Chinese Journal of Tissue Engineering Research ›› 2025, Vol. 29 ›› Issue (17): 3537-3547.doi: 10.12307/2025.708
Previous Articles Next Articles
AAV-mediated expression of p65shRNA and bone morphogenetic protein 4 synergistically enhances chondrocyte regeneration
Yu Yangyi1 , Song Zhuoyue2, Lian Qiang1, Ding Kang3, Li Guangheng1
- 1Shenzhen Key Laboratory of Musculoskeletal Tissue Reconstruction and Function Restoration, Division of Adult Joint Reconstruction and Sports Medicine, Department of Orthopedic Surgery, Shenzhen People’s Hospital (The Second Clinical Medical College Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen 518020, Guangdong Province, China; 2Department of Orthopedics, Zhengzhou Orthopedics Hospital, Zhengzhou 450000, Henan Province, China; 3Department of Orthopedics, Shenzhen Pingle Orthopedics Hospital, Shenzhen 518000, Guangdong Province, China
-
Received:2024-06-11Accepted:2024-09-19Online:2025-06-18Published:2024-10-30 -
Contact:Li Guangheng, MD, Chief physician, Shenzhen Key Laboratory of Musculoskeletal Tissue Reconstruction and Function Restoration, Division of Adult Joint Reconstruction and Sports Medicine, Department of Orthopedic Surgery, Shenzhen People’s Hospital (The Second Clinical Medical College Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen 518020, Guangdong Province, China -
About author:Yu Yangyi, Attending physician, Shenzhen Key Laboratory of Musculoskeletal Tissue Reconstruction and Function Restoration, Division of Adult Joint Reconstruction and Sports Medicine, Department of Orthopedic Surgery, Shenzhen People’s Hospital (The Second Clinical Medical College Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen 518020, Guangdong Province, China Song Zhuoyue, Department of Orthopedics, Zhengzhou Orthopedics Hospital, Zhengzhou 450000, Henan Province, China Yu Yangyi and Song Zhuoyue contributed equally to this work. -
Supported by:the National Natural Science Foundation of China (General Program), No. 81472136 (to LGH)
CLC Number:
Cite this article
Yu Yangyi , Song Zhuoyue, Lian Qiang, Ding Kang, Li Guangheng . AAV-mediated expression of p65shRNA and bone morphogenetic protein 4 synergistically enhances chondrocyte regeneration[J]. Chinese Journal of Tissue Engineering Research, 2025, 29(17): 3537-3547.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
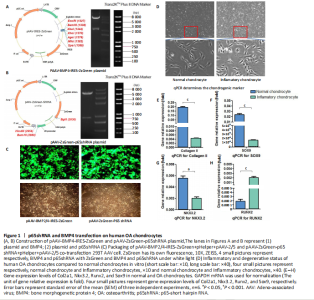
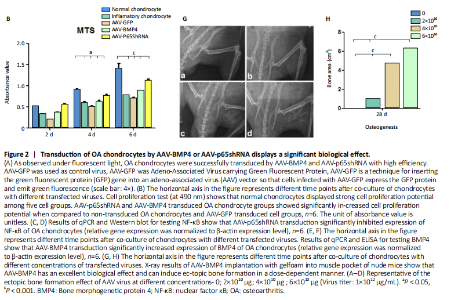
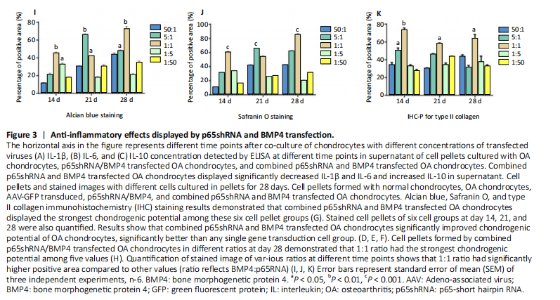
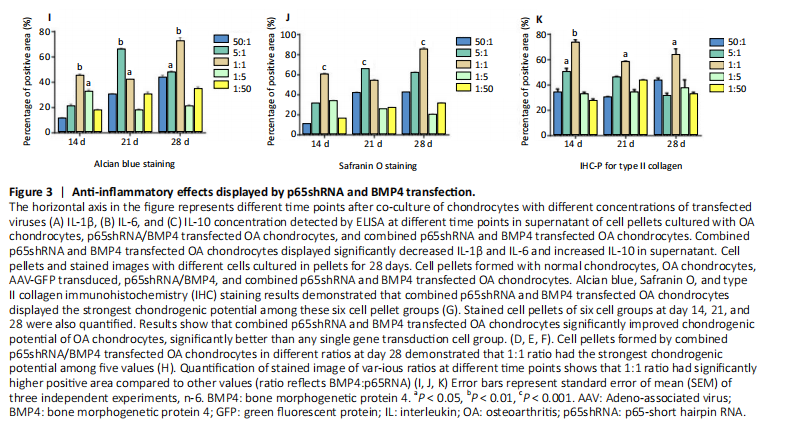
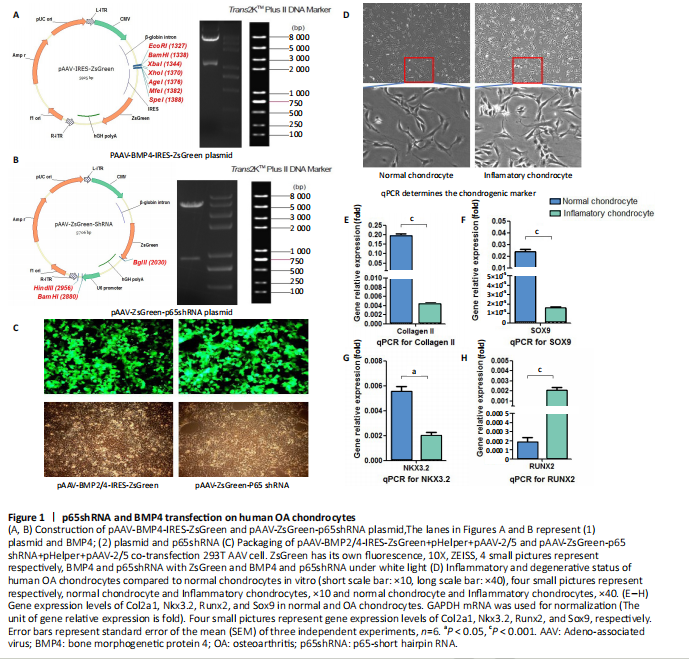
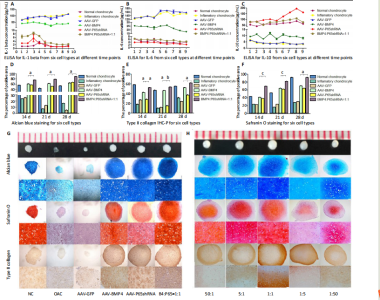
RESULTS In vitro study of human OA chondrocytes Chondrocytes from OA patients were isolated and cultured in vitro (Figure 1D). The related gene expression of chondrocytes was analyzed by real-time quantitative PCR (Figure 1E?H). The results showed that chondrocyte marker genes Col II, Nkx3.2, RUNX2 and Sox9 in OA chondrocytes were significantly downregulated, indicating the degenerative status of OA chondrocytes. In addition, the expression level of Runx2 was significantly increased in OA chondrocytes. Therefore, the OA chondrocytes presented an inflammatory, degenerative, hypertrophic status. Effects of p65shRNA and BMP4 transfection on human OA chondrocytes OA chondrocytes were transfected by AAV containing p65shRNA or BMP4. The success of the transfection was confirmed by real-time quantitative PCR and western blot/ELISA. The results show that p65 was significantly downregulated in p65shRNA transfected cells and significantly upregulated in BMP4 transfected cells. When AAV-BMP4 was implanted with gelfoam into the muscle pocket of nude rat, more bone formation could be found on X-ray, which was further confirmed by micro-CT analysis (Figure 2A?H). Combination effect of BMP4 and p65 and optimization of the mixed ratio in vitro The cell numbers were the same for the combined group and the three unmixed cell groups, which were composed of non-transfected OA chondrocytes, p65shRNA trans-fected cells, and BMP4 transfected cells. On days 14, 21, and 28, cell culture medium and cell pellets were collected for analysis. The concentrations of IL-1β, IL-6, and IL-10 in the culture medium were measured to reveal the modulation of inflammation. As shown in Figure 2C (day 21), OA chondrocytes presented a pro-inflammatory status with high levels of IL-1β and IL-6, but a low level of IL-10. Compared to the non-transfected OA chondrocytes, the p65shRNA transfected cells presented an improved inflammatory status. For the combined group, the pro-inflammatory factors were significantly decreased, but slightly higher than the p65shRNA alone group. Thus, transfection with both p65shRNA and BMP4 could suppress the inflammatory reaction of OA chondrocytes. The combined group showed slightly less suppression than the p65shRNA group, which resulted from the proportion of BMP4 transfected cells. The effects of p65shRNA and BMP4 on OA chondrocytes were further analyzed based on the promotion of anabolic activity. Growing from the same number of cells, the cell pellets in different groups clearly had various sizes after 21 days (Figure 2A). The non-transfected OA chondrocytes formed very small aspheric pellets, while the transfected cells formed large pellets. The combined group was the largest among the cell groups. These observations indicate that combined p65shRNA and BMP4 transfection could promote the proliferation or extracellular matrix (ECM) synthesis of OA chondrocytes, and the combination of the two viruses exert a synergistic effect. The amount of ECM synthesized in each cell group was then assessed by Alcian blue, Safranin O, and type II collagen immunohistochemical staining (Figure 2B?E). The non-transfected control group had the lightest staining. In p65shRNA and BMP4 transfected cells, the staining strength increased, and the combined group had the heaviest staining. The quantified statistical analysis of staining (Figure 2E) shows that both p65shRNA and BMP4 transfection increased the ECM synthesis activity of OA chondrocytes. After confirming the significant effect of the combined strategy, we further optimized the repair effects by altering the mixture ratio of the two transfected cells. We tested five different mixture combinations (Figure 3). After 21 days, cell pellets in different groups demonstrated various sizes; the 1:1 group had the largest sizes, the 5:1 and 1:5 groups had the second largest, and the 50:1 and 1:50 groups had the smallest (Figure 3H). With Alcian blue and Safranin O staining, the 1:5 group showed the heaviest staining, while the 1:1 group showed the second heaviest (Figure 3H). This indicated that BMP4 promoted the synthesis of proteoglycan. For type II collagen immunohistochemistry staining, the 1:1 group had significantly heavier staining than the others, indicating that the presence of p65shRNA BMP4 promoted the synthesis "
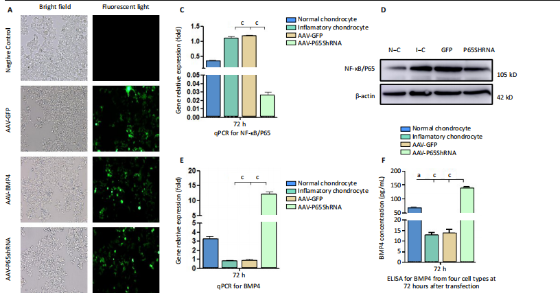
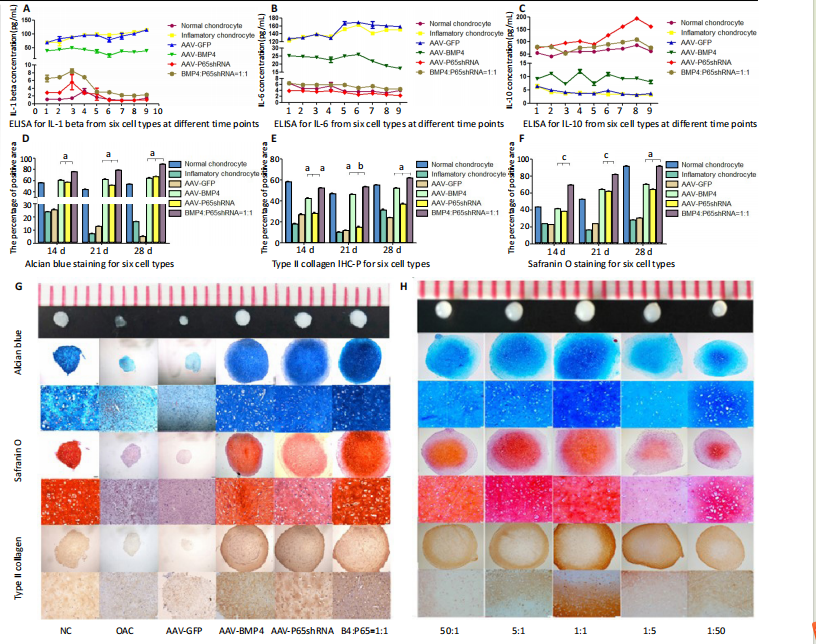
of type II collagen, which is a marker of hyaline cartilage formation. The statistical analysis (Figure 3I?K) confirmed our observations of the staining images. BMP4 contributed more to ECM synthesis than p65shRNA. However, with the same extent of inflammation modulation by p65shRNA, BMP4 synthesized the largest amount of type II collagen. Therapeutic effects of combined AAV-p65shRNA and AAV-BMP4 injection in the rat OA model in vivo The synergistic effect of AAV-p65shRNA and AAV-BMP4 in vivo was also tested by injecting the processed virus into the joints of SD rats with OA. As mixing an equal amount of p65shRNA and BMP4 transfected OA chondrocytes presented the best re-generative effects in vitro, we injected equal amounts of AAV-p65shRNA and AAV-BMP4 virus particles (0.5×1010) into the rat joints. The rats were sacrificed 12 weeks after injection, and hind limb knee joints were harvested. By gross observation, we found that the OA control rats had severely destroyed joints (Figure 4). Injection of AAV-p65shRNA virus particles (1×1010) greatly inhibited the inflammation but did not generate more cartilage when compared to the BMP4 and combined groups. With AAV-BMP4, there was regenerated hyaline-like cartilage. For the combined group, joints showed less inflammation and obvious hyaline-like cartilage formation. Therefore, the same as the in vitro results, the application of both viruses combined also presented the strongest regenerative potential in vivo. We further performed histological staining of the cartilage specimen (Figure 4). It showed that the OA rats had no cartilage left. The rats injected with virus showed obvious"

cartilage regeneration, and the combined group had the most robust ECM synthesis. At higher magnification, many chondrocytes were found to be distributed in the cartilage layer in samples from the AAV-BMP4 and combined groups; only the combined group had chondrocytes distributed all around the whole cartilage layer. Twelve weeks after virus injection, robust cartilage regenerative potential was observed in all virus-injected groups; the combined group had the best effect and BMP4 exhibited a better repair effect than p65shRNA."
| References [1] MANIVONG S, CULLIER A, AUDIGIé F, et al. New trends for osteoarthritis: biomaterials, models and modeling. Drug Discov Today. 2023;28(3):103488. [2] EMAMI A, NAMDARI H, PARVIZPOUR F, et al. Challenges in osteoarthritis treatment. Tissue Cell. 2023;80:101992. [3] HERMANN W, LAMBOVA S, MULLER-LADNER U. Current treatment options for osteoarthritis. Curr Rheumatol Rev. 2018;14(2):108-116. [4] CHULAY JD, YE GJ, THOMAS DL, et al. Preclinical evaluation of a recombinant adeno-associated virus vector expressing human alpha-1 antitrypsin made using a recombinant herpes simplex virus production method. Hum Gene Ther. 2011;22(2):155-165. [5] KAPLITT MG, FEIGIN A, TANG C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007; 369(9579):2097-2105. [6] MADRY H, CUCCHIARINI M, TERWILLIGER EF, et al. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum Gene Ther. 2003;14(4):393-402. [7] MADRY H, VENKATESAN JK, SCHMITT G, et al. rAAV vectors as safe and efficient tools for the stable delivery of genes to primary human chondrosarcoma cells in vitro and in situ. Sarcoma. 2012;2012:347417. [8] WATSON LEVINGS RS, BROOME TA, SMITH AD, et al. Gene therapy for osteoarthritis: pharmacokinetics of intra-articular self-complementary adeno-associated virus interleukin-1 receptor antagonist delivery in an equine model. Hum Gene Ther Clin Dev. 2018;29(2):90-100. [9] LIM CL, LEE YJ, CHO JH, et al. Immunogenicity and immunomodulatory effects of the human chondrocytes, hChonJ. BMC Musculoskelet Disord. 2017;18(1):199. [10] SOKOLOVE J, LEPUS CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77-94. [11] RIGOGLOU S, PAPAVASSILIOU AG. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45(11):2580-2584. [12] HENROTIN Y, CLUTTERBUCK AL, ALLAWAY D, et al. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18(2):141-149. [13] SHAKIBAEI M, JOHN T, SCHULZE-TANZIL G, et al. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73(9):1434-1445. [14] ROMAN-BLAS JA, JIMENEZ SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14(9):839-848. [15] CHOI MC, JO J, PARK J, et al. NF-κB signaling pathways in osteoarthritic cartilage destruction. Cells. 2019;8(7):734. [16] EVANS CH, GHIVIZZANI SC, ROBBINS PD. Osteoarthritis gene therapy in 2022. Curr Opin Rheumatol. 2023;35(1):37-43. [17] KURODA R, USAS A, KUBO S, et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54(2):433-442. [18] MARCU KB, OTERO M, OLIVOTTO E, et al. NF-kappaB signaling: multiple angles to target OA. Curr Drug Targets. 2010;11(5):599-613. [19] MATSUMOTO T, COOPER GM, GHARAIBEH B, et al. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60(5):1390-1405. [20] XIAO X, LI J, SAMULSKI RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72(3):2224-2232. [21] LI J, SAMULSKI RJ, XIAO X. Role for highly regulated rep gene expression in adeno-associated virus vector production. J Virol. 1997;71(7): 5236-5243. [22] WANG B, LI J, FU FH, et al. Systemic human minidystrophin gene transfer improves functions and life span of dystrophin and dystrophin/utrophin-deficient mice. J Orthop Res. 2009;27(4):421-426. [23] AISENBREY EA, BILOUSOVA G, PAYNE K, et al. Dynamic mechanical loading and growth factors influence chondrogenesis of induced pluripotent mesenchymal progenitor cells in a cartilage-mimetic hydrogel. Biomater Sci. 2019;7(12):5388-5403. [24] TIAN K, QI M, WANG L, et al. Two-stage therapeutic utility of ectopically formed bone tissue in skeletal muscle induced by adeno-associated virus containing bone morphogenetic protein-4 gene. J Orthop Surg Res. 2015;10:86. [25] JEON OH, KIM C, LABERGE RM, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23(6): 775-781. [26] CHAGANTI RK, LANE NE. Risk factors for incident osteoarthritis of the hip and knee. Curr Rev Musculoskelet Med. 2011;4(3):99-104. [27] HARRIS H, CRAWFORD A. Recognizing and managing osteoarthritis. Nursing. 2015;45(1):36-42; quiz 42-33. [28] KHEZRI K, MALEKI DIZAJ S, RAHBAR SAADAT Y, et al. Osteogenic differentiation of mesenchymal stem cells via curcumin-containing nanoscaffolds. Stem Cells Int. 2021;2021:1520052. [29] VAHEDI P, MOGHADDAMSHAHABI R, WEBSTER TJ, et al. The use of infrapatellar fat pad-derived mesenchymal stem cells in articular cartilage regeneration: a review. Int J Mol Sci. 2021;22(17):9215. [30] MARTEL-PELLETIER J, PELLETIER JP, FAHMI H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin Arthritis Rheum. 2003; 33(3):155-167. [31] YANG Q, TANG Y, IMBROGNO K, et al. AAV-based shRNA silencing of NF-κB ameliorates muscle pathologies in mdx mice. Gene Ther. 2012;19(12):1196-1204. [32] ZHANG Y, PIZZUTE T, PEI M. Anti-inflammatory strategies in cartilage repair. Tissue Eng Part B Rev. 2014;20(6):655-668. [33] MILJKOVIC ND, COOPER GM, MARRA KG. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthritis Cartilage. 2008;16(10):1121-1130. [34] JANE JA JR, DUNFORD BA, KRON A, et al. Ectopic osteogenesis using adenoviral bone morphogenetic protein (BMP)-4 and BMP-6 gene transfer. Mol Ther. 2002;6(4):464-470. [35] BRAMLAGE CP, HäUPL T, KAPS C, et al. Decrease in expression of bone morphogenetic proteins 4 and 5 in synovial tissue of patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2006; 8(3):R58. [36] WANG D, PRAKASH J, NGUYEN P, et al. Bone morphogenetic protein signaling in vascular disease: anti-inflammatory action through myocardin-related transcription factor A. J Biol Chem. 2012;287(33): 28067-28077. [37] VAN DER KRAAN PM, VAN DEN BERG WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20(3):223-232. [38] BEHRENDT P, FELDHEIM M, PREUSSE-PRANGE A, et al. Chondrogenic potential of IL-10 in mechanically injured cartilage and cellularized collagen ACI grafts. Osteoarthritis Cartilage. 2018;26(2):264-275. |
| [1] | Lai Pengyu, Liang Ran, Shen Shan. Tissue engineering technology for repairing temporomandibular joint: problems and challenges [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(在线): 1-9. |
| [2] | Yin Lu, Jiang Chuanfeng, Chen Junjie, Yi Ming, Wang Zihe, Shi Houyin, Wang Guoyou, Shen Huarui. Effect of Complanatoside A on the apoptosis of articular chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(8): 1541-1547. |
| [3] | Xu Tianjie, Fan Jiaxin, Guo Xiaoling, Jia Xiang, Zhao Xingwang, Liu kainan, Wang Qian. Metformin exerts a protective effect on articular cartilage in osteoarthritis rats by inhibiting the PI3K/AKT/mTOR pathway [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1003-1012. |
| [4] | Wu Guangtao, Qin Gang, He Kaiyi, Fan Yidong, Li Weicai, Zhu Baogang, Cao Ying . Causal relationship between immune cells and knee osteoarthritis: a two-sample bi-directional Mendelian randomization analysis [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(5): 1081-1090. |
| [5] | Shui Jing, He Yu, Jiang Nan, Xu Kun, Song Lijuan, Ding Zhibin, Ma Cungen, Li Xinyi. Astrocytes regulate remyelination in central nervous system [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7889-7897. |
| [6] | Ma Weibang, Xu Zhe, Yu Qiao, Ouyang Dong, Zhang Ruguo, Luo Wei, Xie Yangjiang, Liu Chen. Screening and cytological validation of cartilage degeneration-related genes in exosomes from osteoarthritis synovial fluid [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(36): 7783-7789. |
| [7] | Zhang Xiaoyu, Wei Shanwen, Fang Jiawei, Ni Li. Prussian blue nanoparticles restore mitochondrial function in nucleus pulposus cells through antioxidation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(34): 7318-7325. |
| [8] | Zhao Xuemei, Wang Rui, Ao · Wuliji, Bao Shuyin, Jiang Xiaohua. Effects of Agiophyllum Oligo Saccharides on inflammation and apoptosis of mouse synovial cells [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 6939-6946. |
| [9] | Wu Zhenhua, Zhang Xiwei, Wang Yipin, Li Qianqian. Relationship between seven serum lipid traits and osteoarthritis: a large sample analysis of European population in IEU OPEN GWAS database [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(32): 7004-7014. |
| [10] | Fan Jiaxin, Jia Xiang, Xu Tianjie, Liu Kainan, Guo Xiaoling, Zhang Hui, Wang Qian . Metformin inhibits ferroptosis and improves cartilage damage in osteoarthritis model rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6398-6408. |
| [11] | Wang Wanchun, , Yi Jun, Yan Zhangren, Yang Yue, Dong Degang, Li Yumei. 717 Jiedu Decoction remodels homeostasis of extracellular matrix and promotes repair of local injured tissues in rats after Agkistrodon halys bite [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(30): 6457-6465. |
| [12] | Song Yuting, Wen Chunlei, Li Yi, Bai Xue, Gao Hong, Hu Tingju, Wang Zijun, Yan Xu. Effects of myocardial extracellular matrix remodeling on connexin 43 and its Ser368 phosphorylation and electrical conduction [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(29): 6212-6218. |
| [13] | Sun Yahui, Wang Yufeng, Guo Chao, Yao Junjie, Ji Yuanyuan, Li Zhongxu, Lou Huijuan, Jiang Jinglei, Sun Yiping, Xu Jing, Cong Deyu. Effect of massage on extracellular matrix collagen deposition in skeletal muscle of type 2 diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5549-5555. |
| [14] | Hao Maochen, Ma Chao, Liu Kai, Liu Kexin, Meng Lingting, Wang Xingru, Wang Jianzhong. Bioinformatics screening of key genes for endoplasmic reticulum stress in osteoarthritis and experimental validation [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(26): 5632-5641. |
| [15] | Ji Yaqiong, Ning Zhongping. Protective effect of paeoniflorin on angiotensin II-induced fibrosis in cardiac fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(25): 5382-5389. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||