Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (17): 3529-3536.doi: 10.12307/2025.706
Previous Articles Next Articles
Astragaloside IV alleviates oxidative stress injury and promotes osteogenesis in MC3T3-E1 cells
Zhang Jiahao1, Li Jiacheng2, Wen Mingtao1, Guo Yanbo1, 2, Luo Di2, Li Gang2
- 1The First Clinical Medical College of Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China; 2The Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China
-
Received:2024-06-13Accepted:2024-08-21Online:2025-06-18Published:2024-10-30 -
Contact:Li Gang, MD, Professor, Chief physician, Doctoral supervisor, The First Clinical Medical College of Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China -
About author:Zhang Jiahao, Master candidate, The First Clinical Medical College of Shandong University of Traditional Chinese Medicine, Jinan 250014, Shandong Province, China -
Supported by:Research and Development Program (Major Science and Technology Innovation Project) of Shandong Province, No. 2021CXGC010501 (to LG); Natural Science Foundation of Shandong Province, No. ZR2022LZY003 (to LJC)
CLC Number:
Cite this article
Zhang Jiahao, Li Jiacheng, Wen Mingtao, Guo Yanbo, Luo Di, Li Gang. Astragaloside IV alleviates oxidative stress injury and promotes osteogenesis in MC3T3-E1 cells[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(17): 3529-3536.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
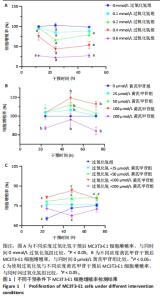
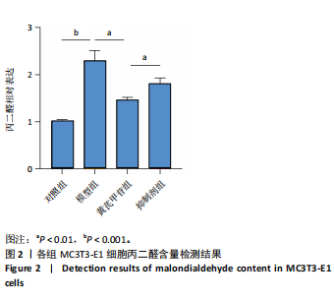
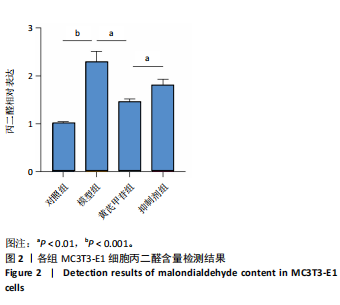
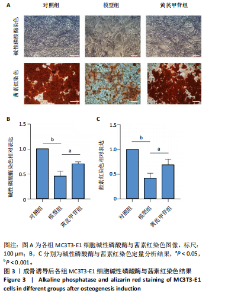
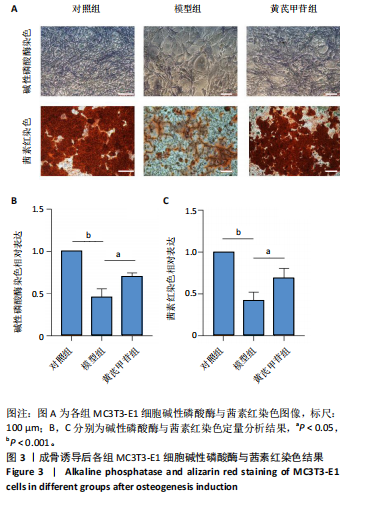
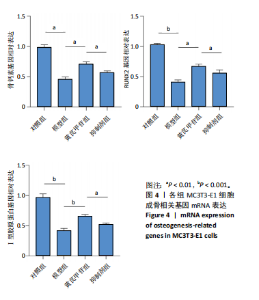
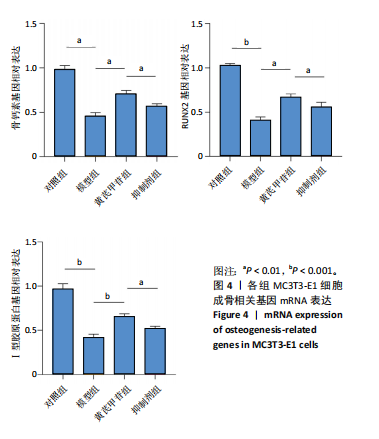
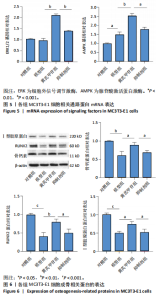
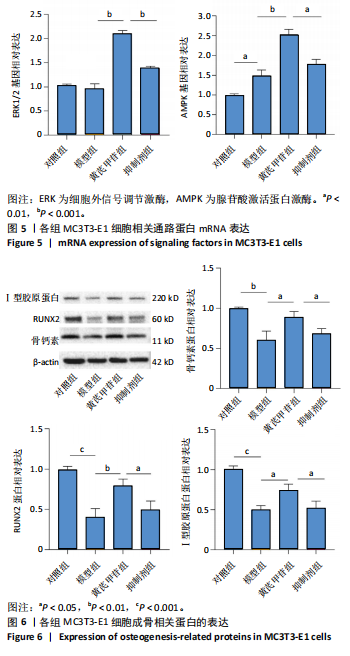
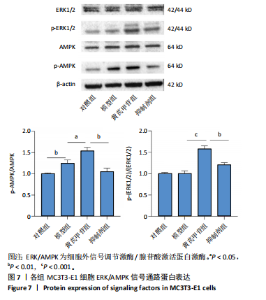
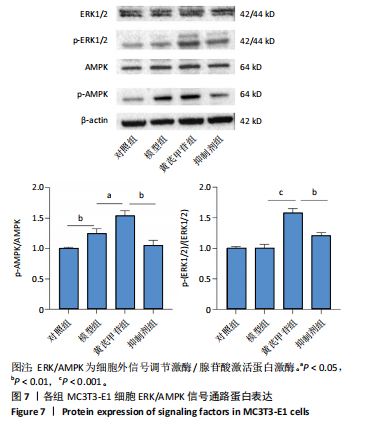
2.1 不同浓度过氧化氢对MC3T3-E1细胞增殖活力的影响 干预12 h后,0.4,0.6 mmol/L过氧化氢组细胞增殖率低于对照组;干预24 h后,0.1,0.2,0.4,0.6 mmol/L过氧化氢组细胞增殖率均低于对照组;干预48 h后,0.2,0.4,0.6 mmol/L过氧化氢组细胞增殖率均低于对照组,并且各组干预24 h的细胞增殖率较低,见图1A。后续实验选择0.2 mmol/L过氧化氢干预24 h作为氧化应激造模条件。 2.2 不同浓度黄芪甲苷组对MC3T3-E1细胞增殖活力的影响 干预24 h后,25,50,100 μmol/L黄芪甲苷组细胞增殖率均高于对照组,200 μmol/L黄芪甲苷组细胞增殖率低于对照组;干预48 h后,25,50,100 μmol/L黄芪甲苷组细胞增殖率较干预24 h后升高,并且均高于对照组,200 μmol/L黄芪甲苷组细胞增殖率低于对照组;干预72 h后,25,50,100 μmol/L黄芪甲苷组细胞增殖率较干预48 h后降低,但仍高于对照组,200 μmol/L黄芪甲苷组细胞增殖率低于对照组,见图1B。说明100 μmol/L以内的黄芪甲苷对MC3T3-E1细胞无毒性作用。 2.3 黄芪甲苷对氧化应激条件下MC3T3-E1细胞增殖活力的影响 在加入过氧化氢基础上,干预24 h后,25,50,100,200 μmol/L黄芪甲苷提高了MC3T3-E1细胞增殖率;干预48 h后,25,50,100 μmol/L黄芪甲苷提高了MC3T3-E1细胞增殖率,而200 μmol/L黄芪甲苷降低了MC3T3-E1细胞增殖率;干预72 h后,25,50,100 μmol/L黄芪甲苷提高了MC3T3-E1细胞增殖率,而200 μmol/L黄芪甲苷降低了MC3T3-E1细胞增殖率,见图1C。并且可见过氧化氢+100 μmol/L黄芪甲苷组干预48 h的细胞增殖率最高。 综合2.2、2.3实验结果,在保证MC3T3-E1细胞足够活性的条件下,选择100 μmol/L黄芪甲苷干预48 h进行实验。 2.4 黄芪甲苷可增强氧化应激条件下MC3T3-E1细胞的成骨与矿化能力 2.4.1 丙二醛含量检测结果 模型组细胞内丙二醛含量高于对照组、黄芪甲苷组,抑制剂组细胞内丙二醛含量高于黄芪甲苷组,见图2。 2.4.2 碱性磷酸酶染色与茜素红染色结果 模型组碱性磷酸酶染色较对照组浅,黄芪甲苷组碱性磷酸酶染色较模型组加深,表明黄芪甲苷可促进氧化应激条件下MC3T3-E1细胞的成骨分化;定量分析结果显示,模型组碱性磷酸酶含量低于对照组、黄芪甲苷组(P < 0.001,P < 0.05),见图3。茜素红染色及定量分析结果显示,模型组成骨矿化结节形成少于对照组、黄芪甲苷组(P < 0.001,P < 0.05),见图3。表明黄芪甲苷能够促进过氧化氢诱导MC3T3-E1细胞的成骨分化与矿化能力。 2.4.3 RT-qPCR检测结果 模型组骨钙素、RUNX2、Ⅰ型胶原蛋白mRNA表达均低于对照组(P < 0.01,P < 0.001),黄芪甲苷组骨钙素、RUNX2、Ⅰ型胶原蛋白mRNA表达均高于模型组(P < 0.01,P < 0.001),抑制剂组骨钙素、RUNX2、Ⅰ型胶原蛋白mRNA表达均低于黄芪甲苷组(P < 0.01),见图4。表明黄芪甲苷可以明显提高氧化应激条件下MC3T3-E1细胞成骨相关基因mRNA的表达,并且ERK抑制剂可部分拮抗黄芪甲苷的作用。 模型组AMPK mRNA表达高于对照组(P < 0.01),黄芪甲苷组ERK1/2、AMPK mRNA表达均高于模型组(P < 0.001),抑制剂组ERK1/2、AMPK mRNA表达均低于黄芪甲苷组(P < 0.001,P < 0.01),见图5。 2.4.4 Western blot检测结果 模型组骨钙素、RUNX2、Ⅰ型胶原蛋白的蛋白表达均低于对照组(P < 0.01,P < 0.001),黄芪甲苷组骨钙素、RUNX2、Ⅰ型胶原蛋白的蛋白表达均高于模型组(P < 0.05,P < 0.01),抑制剂组骨钙素、RUNX2、Ⅰ型胶原蛋白的蛋白表达均低于黄芪甲苷组(P < 0.05),见图6。 各组ERK1/2蛋白表达无差异,模型组、黄芪甲苷组、抑制剂组AMPK蛋白表达高于对照组(P < 0.05),黄芪甲苷组p-AMPK、p-ERK1/2蛋白表达高于模型组(P < 0.05,P < 0.001),抑制剂组p-AMPK、p-ERK1/2蛋白表达低于黄芪甲苷组(P < 0.01),见图7。"

| [1] ENSRUD KE, CRANDALL CJ. Osteoporosis. Ann Intern Med. 2017;167(3): ITC17-ITC32. [2] LANGDAHL BL. Overview of treatment approaches to osteoporosis. Br J Pharmacol. 2021;178(9):1891-1906. [3] 中华医学会骨质疏松和骨矿盐疾病分会.原发性骨质疏松症诊疗指南(2022)[J].中国全科医学,2023,26(14):1671-1691. [4] KIMBALL JS, JOHNSON JP, CARLSON DA. Oxidative Stress and Osteoporosis. J Bone Joint Surg. 2021;103(15):1451-1461. [5] ZHANG C, LI H, LI J, et al. Oxidative stress: A common pathological state in a high-risk population for osteoporosis. Biomed Pharmacother. 2023; 163:114834. [6] RIEGGER J, SCHOPPA A, RUTHS L, et al. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: a narrative review. Cell Mol Biol Lett. 2023;28(1): 76. [7] 曹志威,武晓蓉,邵国,等.MC3T3-E1细胞成骨模型的应用及研究进展[J].生物骨科材料与临床研究,2023,20(5):86-89,93. [8] 蒋微,蒋式骊,刘平.黄芪甲苷的药理作用研究进展[J].中华中医药学刊,2019,37(9):2121-2124. [9] 王嵩,黄思捷,郤庆.含黄芪中药复方制剂中黄芪甲苷含量测定的研究进展[J].上海医药,2021,42(5): 64-68. [10] SUN NY, LIU XL, GAO J, et al. Astragaloside-IV modulates NGF-induced osteoblast differentiation via the GSK3β/β-catenin signalling pathway. Mol Med Rep. 2021;23(1):19. [11] WANG F, QIAN H, KONG L, et al. Accelerated Bone Regeneration by Astragaloside IV through Stimulating the Coupling of Osteogenesis and Angiogenesis. Int J Biol Sci. 2021;17(7):1821-1836. [12] SUGIURA R, SATOH R, TAKASAKI T. ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer. Cells. 2021;10(10):2509. [13] KIM JM, YANG YS, PARK KH, et al. The ERK MAPK Pathway Is Essential for Skeletal Development and Homeostasis. Int J Mol Sci. 2019;20(8):1803. [14] LIU X, ZHANG J, WANG S, et al. Astragaloside IV attenuates the H2O2-induced apoptosis of neuronal cells by inhibiting α-synuclein expression via the p38 MAPK pathway. Int J Mol Med. 2017;40(6):1772-1780. [15] LIU Y, CHONG L, LI X, et al. Astragaloside IV rescues MPP+-induced mitochondrial dysfunction through upregulation of methionine sulfoxide reductase A. Exp Ther Med. 2017;14(3):2650-2656. [16] 刘海萌,付冠.黄芪甲苷增强骨髓间充质干细胞修复大鼠脑缺血再灌注损伤的实验研究[J].中国免疫学杂志,2020,36(11):1313-1317. [17] JIN H, DU J, REN H, et al. Astragaloside IV protects against iron loading-induced abnormal differentiation of bone marrow mesenchymal stem cells (BMSCs). FEBS Open Bio. 2021;11(4):1223-1236. [18] BLAKE JF, BURKARD M, CHAN J, et al. Discovery of (S)-1-(1-(4-Chloro-3-fluorophenyl)-2-hydroxyethyl)-4-(2-((1-methyl-1H-pyrazol-5-yl)amino)pyrimidin-4-yl)pyridin-2(1H)-one (GDC-0994), an Extracellular Signal-Regulated Kinase 1/2 (ERK1/2) Inhibitor in Early Clinical Development. J Med Chem. 2016;59(12):5650-5660. [19] ROSKOSKI R. Targeting ERK1/2 protein-serine/threonine kinases in human cancers. Pharmacol Res. 2019;142:151-168. [20] FILAIRE E, TOUMI H. Reactive oxygen species and exercise on bone metabolism: friend or enemy? Joint Bone Spine. 2012;79(4):341-346. [21] YANG K, CAO F, QIU S, et al. Metformin Promotes Differentiation and Attenuates H2O2-Induced Oxidative Damage of Osteoblasts via the PI3K/AKT/Nrf2/HO-1 Pathway. Front Pharmacol. 2022;13:829830. [22] ZHAO X, LIN S, LI H, et al. Myeloperoxidase Controls Bone Turnover by Suppressing Osteoclast Differentiation Through Modulating Reactive Oxygen Species Level. J Bone Miner Res. 2021;36(3):591-603. [23] DOMAZETOVIC V, MARCUCCI G, FALSETTI I, et al. Blueberry Juice Antioxidants Protect Osteogenic Activity against Oxidative Stress and Improve Long-Term Activation of the Mineralization Process in Human Osteoblast-Like SaOS-2 Cells: Involvement of SIRT1. Antioxidants (Basel). 2020;9(2):125. [24] AUSTERMANN K, BAECKER N, STEHLE P, et al. Putative Effects of Nutritive Polyphenols on Bone Metabolism In Vivo-Evidence from Human Studies. Nutrients. 2019;11(4):871. [25] MARCUCCI G, DOMAZETOVIC V, NEDIANI C, et al. Oxidative Stress and Natural Antioxidants in Osteoporosis: Novel Preventive and Therapeutic Approaches. Antioxidants (Basel). 2023;12(2):373. [26] FONTANI F, MARCUCCI G, IANTOMASI T, et al. Glutathione, N-acetylcysteine and lipoic acid down-regulate starvation-induced apoptosis, RANKL/OPG ratio and sclerostin in osteocytes: involvement of JNK and ERK1/2 signalling. Calcif Tissue Int. 2015;96(4):335-346. [27] LEÓN-REYES G, ARGOTY-PANTOJA AD, BECERRA-CERVERA A, et al. Oxidative-Stress-Related Genes in Osteoporosis: A Systematic Review. Antioxidants (Basel). 2023;12(4):915. [28] LIU Y, YU X, HUANG A, et al. INTS7-ABCD3 Interaction Stimulates the Proliferation and Osteoblastic Differentiation of Mouse Bone Marrow Mesenchymal Stem Cells by Suppressing Oxidative Stress. Front. Physiol. 2021;12:758607. [29] SHEN Y, WANG H, XIE H, et al. l-arginine promotes angio-osteogenesis to enhance oxidative stress-inhibited bone formation by ameliorating mitophagy. J Orthop Translat. 2024;46:53-64. [30] 梁伟乔,钟诚,李宇明.骨质疏松症的中医病因病机认识与治疗进展[J].中国骨质疏松杂志,2020, 26(1):135-139. [31] 闵甦,关永林,周广超,等.基于气血理论探讨补气补血中药防治骨质疏松症的研究进展[J].中医临床研究,2021,13(9):118-121,139. [32] 刘路,李凯,胡阳,等.黄芪有效成分抗骨质疏松症作用机制的研究进展[J].中草药,2023,54(4): 1321-1328. [33] ZOU Y, LI S, CHEN T, et al. Astragaloside IV ameliorates peripheral immunosuppression induced by cerebral ischemia through inhibiting HPA axis. Int Immunopharmacol. 2022;105:108569. [34] YANG K, XIE Q, TANG T, et al. Astragaloside IV as a novel CXCR4 antagonist alleviates osteoarthritis in the knee of monosodium iodoacetate-induced rats. Phytomedicine. 2023;108:154506. [35] ZHUANG Z, WANG ZH, DENG LH, et al. Astragaloside IV Exerts Cardioprotection in Animal Models of Viral Myocarditis: A Preclinical Systematic Review and Meta-Analysis. Front Pharmacol. 2019;10:1388. [36] CAI B, ZHANG AG, ZHANG X, et al. Promoting Effects on Proliferation and Chondrogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells by Four “Kidney-Tonifying” Traditional Chinese Herbs. Biomed Res Int. 2015;2015:792161. [37] ESSAWY AE, ABD ELKADER HAE, KHAMISS OA, et al. Therapeutic effects of astragaloside IV and Astragalus spinosus saponins against bisphenol A-induced neurotoxicity and DNA damage in rats. PeerJ. 2021;9:e11930. [38] 邵帅,鲁美丽,高秀秋,等.黄芪甲苷对脂多糖诱导的巨噬细胞炎症反应及核因子κB受体活化因子配体/骨保护素系统表达的影响[J].中国医科大学学报,2022,51(4):294-300. [39] 成鹏,白银亮,胡彩莉,等.黄芪甲苷通过调控FoxO3a/Wnt2/β-catenin通路抑制去卵巢大鼠骨质疏松的作用[J].中国实验方剂学杂志,2018,24(15):161-166. [40] ROSKOSKI R. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66(2):105-143. [41] LEE DW, KIM KM, PARK S, et al. Eucalyptol induces osteoblast differentiation through ERK phosphorylation in vitro and in vivo. J Mol Med. 2023;101(9): 1083-1095. [42] YUH D Y, MAEKAWA T, LI X, et al. The secreted protein DEL-1 activates a β3 integrin-FAK-ERK1/2-RUNX2 pathway and promotes osteogenic differentiation and bone regeneration. J Biol Chem. 2020;295(21):7261-7273. [43] CHUNXIA R, XINQING H, LU W, et al. Metformin Carbon Dots for Promoting Periodontal Bone Regeneration via Activation of ERK/AMPK Pathway. Adv Healthcare Mater. 2021;10(12):e2100196. [44] YUE H, LENG Z, BO Y, et al. Novel Peptides from Sea Cucumber Intestinal Enzyme Hydrolysates Promote Osteogenic Differentiation of Bone Mesenchymal Stem Cells via Phosphorylation of PPARγ at Serine 112. Mol Nutr Food Res. 2023;67(9):e2200451. |
| [1] | Zhao Jiyu, Wang Shaowei. Forkhead box transcription factor O1 signaling pathway in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1923-1930. |
| [2] | Zhou Jinhai, Li Jiangwei, Wang Xuquan, Zhuang Ying, Zhao Ying, Yang Yuyong, Wang Jiajia, Yang Yang, Zhou Shilian. Three-dimensional finite element analysis of anterior femoral notching during total knee arthroplasty at different bone strengths [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(9): 1775-1782. |
| [3] | Zhao Jiacheng, Ren Shiqi, Zhu Qin, Liu Jiajia, Zhu Xiang, Yang Yang. Bioinformatics analysis of potential biomarkers for primary osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1741-1750. |
| [4] | Chen Shuai, Jin Jie, Han Huawei, Tian Ningsheng, Li Zhiwei . Causal relationship between circulating inflammatory cytokines and bone mineral density based on two-sample Mendelian randomization [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1556-1564. |
| [5] | Zhang Zhenyu, Liang Qiujian, Yang Jun, Wei Xiangyu, Jiang Jie, Huang Linke, Tan Zhen. Target of neohesperidin in treatment of osteoporosis and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(7): 1437-1447. |
| [6] | Li Yueyao, Zhang Min, Yang Jiaju. Cistanoside A mediates p38/MAPK pathway to inhibit osteoclast activity [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1144-1151. |
| [7] | Zheng Lin, Jin Wenjun, Luo Shanshan, Huang Rui, Wang Jie, Cheng Yuting, An Zheqing, Xiong Yue, Gong Zipeng, Liao Jian. Eucommia ulmoides promotes alveolar bone formation in ovariectomized rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1159-1167. |
| [8] | He Guanghui, Yuan Jie, Ke Yanqin, Qiu Xiaoting, Zhang Xiaoling. Hemin regulates mitochondrial pathway of oxidative stress in mouse chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1183-1191. |
| [9] |
Huang Xiaobin, Ge Jirong, Li Shengqiang, Xie Lihua, Huang Jingwen, He Yanyan, Xue Lipeng.
Mechanisms of different yin nourishing and kidney tonifying methods on osteoclastysis pathway in ovariectomized rats #br#
#br#
[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1214-1219.
|
| [10] | He Bo, Chen Wen, Ma Suilu, He Zhijun, Song Yuan, Li Jinpeng, Liu Tao, Wei Xiaotao, Wang Weiwei, Xie Jing . Pathogenesis and treatment progress of flap ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1230-1238. |
| [11] | Qian Kun, Li Ziqing, Sun Shui . Endoplasmic reticulum stress in the occurrence and development of common degenerative bone diseases [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(6): 1285-1295. |
| [12] | Lan Shuangli, Xiang Feifan, Deng Guanghui, Xiao Yukun, Yang Yunkang, Liang Jie. Naringin inhibits iron deposition and cell apoptosis in bone tissue of osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 888-898. |
| [13] | Lu Ranran, Zhou Xu, Zhang Lijie, Yang Xinling. Dimethyl fumarate alleviates nerve damage in a mouse model of Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 989-994. |
| [14] | Wang Dongyang, Yang Qiaohui, Lin Xinchao. Relationship between vitamin D levels and reproductive characteristics and exercise dietary situation in postmenopausal women [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1021-1025. |
| [15] | Zhang Lichuang, Yang Wen, Ding Guangjiang, Li Peikun, Xiao Zhongyu, Chen Ying, Fang Xue, Zhang Teng. Dispersion effect of bone cement after vertebroplasty using individualized unilateral external pedicle approach and bilateral pedicle approach [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(4): 800-808. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||