term safety and efficacy of CART-20 cells in patients with refractory or relapsed B-cell non-Hodgkin lymphoma: 5-years follow-up results of the phase I and IIa trials. Signal Transduct Target Ther. 2017;2:17054. [76] SANG W, SHI M, YANG J, et al. Phase II trial of co-administration of CD19- and CD20-targeted chimeric antigen receptor T cells for relapsed and refractory diffuse large B cell lymphoma. Cancer Med. 2020;9(16):5827-5838. [77] PAN J, NIU Q, DENG B, et al. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia. 2019;33(12): 2854-2866. [78] WANG N, HU X, CAO W, et al. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood. 2020;135(1):17-27. [79] YAN LE, ZHANG H, WADA M, et al. Targeting Two Antigens Associated with B-ALL with CD19-CD123 Compound Car T Cell Therapy. Stem Cell Rev Rep. 2020;16(2):385-396. [80] SUN C, MAHENDRAVADA A, BALLARD B, et al. Safety and efficacy of targeting CD138 with a chimeric antigen receptor for the treatment of multiple myeloma. Oncotarget. 2019;10(24):2369-2383. [81] VAN DE DONK NW, JANMAAT ML, MUTIS T, et al. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016;270(1):95-112. [82] MEI H, LI C, JIANG H, et al. A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. J Hematol Oncol. 2021;14(1):161. [83] TANG Y, YIN H, ZHAO X, et al. High efficacy and safety of CD38 and BCMA bispecific CAR-T in relapsed or refractory multiple myeloma. J Exp Clin Cancer Res. 2022;41(1):2. [84] RADHAKRISHNAN SV, LUETKENS T, SCHERER SD, et al. CD229 CAR T cells eliminate multiple myeloma and tumor propagating cells without fratricide. Nat Commun. 2020; 11(1):798. [85] BRUDNO JN, KOCHENDERFER JN. Current understanding and management of CAR T cell-associated toxicities. Nat Rev Clin Oncol. 2024; 21(7):501-521. [86] PORTER DL, HWANG WT, FREY NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. [87] NEELAPU SS, TUMMALA S, KEBRIAEI P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47-62. [88] LEE DW, SANTOMASSO BD, LOCKE FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4): 625-638. [89] BULLOCK TNJ. CD40 stimulation as a molecular adjuvant for cancer vaccines and other immunotherapies. Cell Mol Immunol. 2022;19(1):14-22. [90] RIEGLER LL, JONES GP, LEE DW. Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Ther Clin Risk Manag. 2019;15:323-335. [91] JIANG H, LIU L, GUO T, et al. Improving the safety of CAR-T cell therapy by controlling CRS-related coagulopathy. Ann Hematol. 2019;98(7):1721-1732. [92] WANG Y, QI K, CHENG H, et al. Coagulation Disorders after Chimeric Antigen Receptor T Cell Therapy: Analysis of 100 Patients with Relapsed and Refractory Hematologic Malignancies. Biol Blood Marrow Transplant. 2020;26(5):865-875. [93] DONG R, JIANG S, CHEN Y, et al. Prognostic Significance of Cytokine Release Syndrome in B Cell Hematological Malignancies Patients After Chimeric Antigen Receptor T Cell Therapy. J Interferon Cytokine Res. 2021;41(12):469-476. [94] LIU Y, CHEN X, WANG D, et al. Hemofiltration Successfully Eliminates Severe Cytokine Release Syndrome Following CD19 CAR-T-Cell Therapy. J Immunother. 2018;41(9):406-410. [95] TORRE M, SOLOMON IH, SUTHERLAND CL, et al. Neuropathology of a Case With Fatal CAR T-Cell-Associated Cerebral Edema. J Neuropathol Exp Neurol. 2018;77(10): 877-882. [96] WANG Z, HAN W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res. 2018;6:4. [97] DENG Q, HAN G, PUEBLA-OSORIO N, et al. Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat Med. 2020;26(12):1878-1887. [98] MORRIS EC, NEELAPU SS, GIAVRIDIS T, et al. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022;22(2):85-96. [99] HUNTER BD, JACOBSON CA. CAR T-Cell Associated Neurotoxicity: Mechanisms, Clinicopathologic Correlates, and Future Directions. J Natl Cancer Inst. 2019;111(7): 646-654. [100] SCHUBERT ML, SCHMITT M, WANG L, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32(1):34-48. [101] FLUGEL CL, MAJZNER RG, KRENCIUTE G, et al. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat Rev Clin Oncol. 2023;20(1):49-62. [102] PARKER KR, MIGLIORINI D, PERKEY E, et al. Single-Cell Analyses Identify Brain Mural Cells Expressing CD19 as Potential Off-Tumor Targets for CAR-T Immunotherapies. Cell. 2020;183(1): 126-142.e17. [103] GANATRA S, REDD R, HAYEK SS, et al. Chimeric Antigen Receptor T-Cell Therapy-Associated Cardiomyopathy in Patients With Refractory or Relapsed Non-Hodgkin Lymphoma. Circulation. 2020;142(17):1687-1690. [104] WUDHIKARN K, PENNISI M, GARCIA-RECIO M, et al. DLBCL patients treated with CD19 CAR T cells experience a high burden of organ toxicities but low nonrelapse mortality. Blood Adv. 2020;4(13):3024-3033. [105] VAN OEKELEN O, ALEMAN A, UPADHYAYA B, et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat Med. 2021;27(12):2099-2103. [106] STRATI P, AHMED S, FURQAN F, et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood. 2021;137(23): 3272-3276. [107] RODDIE C, DIAS J, O’REILLY MA, et al. Durable Responses and Low Toxicity After Fast Off-Rate CD19 Chimeric Antigen Receptor-T Therapy in Adults With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia. J Clin Oncol. 2021;39(30):3352-3363. [108] FREY NV, SHAW PA, HEXNER EO, et al. Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults With Acute Lymphoblastic Leukemia. J Clin Oncol. 2020;38(5):415-422. [109] LE RQ, LI L, YUAN W, et al. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist. 2018;23(8):943-947. [110] LIU S, DENG B, YIN Z, et al. Corticosteroids do not influence the efficacy and kinetics of CAR-T cells for B-cell acute lymphoblastic leukemia. Blood Cancer J. 2020;10(2):15. [111] GARDNER RA, CEPPI F, RIVERS J, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood. 2019;134(24):2149-2158. [112] PARK JH, SAUTER CS, PALOMBA ML, et al. A Phase II Study of Prophylactic Anakinra to Prevent CRS and Neurotoxicity in Patients Receiving CD19 CAR T Cell Therapy for Relapsed or Refractory Lymphoma. Blood. 2021;138(Supplement 1):96. [113] AUTIO KA, BONI V, HUMPHREY RW, et al. Probody Therapeutics: An Emerging Class of Therapies Designed to Enhance On-Target Effects with Reduced Off-Tumor Toxicity for Use in Immuno-Oncology. Clin Cancer Res. 2020;26(5):984-989. [114] LI X, ZHOU J, ZHANG W, et al. Pan-Cancer Analysis Identifies Tumor Cell Surface Targets for CAR-T Cell Therapies and Antibody Drug Conjugates. Cancers (Basel). 2022;14(22):5674. [115] SESQUES P, KIRKWOOD AA, KWON M, et al. Novel prognostic scoring systems for severe CRS and ICANS after anti-CD19 CAR T cells in large B-cell lymphoma. J Hematol Oncol. 2024;17(1):61. [116] GRITTI G, BELOUSOV A, RELF J, et al. Predictive model for the risk of cytokine release syndrome with glofitamab treatment for diffuse large B-cell lymphoma. Blood Adv. 2024;8(14):3615-3618. [117] WANG Z, WU Z, LIU Y, et al. New development in CAR-T cell therapy. J Hematol Oncol. 2017; 10(1):53. [118] HAY KA, GAUTHIER J, HIRAYAMA AV, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019; 133(15):1652-1663. [119] MAUDE SL, LAETSCH TW, BUECHNER J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439-448. [120] TURTLE CJ, HANAFI LA, BERGER C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016; 126(6):2123-2138. [121] ALI SA, SHI V, MARIC I, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688-1700. [122] TONG C, ZHANG Y, LIU Y, et al. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B-cell lymphoma. Blood. 2020;136(14):1632-1644. [123] ZANETTI SR, VELASCO-HERNANDEZ T, GUTIERREZ-AGÜERA F, et al. A novel and efficient tandem CD19- and CD22-directed CAR for B cell ALL. Mol Ther. 2022; 30(2):550-563. [124] DAI H, WU Z, JIA H, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13(1):30. [125] FOUSEK K, WATANABE J, JOSEPH SK, et al. CAR T-cells that target acute B-lineage leukemia irrespective of CD19 expression. Leukemia. 2021;35(1):75-89. [126] SCHNEIDER D, XIONG Y, WU D, et al. Trispecific CD19-CD20-CD22-targeting duoCAR-T cells eliminate antigen-heterogeneous B cell tumors in preclinical models. Sci Transl Med. 2021; 13(586):eabc6401. [127] GAUTHIER J, BEZERRA ED, HIRAYAMA AV, et al. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B-cell malignancies. Blood. 2021; 137(3):323-335. [128] JAIN N, KANTARJIAN H, SOLOMON SR, et al. Preliminary Safety and Efficacy of PBCAR0191, an Allogeneic ‘Off-the-Shelf’ CD19-Directed CAR-T for Patients with Relapsed/Refractory (R/R) CD19+ B-ALL. Blood. 2021; 138(Supplement 1):650. [129] ZHANG Y, CHEN H, SONG Y, et al. Chimeric antigens receptor T cell therapy as a bridge to haematopoietic stem cell transplantation for refractory/ relapsed B-cell acute lymphomalastic leukemia. Br J Haematol. 2020;189(1):146-152. [130] HU Y, ZHANG M, YANG T, et al. Sequential CD7 CAR T-Cell Therapy and Allogeneic HSCT without GVHD Prophylaxis. N Engl J Med. 2024; 390(16):1467-1480. [131] PONT MJ, HILL T, COLE GO, et al. γ-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood. 2019;134(19): 1585-1597. |
| [1] | Yu Weijie, Cao Dongdong, Guo Tianci, Niu Puyu, Yang Jialin, Wang Simin, Liu Aifeng. Risk prediction models of recurrence after percutaneous endoscopic lumbar discectomy: a systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 749-759. |
| [2] | Xue Hui, Li Dongnan, Zhao Yadi, Chen Chao, Xie Zongyuan. Relationship between BCR/ABL gene expression and recurrence before and after allogeneic transplantation in Ph chromosome positive acute lymphoblastic leukemia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(1): 139-144. |
| [3] | Jing Ruyi, Chen Yingxin, Cao Lei . Prognosis of deep lamellar keratoplasty versus penetrating keratoplasty in the treatment of stromal corneal dystrophy [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1626-1633. |
| [4] | Wang Jianxu, Dong Zihao, Huang Zishuai, Li Siying, Yang Guang. Interaction between immune microenvironment and bone aging and treatment strategies [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6509-6519. |
| [5] | Wu Lijuan, Wang Zhenfei, Tan Xiaohui, Wu Yingcai, Zheng Yanling, Dai Fengxue. Effect of extracellular matrix stiffness on tumor progression and treatment strategies [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(20): 4286-4294. |
| [6] | Liu Jiwei, Liu Weici, Mao Wenjun. Research, development and advance in precise screening of lung cancer drugs with tumor organoids [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 5078-5084. |
| [7] | Wang Huida, Sun Xiaotong, Bi Lan, Wang Zixuan, Zhang Ronghe. Effect of astragalus polysaccharides on orthodontic bone remodeling [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(32): 5214-5218. |
| [8] | Gan Tian, Wang Wenyuan, Yan Shujin, Hao Lan, Ran Haitao, Wang Zhigang, Xia Jizhu. Near infrared photoresponsive nanoparticles loaded with LXR agonists for photothermal immunotherapy [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(12): 1863-1869. |
| [9] | Ci Wentao, Zhang Xinlong, Yan Shi, Wang Zhao. Reducing the recurrence of infection after the application of Masquelet technique for osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(28): 4546-4552. |
| [10] | Cai Shengsheng, Mei Heng, Zhang Xuequan, Deng Jin, Cao Jun, He Bin. Prepared HPe6DF composite nanoparticles enhance the effect of photodynamic therapy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1566-1573. |
| [11] | Wang Yanjiao, Wang Rui, Sun Luning. Bankart pepair versus Bristow-Latarjet procedure for recurrent anterior instability of the shoulder: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(21): 3423-3430. |
| [12] | Tian Lin, Shi Xiaoqing, Mao Jun, Zhang Nongshan, Zhang Li, Xing Runlin, Wang Peimin. Meta-analysis of vacuum-sealing drainage combined with antibiotic bone cement in the treatment of chronic osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2618-2624. |
| [13] | Qian Yuzhang, Wang Nan, Dong Yuqi, Xie Lin, Kang Ran. Factors for the recurrence of lumbar disc herniation after percutaneous transforaminal endoscopic discectomy: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(36): 5886-5896. |
| [14] | Wang Jinchun, Liu Huiying, Cao Yunpeng. tau protein and Alzheimer’s disease [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(17): 2775-2781. |
| [15] | Liu Zhiling, Gao Minghong, Chen Yingxin. Bio-engineering cornea versus human donor cornea in the treatment of fungal corneal ulcer [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(10): 1563-1569. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||