Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (11): 2201-2209.doi: 10.12307/2025.368
Regulatory mechanism of urolithin B in osteoclastic differentiation of bone marrow-derived macrophages
Luo Xi, Chen Jianquan
- Institute of Orthopaedic Surgery, Suzhou Medical College, Soochow University, Suzhou 215000, Jiangsu Province, China
-
Received:2024-03-23Accepted:2024-05-21Online:2025-04-18Published:2024-08-10 -
Contact:Chen Jianquan, Professor, Doctoral supervisor, Institute of Orthopaedic Surgery, Suzhou Medical College, Soochow University, Suzhou 215000, Jiangsu Province, China -
About author:Luo Xi, Master candidate, Institute of Orthopaedic Surgery, Suzhou Medical College, Soochow University, Suzhou 215000, Jiangsu Province, China -
Supported by:the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPA) (to CJQ [project participant])
CLC Number:
Cite this article
Luo Xi, Chen Jianquan. Regulatory mechanism of urolithin B in osteoclastic differentiation of bone marrow-derived macrophages[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(11): 2201-2209.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
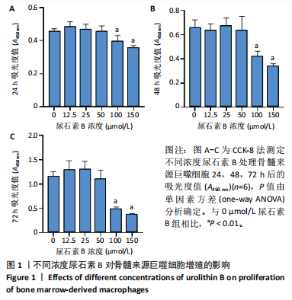
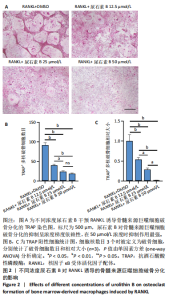
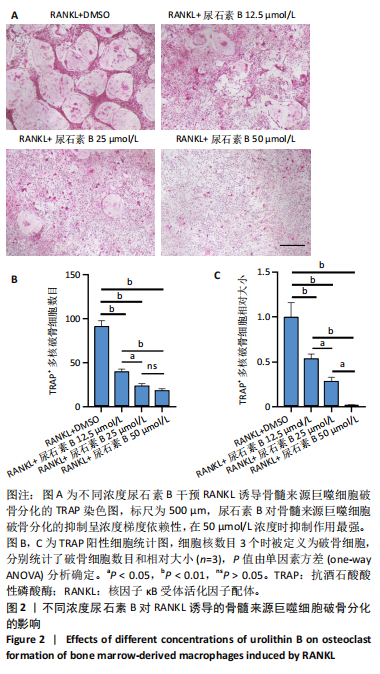
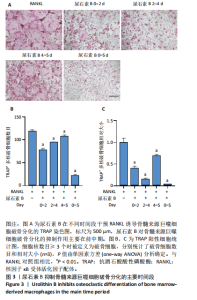
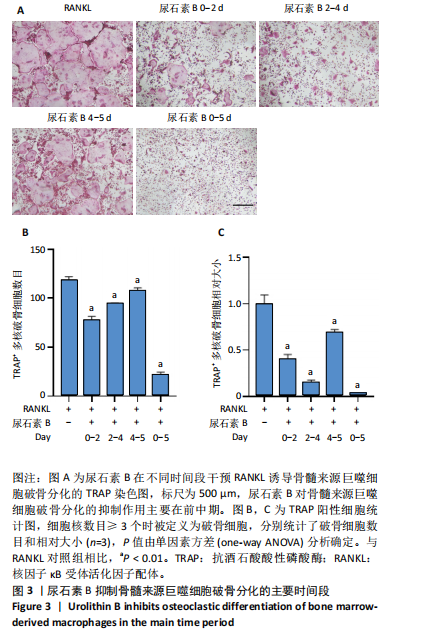
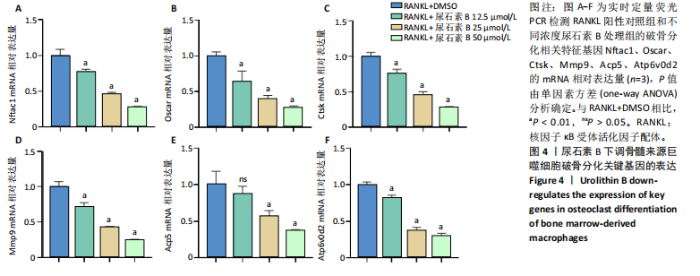
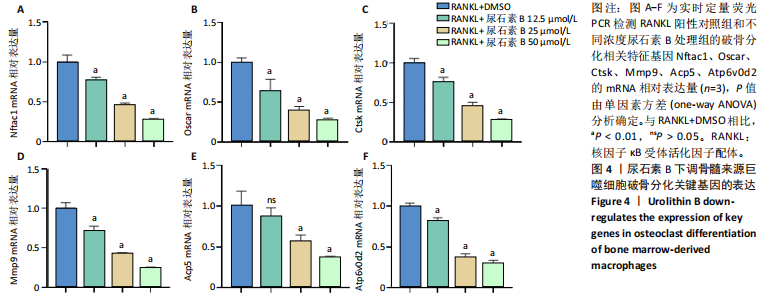
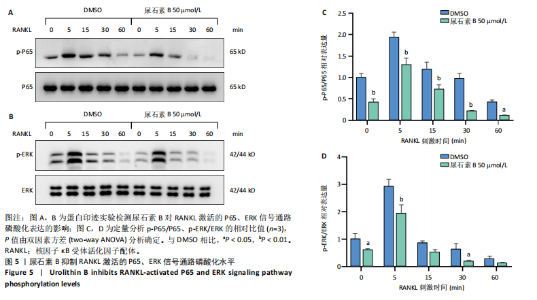
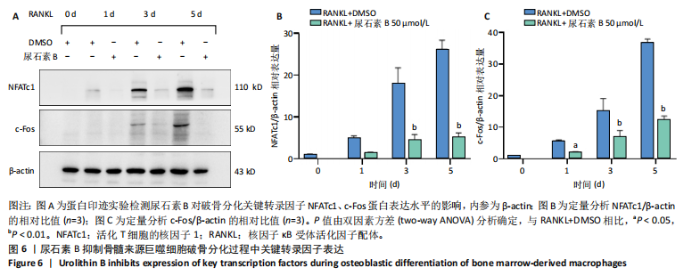
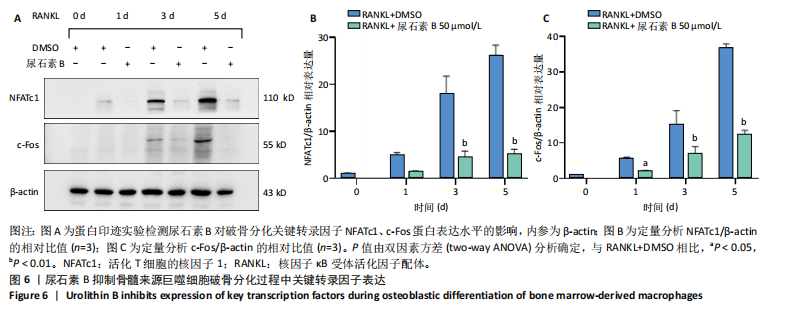
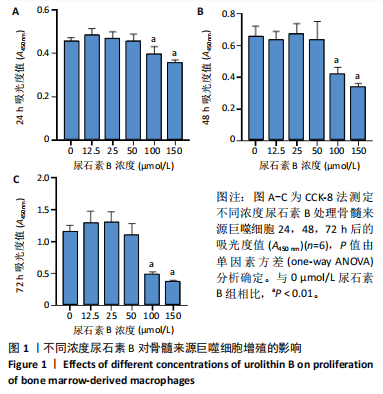
2.1 尿石素B对骨髓来源巨噬细胞增殖的影响 首先利用CCK-8法来评估不同浓度尿石素B(0,12.5,25,50,100和150 μmol/L)尿石素B对骨髓来源巨噬细胞增殖的影响。用不同浓度尿石素B处理24,48,72 h,CCK-8结果显示在尿石素B剂量在50 μmol/L及以内时,对骨髓来源巨噬细胞增殖无明显影响,高浓度尿石素B(100,150 μmol/L)与对照组(0 μmol/L)相比差异有显著性意义(P < 0.05),对骨髓来源巨噬细胞增殖有抑制作用,具体结果见图1。因此在后续实验中,选取50 μmol/L及以下浓度探究尿石素B对骨髓来源巨噬细胞破骨分化的影响。 2.2 尿石素B抑制RANKL诱导骨髓来源巨噬细胞的破骨分化 CCK-8检测结果显示50 μmol/L及以下浓度的尿石素B对骨髓来源巨噬细胞增殖无明显抑制作用,为了进一步探究尿石素B对骨髓来源巨噬细胞破骨分化的影响,此次研究在体外细胞实验使用不同浓度(0,12.5,25和50 μmol/L)的尿石素B干预RANKL刺激骨髓来源巨噬细胞破骨分化过程。RANKL刺激5 d后,观察到RANKL阳性对照组出现较多密且大的多核破骨细胞。抗酒石酸酸性磷酸酶染色结果显示,当尿石素B为12.5 μmol/L时骨髓来源巨噬细胞破骨分化受到明显抑制,破骨细胞数量及大小明显减少,增加到50 μmol/L几乎完全抑制了破骨细胞的生成,差异有显著性意义(P < 0.01),见图2。这一结果表明尿石素B能够抑制体外RANKL诱导的骨髓来源巨噬细胞破骨分化,并呈浓度依赖性。 2.3 尿石素B主要在骨髓来源巨噬细胞的破骨分化前中期产生抑制作用 在RANKL诱导骨髓来源巨噬细胞过程中,诱导第二三天时,骨髓来源巨噬细胞会出现部分细胞逐渐有融合的趋势;诱导的第4天,出现小块细胞融合区域,破骨细胞开始形成,呈圆形或类圆形;到第5天出现大的边界清晰的呈煎饼状的成熟破骨细胞。为了检测尿石素B对RANKL诱导的破骨细胞形成的主要影响阶段,此次研究使用50 μmol/L尿石素B对RANKL诱导的骨髓来源巨噬细胞进行了不同时间段的干预。分别在RANKL刺激的早期(0-2 d)、中期(2-4 d)、晚期(4-5 d)、全程(0-5 d)分别给予50 μmol/L浓度的尿石素B干预骨髓来源巨噬细胞破骨分化过程。抗酒石酸酸性磷酸酶染色结果表明,在破骨细胞形成前中期加入尿石素B较显著地减少了破骨细胞的数量和大小,见图3。 2.4 尿石素B抑制破骨细胞分化相关特征基因的mRNA表达 为了进一步探讨尿石素B对破骨细胞分化的抑制作用,此次研究通过荧光定量PCR评估在体外其对Nfatc1、Oscar、Ctsk、Mmp9、Acp5和Atp6V0d2等破骨分化特征基因表达的影响。在mRNA水平上检测尿石素B对RANKL诱导5 d骨髓来源巨噬细胞后破骨分化特征基因的表达情况,以18s表达量为标准对照。实时定量荧光PCR结果显示:经尿石素B干预上述破骨分化特征基因表达均有所下降,50 μmol/L浓度的尿石素B处理组与对照组相比,破骨分化基因表达差异有显著性意义(P < 0.01),见图4。这说明尿石素B通过下调破骨分化特征基因的转录激活与表达,抑制了破骨细胞的形成与成熟。 2.5 尿石素B抑制RANKL介导的P65、ERK信号通路激活 此次研究采用抑制作用最强的浓度50 μmol/L来研究尿石素B对RANKL诱导的骨髓来源巨噬细胞中核因子κB(P65)、MAPK(ERK)信号通路的影响。用RANKL在0,5,15,30,60 min不同时间梯度来刺激骨髓来源巨噬细胞。结果表明,RANKL刺激显著提高了p-P65、p-ERK的表达水平,呈现出先升高后降低的趋势,p-P65、p-ERK在5 min达到峰值。与RANKL阳性对照组对比,尿石素B处理组主要在5 min降低了磷酸化ERK相对于其总蛋白的表达水平,在各时间点均抑制了磷酸化P65相对于其总蛋白的表达水平。这表明尿石素B抑制了RANKL诱导的P65和ERK信号通路的磷酸化情况,从而干预破骨细胞分化,见图5。 2.6 骨髓来源巨噬细胞破骨分化过程中NFATc1、c-Fos表达变化及尿石素B对其的抑制作用 此次研究采用抑制作用最强的浓度50 μmol/L来研究尿石素B对骨髓来源巨噬细胞破骨分化过程中破骨关键转录因子NFATc1、c-Fos表达的影响。在RANKL诱导0,1,3或5 d,收集了阳性对照组和尿石素B干预组的骨髓来源巨噬细胞。通过蛋白印迹实验发现,在RANKL处理骨髓来源巨噬细胞破骨分化过程中,NFATc1和c-Fos蛋白表达水平逐渐上升,在第5天达到峰值。在50 μmol/L浓度尿石素B处理后这些蛋白表达水平受到明显抑制,尤其是第3,5天。由此可知,尿石素B抑制了NFATc1和c-Fos的活性,从而影响RANKL诱导的骨髓来源巨噬细胞破骨分化过程,见图6。"
| [1] SALHOTRA A, SHAH HN, LEVI B, et al. Mechanisms of bone development and repair. Nat Rev Mol Cell Biol. 2020;21(11):696-711. [2] TAO H, LI X, WANG Q, et al. Redox signaling and antioxidant defense in osteoclasts. Free Radic Biol Med. 2024;212:403-414. [3] BOYLE, WILLIAM J, SIMONET W, et al. Osteoclast differentiation and activation. Nature.2003;423(6937):337-342. [4] BI H, CHEN X, GAO S, et al. Key Triggers of Osteoclast-Related Diseases and Available Strategies for Targeted Therapies: A Review. Front Med. 2017;4:234. [5] STEGEN S, MOERMANS K, STOCKMANS I, et al. The serine synthesis pathway drives osteoclast differentiation through epigenetic regulation of NFATc1 expression. Nat Metab. 2024;6(1):141-152. [6] ANESI A, GENERALI L, SANDONI L, et al. From Osteoclast Differentiation to Osteonecrosis of the Jaw: Molecular and Clinical Insights. Int J Mol Sci. 2019;20(19):4925. [7] BROWN JP, MORIN S, LESLIE W, et al. Bisphosphonates for treatment of osteoporosis: expected benefits, potential harms, and drug holidays. Can Fam Physician. 2014;60(4):324-333. [8] DEEKS ED. Denosumab: A Review in Postmenopausal Osteoporosis. Drugs Aging. 2018;35(2):163-173. [9] KHOSLA S, HOFBAUER LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5(17):898-907. [10] REID IR, BILLINGTON EO. Drug therapy for osteoporosis in older adults. Lancet. 2022;399(10329):1080-1092. [11] ASADIPOOYA K, WEINSTOCK A. Cardiovascular Outcomes of Romosozumab and Protective Role of Alendronate. Arterioscler Thromb Vasc Biol. 2019;39(7):1343-1350. [12] TAKITO J, NONAKA N. Osteoclasts at Bone Remodeling: Order from Order. Results Probl Cell Differ. 2024;71:227-256. [13] VEIS DJ, O’BRIEN CA. Osteoclasts, Master Sculptors of Bone. Annu Rev Pathol. 2023;18:257-281. [14] FENG X, TEITELBAUM SL. Osteoclasts: New Insights. Bone Res. 2013; 1(1):11-26. [15] TAKITO J, NONAKA N. Osteoclasts at Bone Remodeling: Order from Order. Results Probl Cell Differ. 2021;71:227-256. [16] TAKAYANAGI H. RANKL as the master regulator of osteoclast differentiation. J Bone Miner Metab. 2021;39(1):13-18. [17] HE J, ZHENG L, LI X, et al. Obacunone targets macrophage migration inhibitory factor (MIF) to impede osteoclastogenesis and alleviate ovariectomy-induced bone loss. J Adv Res. 2023;53:235-248. [18] TAKAYANAGI H ASAGIRI M. The molecular understanding of osteoclast differentiation. Bone. 2007;40(2):251-264. [19] GANDHI GR, ANTONY PJ, CEASAR SA, et al. Health functions and related molecular mechanisms of ellagitannin-derived urolithins. Crit Rev Food Sci Nutr. 2024;64(2):280-310.
20] ZHENG D, LIU Z, ZHOU Y, et al. Urolithin B, a gut microbiota metabolite, protects against myocardial ischemia/reperfusion injury via p62/Keap1/Nrf2 signaling pathway. Pharmacol Res. 2020;153:104655. [21] REDDY MK, GUPTA SK, JACOB MR, et al. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007;73(5): 461-467. [22] WANG ST, CHANG WC, HSU C, et al. Antimelanogenic Effect of Urolithin A and Urolithin B, the Colonic Metabolites of Ellagic Acid, in B16 Melanoma Cells. J Agric Food Chem. 2017;65(32):6870-6876. [23] AL-HARBI SA, ABDULRAHMAN AO, ZAMZAMI MA, et al. Urolithins: The Gut Based Polyphenol Metabolites of Ellagitannins in Cancer Prevention, a Review. Front Nutr. 2021;8:647582. [24] LV MY, SHI CJ, PAN FF, et al. Urolithin B suppresses tumor growth in hepatocellular carcinoma through inducing the inactivation of Wnt/β-catenin signaling. J Cell Biochem. 2019;120(10):117273-17282. [25] XUE H, ZHOU H, LOU Q, et al. Urolithin B reduces cartilage degeneration and alleviates osteoarthritis by inhibiting inflammation. Food Funct. 2024;15(7):3552-3565. [26] RAIMUNDO AF, FERREIRA S, POBRE V, et al. Urolithin B: Two-way attack on IAPP proteotoxicity with implications for diabetes. Front Endocrinol. 2022;13:1008418. [27] QU Z, AN H, FENG M, et al. Urolithin B suppresses osteoclastogenesis via inhibiting RANKL-induced signalling pathways and attenuating ROS activities. J Cell Mol Med. 2022;26(16):4428-4439. [28] WU Z, LI C, CHEN Y, et al. Chrysin Protects Against Titanium Particle-Induced Osteolysis by Attenuating Osteoclast Formation and Function by Inhibiting NF-κB and MAPK Signaling. Front Pharmaco. 2022;13: 793087. [29] MIAO J, TU Y, JIANG J, et al. VSIG4 inhibits RANKL-induced osteoclastogenesis by enhancing Nrf2-dependent antioxidant response against reactive oxygen species production. Int J Biol Macromol. 2024; 260:129357. [30] 中华医学会骨质疏松和骨矿盐疾病分会. 原发性骨质疏松症诊疗指南[J]. 中国全科医学,2023,26(14):1671-1691. [31] JIANG T, XIA T, QIAO F, et al. Role and Regulation of Transcription Factors in Osteoclastogenesis. Int J Mol Sci. 2023;24(22):16175. [32] LIU CL, HO TL, FANG SY, et al. Ugonin L inhibits osteoclast formation and promotes osteoclast apoptosis by inhibiting the MAPK and NF-κB pathways. Biomed Pharmacother. 2023;166:115392. [33] 梅良伟, 桑文华, 陈富春, 等. RANK 信号调控破骨细胞分化与成熟的研究进展[J]. 中国骨质疏松杂志,2018,24(12):1652-1656. [34] QIU Z, LI L, HUANG Y, et al. Puerarin specifically disrupts osteoclast activation via blocking integrin-β3 Pyk2/Src/Cbl signaling pathway. J Orthop Translat. 2022;33:55-69. [35] ZHANG L, YANG Y, LIAO Z, et al. Genetic and pharmacological activation of Hedgehog signaling inhibits osteoclastogenesis and attenuates titanium particle-induced osteolysis partly through suppressing the JNK/c-Fos-NFATc1 cascade. Theranostics. 2020;10(15):6638-6660. [36] XU H, CHEN F, LIU T, et al. Ellagic acid blocks RANKL-RANK interaction and suppresses RANKL-induced osteoclastogenesis by inhibiting RANK signaling pathways. Chem Biol Interact. 2020;331:109235. [37] LIN X, YUAN G, LI Z, et al. Ellagic acid protects ovariectomy-induced bone loss in mice by inhibiting osteoclast differentiation and bone resorption. J Cell Physiol. 2020;235(9):5951-5961. [38] LI Y, ZHUANG Q, TAO L, et al. Urolithin B suppressed osteoclast activation and reduced bone loss of osteoporosis via inhibiting ERK/ NF-κB pathway. Cell Prolif. 2022;55(10):e13291. [39] MA L, ZHANG L, LIAO Z, et al. Pharmacological inhibition of protein S-palmitoylation suppresses osteoclastogenesis and ameliorates ovariectomy-induced bone loss. J Orthop Translat. 2023;42:1-14. [40] GHOSH S, HAYDEN MS. Shared principles in NF-kappaB signaling. Cell, 2008;132(3):344-362. [41] TIAN M, HAN YB, YANG GY, et al. The role of lactoferrin in bone remodeling: evaluation of its potential in targeted delivery and treatment of metabolic bone diseases and orthopedic conditions. Front Endocrinol. 2023;14:1218148. [42] YANG Y, LIU Z, WU J, et al. Nrf2 Mitigates RANKL and M-CSF Induced Osteoclast Differentiation via ROS-Dependent Mechanisms. Antioxidants. 2023;12(12):2094. [43] LUO N, ZHANG L, XIU C, et al. Piperlongumine, a Piper longum-derived amide alkaloid, protects mice from ovariectomy-induced osteoporosis by inhibiting osteoclastogenesis via suppression of p38 and JNK signaling. Food Funct. 2024;15(4):2154-2169. [44] LIU C, WALTER TS, HUANG P, et al. Structural and functional insights of RANKL-RANK interaction and signaling. J Immunol. 2010;184(12): 6910-6919. |
| [1] | Xia Zhaoxin, Gao Yichen, Deng Yuyao, Wang Xia, Lan Xiaorong, He Yun, Chen Junliang. Three-dimensional finite element analysis of different material implants for replacing single missing anterior tooth [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(22): 4687-4693. |
| [2] | Hou Chengzhi, Han Jiatong, Wei Guangcheng, Zhuo Zechuan, Li Qiuyue, Zhao Yong, Yu Zhangjingze . Gushukang interferes with osteoclasts: activation of nuclear factor erythroid 2-related factor 2 regulates the c-Fos/NFATc1 pathway in the treatment of osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(2): 279-285. |
| [3] | Zhang Jiahao, Li Jiacheng, Wen Mingtao, Guo Yanbo, Luo Di, Li Gang. Astragaloside IV alleviates oxidative stress injury and promotes osteogenesis in MC3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(17): 3529-3536. |
| [4] | Yu Yangyi , Song Zhuoyue, Lian Qiang, Ding Kang, Li Guangheng . AAV-mediated expression of p65shRNA and bone morphogenetic protein 4 synergistically enhances chondrocyte regeneration [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(17): 3537-3547. |
| [5] | Zhou Guanjin, Li Yanan, Li Tao. Neferine inhibits interleukin-1beta-induced chondrocyte apoptosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5624-5629. |
| [6] | Cao Yu, Wu Dang, Ouyang Mingxing, Deng Linhong. Effect of cell mechanics on morphogenesis of MDCK lobular organoid [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 5003-5009. |
| [7] | Wu Qixiang, Fang Chenyu, Zhang Lei. Interleukin-1beta enhances migration and adhesion of mesenchymal stem cells in inflammatory environments [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 5048-5054. |
| [8] | Liu Yang, Cao Youhui, Bao Xuemei. Polyetheretherketone and its composite materials in post core crown restoration: problems and clinical application value [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(29): 4691-4696. |
| [9] | Zuo Jun, Ma Shaolin. Mechanism of beta-sitosterol on hypertrophic scar fibroblasts: an analysis based on network pharmacology [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 216-223. |
| [10] | Yang Xiaoguang, Shi Yancheng, Ma Tao, Zhang Jimin, Zhang Wei. Effects of biological amniotic membrane on tendon adhesion and healing in ruptured Achilles tendon rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(15): 2297-2301. |
| [11] | Hua Xiaoqiong, Li Yanjie, Qin Hewei, Jin Xiaoqin, Ren Kun, Zhang Zhixin, Zhu Bochao, Wang Yupu. Acupoint dressing therapy using Expectorant Choking Formula for swallowing disorders in rats after cerebral ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(32): 5155-5161. |
| [12] | Mo Yaomin, Liu Pan, Ma Ruixin, Zeng Gaofeng, Zong Shaohui. Mechanism of paroxetine on osteoclast differentiation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(32): 5184-5190. |
| [13] | Liao Zirui, Chen Jianquan. Effect of inhibitor of Wnt production 2 on osteoclast differentiation [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(31): 4990-4995. |
| [14] | Chen Jingtao, Chen You, Li Yujing, Liu Zhigang. Astragalus polysaccharide inhibits Toll-like receptor 4/nuclear factor kappaB p65 pathway in the treatment of knee osteoarthritis in rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(31): 5002-5008. |
| [15] | Zhai Hongjie, Han Guanda, Li Lei, Dong Xiaohui, Jiang Zhiquan, Lou Feiyun. 3D printed polyetheretherketone material for skull defect repair [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(3): 380-384. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||