Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (31): 5003-5009.doi: 10.12307/2024.725
Previous Articles Next Articles
Effect of cell mechanics on morphogenesis of MDCK lobular organoid
Cao Yu1, 2, Wu Dang1, Ouyang Mingxing1, Deng Linhong1
- 1Institute of Biomedical Engineering and Health Sciences, School of Medical and Health Engineering, 2School of Pharmacy & School of Biological and Food Engineering, Changzhou University, Changzhou 213164, Jiangsu Province, China
-
Received:2023-09-09Accepted:2023-11-01Online:2024-11-08Published:2024-01-22 -
Contact:Deng Linhong, PhD, Professor, Institute of Biomedical Engineering and Health Sciences, School of Medical and Health Engineering, Changzhou University, Changzhou 213164, Jiangsu Province, China Ouyang Mingxing, PhD, Professor, Institute of Biomedical Engineering and Health Sciences, School of Medical and Health Engineering, Changzhou University, Changzhou 213164, Jiangsu Province, China -
About author:Cao Yu, Master candidate, Institute of Biomedical Engineering and Health Sciences, School of Medical and Health Engineering, and School of Pharmacy & School of Biological and Food Engineering, Changzhou University, Changzhou 213164, Jiangsu Province, China -
Supported by:National Natural Science Foundation of China, No. NSFC12372312, No. NSFC11872129 (to OYMX); National Natural Science Foundation of China, No. 11532003 (to DLH)
CLC Number:
Cite this article
Cao Yu, Wu Dang, Ouyang Mingxing, Deng Linhong. Effect of cell mechanics on morphogenesis of MDCK lobular organoid[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 5003-5009.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
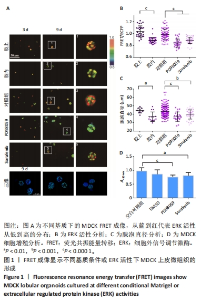
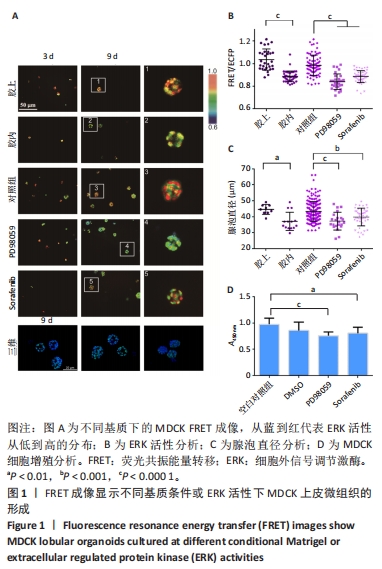
2.1 通过FRET成像显示MDCK上皮微组织的形成 MDCK细胞是一种常用的研究上皮形态发生的模型细胞系,由Rab GTP酶调控顶基底极性的形成[35]。基于FRET技术可视化观测MDCK微组织发生和ERK活性变化,构建了稳定表达ERK FRET探针的细胞株。将细胞培养在100% Matrigel凝胶上(模拟基底膜)、包埋在Matrigel凝胶中,以及培养基含有MEK抑制剂(PD98059,10 μmol/L)、RAF抑制剂(Sorafenib,5 μmol/L)的凝胶上。这2种抑制剂均是ERK上游信号分子特异性抑制剂,对照组为添加溶剂二甲基亚砜。使用含2% Matrigel的DMEM高糖培养基进行培养,每隔2 d更换1次培养基。在生长的第3,9天进行活细胞FRET成像(20倍物镜),通过FluoCell软件程序分析FRET荧光比值(FRET/ECFP),以及Image J软件计算细胞团的直径。其中,MDCK微组织的颜色代表FRET/ECFP的相对荧光比值,从蓝到红代表ERK活性从低到高的分布。 培养第9天之后,与包埋在Matrigel凝胶中相比,在Matrigel凝胶上生长的微组织FRET比值更高,代表了更高的ERK活性(图1A,B),细胞团的直径更大(图1C),提示改变培养的基质环境对MDCK微组织的形态发生有重要影响。用共聚焦显微镜拍摄培养第9天后的微组织图片,证明已形成了类似三维组织结构。使用PD98059、Sorafenib处理的MDCK微组织与对照组相比,ERK活性降低(图1A,B),直径更小(图1C);通过CCK-8实验分析,这2种抑制剂对MDCK细胞增殖有一定抑制作用(图1D)。这些结果表明ERK活性对MDCK上皮微组织的生长有重要调控作用。"

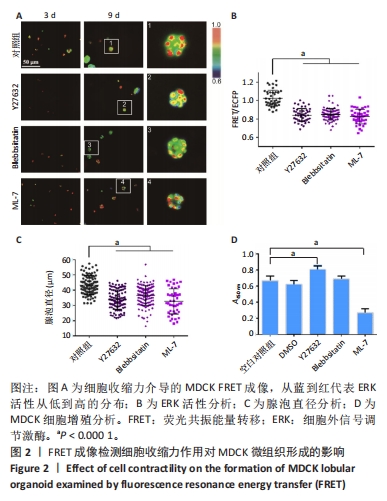
2.2 细胞收缩力调节MDCK上皮微组织的形成 基于以上实验,进一步检测细胞肌动蛋白收缩力是否参与调节MDCK微组织的发生。Y27632抑制Rho下游的ROCK信号通路[36],Blebbstanin和ML-7分别抑制非肌肉肌球蛋白Ⅱ型ATP酶和肌球蛋白Ⅱ轻链激酶(MLCK),从而抑制细胞中的收缩力作用[37-38]。将MDCK细胞分别培养在含二甲基亚砜(0.1%)、Y27632(40 μmol/L)、Blebbsitatin(40 μmol/L)、ML-7(40 μmol/L)的培养基中,每隔2 d更换1次培养基(含2% Matrigel)。 培养第9天后,均形成了MDCK微组织(图2A),与对照组(二甲基亚砜)相比,加入3种抑制剂之后ERK活性降低(图2A,B),细胞团变小(图2C)。通过CCK-8实验检测细胞增殖活性,发现Y27632对细胞增殖有一定促进作用、Blebbsitatin对细胞没有影响、ML-7对细胞增殖有一定抑制作用(图2D)。这些结果表明,抑制细胞内收缩力后,ERK活性降低,微组织体积变小,显示细胞收缩力作用是该微组织正常生长的必要条件。"
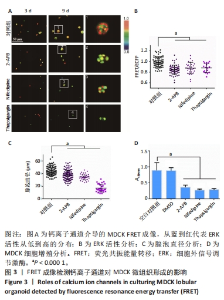
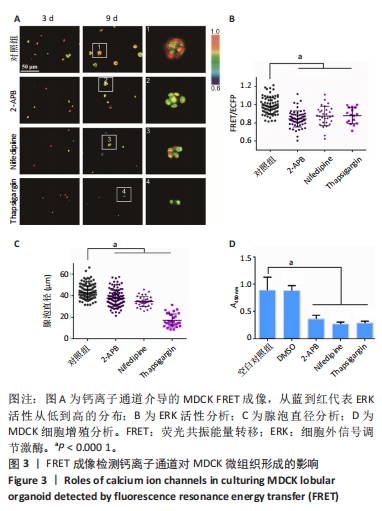
2.3 钙离子通道对MDCK微组织发生的调节作用 细胞钙信号常见于力学信号转导过程[39],课题组近期工作表明内质网膜上的钙通道在细胞-细胞力学通讯中至关重要[40]。该研究通过使用多种阻滞剂来研究Ca2+通道是否参与调控MDCK微组织的形成。2-APB能够选择性地抑制内质网上IP3R Ca2+ 通道,硝苯地平(Nifedipine)能够抑制质膜上L型Ca2+ 通道,Thapsigargin选择性地抑制内质网上的SERCA钙泵。将MDCK细胞分别培养在含二甲基亚砜(0.1%)、2-APB(10 μmol/L)、Nifedipine(10 μmol/L)、Thapsigargin(10 μmol/L)的培养基中,每隔2 d更换1次培养基(含2% Matrigel)。 培养第9天之后,用2-APB和Nifedipine处理的细胞能够形成微组织形态,而Thapsigargin处理的细胞没有组织形态的发生(图3A)。与对照组(二甲基亚砜)相比,2-APB和Nifedipine处理后ERK活性和细胞团大小显著下降(图3B,C)。CCK-8实验显示这3种抑制剂对细胞增殖都有明显抑制作用(图3D)。该结果显示选择性抑制IP3R Ca2+ 通道和质膜上L型Ca2+通道导致MDCK微组织变小,而Thapsigargin对细胞的毒性较大,影响微组织的正常形态发生。"
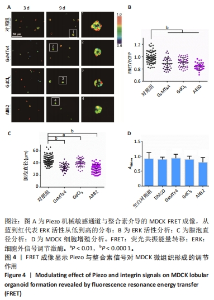
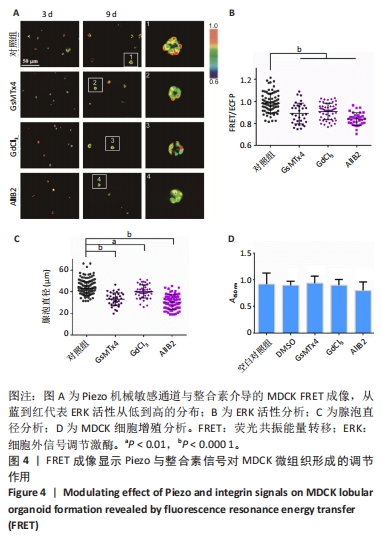
2.4 机械敏感离子通道Piezo与整合素信号调控MDCK上皮微组织的生长 Piezo是细胞膜上的一种机械敏感离子通道,可以将机械信号转化为生物信号,并导致多种细胞反应[41]。整合素是由亚基α和β组成的二聚体,作为细胞膜上的受体与胞外基质配体相互作用,调节多种动态细胞过程,如细胞迁移、吞噬作用以及生长和发育的细胞内分子信号[42]。该研究进一步检测细胞质膜上机械敏感受体分子Piezo和整合素对MDCK微组织发生的影响。GsMTx4可以选择性抑制Piezo,GdCl3是力学敏感型钙离子通道阻滞剂;AIIB2抗体可以作用于整合素代表型β1亚基,起到一定的阻滞作用。将MDCK细胞分别培养在含二甲基亚砜(0.1%)、GsMTx4(5 μmol/L)、GdCl3(25 μmol/L)、AIIB2(2 μg/mL)的DMEM高糖培养基中,每隔2 d更换1次培养基(含2% Matrigel)。 培养第9天后,与对照组(二甲基亚砜)相比,加入Piezo抑制剂的实验组ERK活性更低(图4A,B)、直径更小(图4C)。CCK-8实验分析显示该抑制剂对细胞增殖没有显著影响(图4D)。这些结果初步表明Piezo1力敏感通道对MDCK细胞中的ERK活性和组织形态大小发挥有效调控作用。加入AIIB2抗体后,实验组中仅形成没有完整组织形态的细胞簇,CCK-8实验分析表明AIIB2对MDCK细胞增殖没有显著影响(图4A-D),这些结果证实整合素在该组织形态发生中具有重要作用。"

2.5 不同细胞外基质环境介导的肾小管形成 基于以上细胞力敏感受体的作用,该研究进一步检测不同胞外基质环境对MDCK微组织生长的影响。将细胞分别培养在100% Matrigel凝胶、用PBS稀释过1倍的50% Matrigel凝胶、50%Matrigel基质胶+Ⅰ型胶原(0.5 mg/mL)、50%Matrigel基质胶+Ⅰ型胶原(1 mg/mL)和50%Matrigel基质胶+Ⅰ型胶原(2 mg/mL)上。 培养第9天后,与100% Matrigel凝胶相比,50% Matrigel凝胶上MDCK微组织的ERK活性和直径没有显著性差异(图5A-C)。在基质中加入不同质量浓度Ⅰ型胶原后,对MDCK微组织形态发生有显著影响,生成长形的细胞团(图5A)。与50% Matrigel凝胶条件比较,添加Ⅰ型胶原的各组ERK活性没有显著变化(图5B)。通过测量长轴方向的大小,发现Ⅰ型胶原质量浓度越高,形成的细胞团长度越长(图5D)。这些结果表明胞外基质胶的组成对MDCK的形态发生有重要影响。"

| [1] TANNER K, MORI H, MROUE R, et al. Coherent angular motion in the establishment of multicellular architecture of glandular tissues. Proc Natl Acad Sci U S A. 2012;109(6):1973-1978. [2] NARAYANAN V, SCHAPPELL LE, MAYER CR, et al. Osmotic Gradients in Epithelial Acini Increase Mechanical Tension across E-cadherin, Drive Morphogenesis, and Maintain Homeostasis. Curr Biol. 2020;30(4): 624-633.e4. [3] OUYANG M, YU JY, CHEN Y, et al. Cell-extracellular matrix interactions in the fluidic phase direct the topology and polarity of self-organized epithelial structures. Cell Prolif. 2021;54(4):e13014. [4] CHAN CH, LIN P, YANG TY, et al. Epithelial polarization in the 3D matrix requires MST3 signaling to regulate ZO-1 position. PLoS One. 2023; 18(5):e0285217. [5] ZEGERS MM, O’BRIEN LE, YU W, et al. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 2003;13(4):169-176. [6] FESSENDEN TB, BECKHAM Y, PEREZ-NEUT M, et al. Dia1-dependent adhesions are required by epithelial tissues to initiate invasion. J Cell Biol. 2018;217(4):1485-1502. [7] ZUO T, FENG X, ZHANG N, et al. Establishment of a functional secretory IgA transcytosis model system in vitro for functional food screening. Appl Microbiol Biotechnol. 2015;99(13):5535-5545. [8] 关业茂,王庆江,刘静,等.纳米细菌纤维素复合物支架用于MDCK细胞三维培养的研究[J].生物学杂志,2020,37(5):30-34. [9] 蔡国徽,韩云竹,唐富山,等.Matrigel三维培养下卵巢癌细胞生长状况观察[J].江苏大学学报(医学版),2015,25(1):14-18. [10] MAMMOTO T, MAMMOTO A, INGBER DE. Mechanobiology and developmental control. Annu Rev Cell Dev Biol. 2013;29:27-61. [11] PEI B, WANG W, FAN Y, et al. Fiber-reinforced scaffolds in soft tissue engineering. Regen Biomater. 2017;4(4):257-268. [12] ROIGNOT J, PENG X, MOSTOV K. Polarity in mammalian epithelial morphogenesis. Cold Spring Harb Perspect Biol. 2013;5(2):a013789. [13] KHAN LA, JAFARI G, ZHANG N, et al. A tensile trilayered cytoskeletal endotube drives capillary-like lumenogenesis. J Cell Biol. 2019;218(7): 2403-2424. [14] MONTEITH GR, PREVARSKAYA N, ROBERTS-THOMSON SJ. The calcium-cancer signalling nexus. Nat Rev Cancer. 2017;17(6):367-380. [15] PEPPIATT-WILDMAN CM, CRAWFORD C, HALL AM. Fluorescence imaging of intracellular calcium signals in intact kidney tissue. Nephron Exp Nephrol. 2012;121(1-2):e49-e58. [16] DEBNATH J, BRUGGE JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5(9):675-688. [17] SEIFERT A, POSERN G. Tightly controlled MRTF-A activity regulates epithelial differentiation during formation of mammary acini. Breast Cancer Res. 2017;19(1):68. [18] COX CD, BAE C, ZIEGLER L, et al. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun. 2016;7:10366. [19] DALGHI MG, CLAYTON DR, RUIZ WG, et al. Expression and distribution of PIEZO1 in the mouse urinary tract. Am J Physiol Renal Physiol. 2019;317(2):F303-F321. [20] GUDIPATY SA, LINDBLOM J, LOFTUS PD, et al. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature. 2017; 543(7643):118-121. [21] MYLLYMÄKI SM, TERÄVÄINEN TP, MANNINEN A. Two distinct integrin-mediated mechanisms contribute to apical lumen formation in epithelial cells. PLoS One. 2011;6(5):e19453. [22] MARTÍNEZ-ARA G, TABERNER N, TAKAYAMA M, et al. Optogenetic control of apical constriction induces synthetic morphogenesis in mammalian tissues. Nat Commun. 2022;13(1):5400. [23] CAMACHO-GÓMEZ D, GARCÍA-AZNAR JM, GÓMEZ-BENITO MJ. A 3D multi-agent-based model for lumen morphogenesis: the role of the biophysical properties of the extracellular matrix. Eng Comput. 2022;38(5):4135-4149. [24] CUKIERMAN E, BASSI DE. Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. Semin Cancer Biol. 2010; 20(3):139-145. [25] LIPUT DJ, NGUYEN TA, AUGUSTIN SM, et al. A Guide to Fluorescence Lifetime Microscopy and Förster’s Resonance Energy Transfer in Neuroscience. Curr Protoc Neurosci. 2020;94(1):e108. [26] YEH HW, AI HW. Development and Applications of Bioluminescent and Chemiluminescent Reporters and Biosensors. Annu Rev Anal Chem (Palo Alto Calif). 2019;12(1):129-150. [27] 包昆鹭,许琪,曹宏梅,等.基于共振能量转移的生物传感器在食品安全检测中的应用研究进展[J].分析测试学报,2021,40(5):656-661. [28] 马廷政,曹平平,汪徐春,等.利用FRET技术检测膜受体与胞外配体相互作用的优化策略[J].南京医科大学学报(自然科学版), 2022,42(8):1049-1054. [29] KASHIMATA M, SAYEED S, KA A, et al. The ERK-1/2 signaling pathway is involved in the stimulation of branching morphogenesis of fetal mouse submandibular glands by EGF. Dev Biol. 2000;220(2):183-196. [30] KOMATSU N, AOKI K, YAMADA M, et al. Development of an optimized backbone of FRET biosensors for kinases and GTPases. Mol Biol Cell. 2011;22(23):4647-4656. [31] SUGIURA R, SATOH R, TAKASAKI T. ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer. Cells. 2021;10(10):2509. [32] PONSIOEN B, POST JB, BUISSANT DES AMORIE JR, et al. Quantifying single-cell ERK dynamics in colorectal cancer organoids reveals EGFR as an amplifier of oncogenic MAPK pathway signalling. Nat Cell Biol. 2021;23(4):377-390. [33] HINO N, MATSUDA K, JIKKO Y, et al. A feedback loop between lamellipodial extension and HGF-ERK signaling specifies leader cells during collective cell migration. Dev Cell. 2022;57(19):2290-2304.e7. [34] QIN Q, LAUB S, SHI Y, et al. Fluocell for Ratiometric and High-Throughput Live-Cell Image Visualization and Quantitation. Front Phys. 2019;7:154. [35] MROZOWSKA PS, FUKUDA M. Regulation of podocalyxin trafficking by Rab small GTPases in 2D and 3D epithelial cell cultures. J Cell Biol. 2016;213(3):355-369. [36] TANG L, DAI F, LIU Y, et al. RhoA/ROCK signaling regulates smooth muscle phenotypic modulation and vascular remodeling via the JNK pathway and vimentin cytoskeleton. Pharmacol Res. 2018;133:201-212. [37] SHI J, TAKAHASHI S, JIN XH, et al. Myosin light chain kinase-independent inhibition by ML-9 of murine TRPC6 channels expressed in HEK293 cells. Br J Pharmacol. 2007;152(1):122-131. [38] LIMOUZE J, STRAIGHT AF, MITCHISON T, et al. Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil. 2004;25(4-5):337-341. [39] DE FELICE D, ALAIMO A. Mechanosensitive Piezo Channels in Cancer: Focus on altered Calcium Signaling in Cancer Cells and in Tumor Progression. Cancers (Basel). 2020;12(7):1780. [40] OUYANG M, ZHU Y, WANG J, et al. Mechanical communication-associated cell directional migration and branching connections mediated by calcium channels, integrin β1, and N-cadherin. Front Cell Dev Biol. 2022;10:942058. [41] LAI A, COX CD, CHANDRA SEKAR N, et al. Mechanosensing by Piezo1 and its implications for physiology and various pathologies. Biol Rev Camb Philos Soc. 2022;97(2):604-614. [42] XU S, ZHANG T, CAO Z, et al. Integrin-α9β1 as a Novel Therapeutic Target for Refractory Diseases: Recent Progress and Insights. Front Immunol. 2021;12:638400. [43] 张斌斌,高全文,李冰,等.骨髓间充质干细胞在Matrigel凝胶支架上的生长与变化[J].中国组织工程研究,2018,22(13):1993-1998. [44] MONTESANO R, MATSUMOTO K, NAKAMURA T, et al. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67(5):901-908. [45] LIU Z, GRECO AJ, HELLMAN NE, et al. Intracellular signaling via ERK/MAPK completes the pathway for tubulogenic fibronectin in MDCK cells. Biochem Biophys Res Commun. 2007;353(3):793-798. [46] TANG H, ZENG R, HE E, et al. Piezo-Type Mechanosensitive Ion Channel Component 1 (Piezo1): A Promising Therapeutic Target and Its Modulators. J Med Chem. 2022;65(9):6441-6453. |
| [1] | Yang Mengxiao, Fu Changxi. Mechanism by which strength training improves bone injury in ovariectomized rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(20): 3150-3156. |
| [2] | Zhu Shijie, Yang Yiting, Cao Yuting, Zheng Liangdong, Lin Kaili, Zhu Rui. Cartilage protective effect of swimming exercise in aged mice with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(20): 3170-3175. |
| [3] | Yang Yunyun, Chen Qiqing, Zhao Jirong, Zhu Bao, Ma Dong, Huang Junkai, An Dehao, Zou Jipeng, Liu Weihang. Research status of traditional Chinese medicine monomer mediating related signaling pathways in treatment of intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(18): 2918-2924. |
| [4] | Liang Chao, Liao Li, Tian Weidong. Application of cell derivative in periodontal regeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(17): 2731-2738. |
| [5] | Zhao Shasha, He Qing, Li Jia, Wu Ying. Effects of recombinant human collagen supplementation on extracellular matrix remodeling in mouse skeletal muscle after eccentric exercise [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(16): 2542-2549. |
| [6] | Yao Siqi, Li Wenzheng, Wang Hong. Transforming growth factor beta/Smad signaling pathway and targeted therapy of keloid scars [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(16): 2619-2624. |
| [7] | Liang Chen, Zhu Tonghe, Zhu Yiyao, Li Ruizhi. Biomimetic design of biomedical scaffolds and their application in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(15): 2416-2422. |
| [8] | Zhao Lixin, Tang Qingxi, Zhang Kezhong. Influence of adipose tissue and its derivative on wound repair and vascularization [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(13): 2120-2125. |
| [9] | Fan Zhihong, Zhang Xian, Li Chao. Wnt signaling pathway in intervertebral disc degeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(12): 1950-1955. |
| [10] | Li Long, Li Guangdi, Shi Hao, Deng Keqi. Circular RNA as a competing endogenous RNA is involved in the regulation of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 751-757. |
| [11] | Teng Jianxiang, Zhu Jisheng, Yuan Daizhu, Wang Zhen, Zhou Yuhu, Tian Xiaobin. Preparation of cartilage decellularized extracellular matrix-loaded composite nanofiber scaffolds based on two-nozzle electrospinning [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(34): 5448-5454. |
| [12] | Jiang Honghui, Kong Yuanyuan, Liu Jing, Wang Zhihong. Preparation and applications of decellularized extracellular matrix bioink in cardiovascular fields [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4904-4911. |
| [13] | Wu Jiang, Yin Han, Yan Zineng, Tian Guangzhao, Ding Zhengang, Yuan Xun, Fu Liwei, Tian Zhuang, Sui Xiang, Liu Shuyun, Guo Quanyi. Preparation of hierarchical porous cartilage composite scaffolds loaded with stromal cell-derived factor-1 using low-temperature deposition 3D printing [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4776-4782. |
| [14] | Yang Shun, Zhao Mingyue, Tu Xiling, Gao Li, Yang Kun, Liu Qi. Application of decellularized extracellular matrix composite scaffolds in tissue regeneration [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(30): 4856-4861. |
| [15] | Chen Cai, Zeng Ping, Liu Jinfu. Long non-coding RNAs regulate osteoarthritis by mediating chondrocyte-related mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(29): 4729-4735. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||