Chinese Journal of Tissue Engineering Research ›› 2013, Vol. 17 ›› Issue (28): 5236-5242.doi: 10.3969/j.issn.2095-4344.2013.28.022
Previous Articles Next Articles
Does Dlx abnormal expression regulate the migration of cranial neural crest cells and development of the first brachial arch?
Liu Zhi-xu, Sun Hao, Wang Xu-dong
- Department of Oral and Maxillofacial Surgery, Ninth People’s Hospital of Shanghai Jiao Tong University School of Medicine, Key Oral Laboratory of Shanghai City, Shanghai 200011, China
-
Online:2013-07-09Published:2013-07-09 -
Contact:Wang Xu-dong, M.D., Chief physician, Department of Oral and Maxillofacial Surgery, Ninth People’s Hospital of Shanghai Jiao Tong University School of Medicine, Key Oral Laboratory of Shanghai City, Shanghai 200011, China -
About author:Liu Zhi-xu, Studying for master’s degree, Department of Oral and Maxillofacial Surgery, Ninth People’s Hospital of Shanghai Jiao Tong University School of Medicine, Key Oral Laboratory of Shanghai City, Shanghai 200011, China liuliu232000@yahoo.com.cn -
Supported by:the Natural Science Foundation of Shanghai City, No. 10ZR1418000*;
the Health Bureau of Shanghai City, No. 2009077*;
the National Natural Science Foundation of China, No. 81271122*
CLC Number:
Cite this article
Liu Zhi-xu, Sun Hao, Wang Xu-dong. Does Dlx abnormal expression regulate the migration of cranial neural crest cells and development of the first brachial arch?[J]. Chinese Journal of Tissue Engineering Research, 2013, 17(28): 5236-5242.
share this article
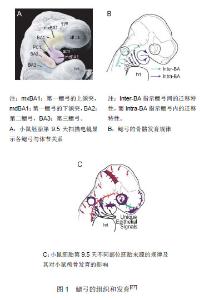
2.1 纳入资料基本概况 纳入文献包括颅神经嵴细胞迁移的文章6篇[15-20],Dlx高表达对颅神经嵴细胞迁移影响的文章12篇[9,13,21-30];细胞周围环境、细胞因子、Dlx基因相互作用有关的文章17篇[9,13,23,29,31-43]。 2.2 纳入资料的研究结果特征 2.2.1 颅神经嵴细胞的迁移 神经嵴细胞是具有多分化潜能的胚胎细胞,它是由神经褶的一些细胞迁移到神经管的背侧,形成的一条神经细胞索,继而分裂为两条,后位于神经管的背外侧。人胚胎第4周末,神经嵴开始分节,其中进入后脑的颅神经嵴细胞进一步分化分区,形成8个菱脑原节,随后迁入第一、第二鳃弓,见图1。其中这8个菱脑原节对颅颌面的发育具有重要意义:①它为颅神经嵴细胞形成数支彼此分隔的迁移流提供了一定的解剖结构基础,其中由偶数菱脑原节迁移出的菱脑原节较多,而由奇数菱脑原节迁出的菱脑原节较少,甚至没有迁出。②菱脑原节的出现,防止了颅颌面部骨与神经等结构发育发生位置错乱。一般来说,特定的菱脑原节的颅神经嵴细胞会迁入特定的鳃弓,如,第一、第二菱脑原节的细胞迁入第一鳃弓,第四菱脑原节的细胞则定向迁入第二鳃弓[15]。因此颅神经嵴细胞的正确分区并迁移对颅颌面发育具有十分重要的意义。"


颅神经嵴细胞从后脑迁出过程至少可以分为3个阶段。第一阶段为颅神经嵴细胞通过与表面外胚层及颅神经嵴细胞邻近的间充质作用,获得沿背外侧迁移的能力;第二阶段,颅神经嵴细胞以松散的结构由背外侧向鳃弓迁移;在最后一个阶段颅神经嵴细胞最终进入鳃弓[16]。 在整个迁移过程中,颅神经嵴细胞的迁移路线具有一定的可塑性。神经嵴细胞迁移路线可以发生改变,并且具有多样性,例如,当第五、第六菱脑节被移除时,正常本应迁入第四鳃弓的第七菱脑节的颅神经嵴细胞则会改变迁移路线,进入第三鳃弓[17]。这个现象说明颅神经嵴细胞的迁移具有可塑性,它的迁移路线是由多个信号分子决定,并且通过迁移过程中细胞-细胞的黏附等相互作用而不断进行调整、改变[18]。 颅神经嵴细胞从迁移的起始,至迁移到最终的位置的整个过程,均有上皮与间充质组织的相互作用,及多个细胞因子通过其时空、时间表达不同,而进行精密的调控。这些调控作用包括黏附因子表达水平的改变,一些趋化因子及诱导因子的相关作用,其中,细胞的黏附在其中起了关键作用[19-20]。 2.2.2 Dlx高表达对颅神经嵴细胞迁移的影响 Dlx基因家族,是编码一组进化过程中保留下来的,与Hox基因相关的转录因子,这些转录因子与早期胚胎发育模式的建立有关系。它属于同源异型盒基因大家族中的歧异同源盒基因,在果蝇中被称为Dll基因,参与果蝇的腿、触角和嘴等附属器的发育。在脊椎动物,Dlx基因与颌面部,尤其是第一鳃弓的发育密切相 关[21]。在人类和小鼠,均存在6个Dlx基因,分别是Dlx1、Dlx2、Dlx3、Dlx4、Dlx5、Dlx6,其中Dlx4又可称之为Dlx7。这些基因在颌面部发育中对颅神经嵴细胞的迁移分化和器官形态发生等起着重要的调控作用。Dlx基因家族成员的作用既有相互独立的部分,又存在相互重叠的部分。因此一个基因表达异常,会出现一定的畸形,但是由于代偿作用的存在,畸形表现将会有一定程度的降低,给研究工作带来一定的复杂性。 Dlx家族基因表达增强,黏附因子生成增加:Dlx家族基因在颅神经嵴细胞中高表达,其时空表达的改变将影响颅颌面的发育。在颅神经嵴细胞迁移的启动阶段,多种细胞黏附因子表达发生改变,比如神经细胞黏附因子、N-钙黏蛋白表达下调[22],并且在颅神经嵴细胞迁移的第二阶段,细胞一般以松散的形式进行迁移。但是,Dlx2高表达将使颅神经嵴细胞黏附成细胞团,并且在神经管基侧聚集,只有少部分高表达Dlx2的颅神经嵴细胞迁移至鳃弓当中[9]。McKeown等通过电穿孔技术,将含小鼠Dlx2基因质粒导入鹌鹑胚颅部体节,他们发现被成功转染了Dlx2的细胞主要分成3种类型:①一小部分Dlx2转染细胞循正常路径进入颅神经节及鳃弓。②大部分的Dlx2转染细胞在神经管内聚集,而它们中的大部分又将迁移至神经管的顶部和基部,最后离开神经管。③数量较少但是很显著的一群Dlx2转染细胞仍以单个细胞的状态存在于神经管中,并呈现梭型的神经外胚层细胞形态。于此同时,Dlx2转染细胞,神经细胞黏附因子,N-钙黏蛋白出现表达增多,这些事实可以在一定程度上说明Dlx2的高表达将通过影响黏附因子而改变颅神经嵴细胞的迁移[9]。而体外实验同样表明,Dlx基因可以控制细胞的黏附[23],并且可以诱导细胞对其周围细胞进行选择及黏附[9]。由于黏附因子表达的改变而导致的间充质的浓聚变化,以及骨形成的相关细胞因子的作用[13],颌面部的发育最后可能发生改变,出现畸形。 Dlx家族基因中,Dlx5的表达增高,将同样促使如神经细胞黏附因子,N-钙黏蛋白等的黏附因子表达增强[9];在Ba/F3细胞中,细胞间黏附分子1和2的表达也会随Dlx4增高而增强[23]。通过这些现象,可以推测,Dlx家族基因通过影响细胞黏附能力而改变细胞的聚集及迁移。 Dlx基因高表达,影响颅神经嵴细胞迁移的可能机制:根据McKeown等[9]的研究,转染了Dlx2或者Dlx5的细胞,迁移至原本无神经嵴细胞的间充质构成的鳃弓中心区域。这可能是由于神经细胞黏附因子,N-钙黏蛋白表达的增多,直接通过细胞间信号分子使原无相关因子表达的颅神经嵴细胞发生迁移改变;也可能是由于黏附因子的表达,使得表达这些黏附因子的颅神经嵴细胞黏附了相关间充质细胞,而使得其发生迁移位点的改变。Msx2与Dlx基因同属同源盒基因,但其常是一种抑制转录因子。Diamond等[24]研究发现Dlx2可以通过与lef-1协调作用,上调Msx2表达。Zhang等[25]研究发现,颅颌面Dlx2与Msx2表达具有一定的关联性:在体外实验中,Dlx可与Msx2形成二聚体,从而抑制Dlx的活性。而Dlx2在颅神经嵴细胞迁移前即有表达,并且其在鳃弓中的表达并未伴有细胞聚集现象发生。据此可以推测,在正常颅神经嵴细胞迁移过程中Dlx的表达会受到其他细胞因子的影响,如Msx2表达降低,从而使得与其相关的黏附因子表达减少,最终帮助正常颅神经嵴细胞正确进入鳃弓。 Dlx基因表达异常导致的畸形表现:Dlx基因转录蛋白可以通过改变神经外胚层与非神经外胚层的边界,细胞周围的细胞间因子而影响其邻近细胞的分化[26]。在组织水平层面,成骨或成软骨的早期步骤包括间充质的浓聚,细胞基质及细胞黏附因子等相关细胞因子的聚集等[13]。成骨或者成软骨的障碍,将导致颌面部畸形的发生。 Dlx2基因敲除小鼠出现畸形。根据Qiu等的研究,当Dlx2-/-纯合子小鼠出生时即死亡,其第一鳃弓上颌骨的相关结构均被累及,出现严重畸形,如蝶基骨、蝶骨大翼、翼板形态异常、腭裂。而Dlx2+/-的杂合子小鼠的表型出现了轻微的改变,如颞翼水平板的分裂。其他Dlx家族基因敲除小鼠,包括纯合子和非纯合子也出现了各自不同的颅颌面部成骨的异常[27]。 Gordon等[13]使用含Dlx2或Dlx5的病毒转染鸡胚中迁移的细胞,或是已迁至第一鳃弓的细胞进行实验。在Dlx2高表达的鸡胚中,出现了异位的骨及软骨的表达。这些异位表达具有一定的模式,其中最常见的为蝶基骨向外伸展的短棒状的软骨,异位软骨结节,由眶突向其前中方向伸展并靠近蝶骨的软骨;在上颌骨可见异位膜内成骨,并在上颌骨的外侧端常见。进一步实验则表明,在这些异位软骨中可以观察到携带了Dlx2的病毒,见图2。而在McKeown等[9]的研究中,将含Dlx2的质粒,通过电转染,转染至鹌鹑颅部体节,会出现大量异位软骨的生成。早前的研究表明,间充质干细胞在向软骨细胞分化的过程中会观察到凋亡现象的产生[28]。Dai等[29]应用转基因技术建立Dlx2条件性激活转基因小鼠(iZEG-Dlx2)获得只在神经嵴细胞高表达Dlx2的小鼠。通过这些高表达Dlx2的转基因小鼠,研究者发现当Dlx2高表达时颅神经嵴细胞出现增殖减少、凋亡增多的现象;随即出现异常软骨生成增加及骨生成的减少,其表型包括唇裂、面裂、神经管缺损、鼻骨、额骨、上颌骨畸形和错牙合畸形。而孙昊等[30]通过体外构建高表达Dlx2的MC3T3-E1-Dlx2,同样也发现Dlx2过表达促进细胞凋亡,及增殖性降低的现象。 2.2.3 与Dlx基因相互作用细胞环境 细胞周围环境可影响Dlx的表达效果,例如,当Gsx1和Ggx2表达缺失,Dlx1/2表达异常所导致的畸形有一定程度的减 轻[31]。颅颌面发育过程中,间充质的浓聚,在正常胚胎中是由外胚层与间充质阶段性的相互作用下诱导形成,这也是使得颅神经嵴细胞分化成骨或软骨的重要步骤[32]。有文献指出,间充质的浓聚程度将决定软骨生成[23]。而间充质的浓聚与黏连蛋白关系密不可分:N-钙黏蛋白表达首先身高,紧接着就是神经细胞黏附因子的表达增加[33]。体外实验也表明,肢芽的N-钙黏蛋白与神经细胞黏附因子高表达使得其间充质浓聚,软骨生成增加[34]。McKeown等[9]进行的通过电转染使得Dlx2或Dlx5高表达的鹌鹑胚胎中,同样出现了神经细胞黏附因子与N-钙黏蛋白表达增加、间充质的浓聚,并且生成大量异位软骨组织;在体外试验中,Dlx2高表达,间充质虽然也发生了浓聚,但与正常颅神经嵴细胞相比,不能表达Ⅱ型胶原,同时缺少软骨的生成。而Xu等[35]研究发现,骨形态发生蛋白2、4可通过上调成软骨细胞中Dlx2的表达来调控Ⅱ型胶原的表达,进而调节软骨的生成。Gordon等[13]进行的胚胎实验表明,Dlx2高表达将诱导其周围结缔组织异常分化,进而出现了异常骨与软骨的生成。因此,根据以上研究,猜测Dlx2诱导骨与软骨的生成,除了需要间充质的浓聚还必须有其他相关细胞因子的参与。 根据相关文献的介绍,Dlx2高表达不仅可以诱导异位软骨的生成[9, 13, 29],还可以增加骨的生 成[13, 36]。在Gordon等[13]的研究中,Dlx2表达增高动物的上颌骨的外侧区,只有异位骨生成的增加,而无异位软骨的生成,而上颌骨的中份则刚好相反。Dlx2高表达细胞群,其周围的环境可能决定其分化的方向:生成软骨或是生成骨。 与Gordon实验所使用的正在迁移的鸡胚细胞或是已迁至第一鳃弓的细胞进行研究不同,孙昊等[36]使用含Dlx2的反转录病毒转染前成骨细胞系MC3T3-E1,并在成骨诱导液下培养细胞,他们发现该细胞系碱性磷酸酶,骨钙素等成骨相关基因表达上调,Dlx2高表达促进了该细胞系向成骨方向发展。这个现象的出现是否是由于成骨诱导液中所含成分与Dlx2相互作用,从而介导细胞系向成骨的方向发展;亦或是由于其选择的前成骨细胞系自身所表达的某些细胞因子与Dlx2相互作用,使得细胞系向成骨方向发展。同时Burns等[37]关于成骨方面的研究也表明,RUNX2/CBFA1, Msx2, Dlx5转录因子共同高表达,并在一定的培养条件下可以促进骨的形成。 成纤维细胞生长因子在软骨分化过程中具有十分重要的作用。Fgf家族中的Fgf3也是诱导软骨的形成的重要因子,如果抑制其表达,将会导致Dlx2表达的下降,但是其表达增多却无法挽救Dlx2表达缺失所造成所有的畸形[38],这可能与Dlx2需要与多个相关细胞因子作用相关。Dlx2可以直接诱导成纤维生长因子家族的成员表达增加,比如betacellulin,而后者可以刺激Fgf受体的表达升高[39]。Fgf9可与Fgf受体FGFR3有高的结合能力[40-41],同时Fgf9高表达能促进软骨生成增多[41]。因此,作者推测Dlx2高表达动物软骨生成增多,可能与其与Fgf家族的相互作用有关。 不仅仅是Fgf,Msx也可与Dlx家族成员相互作用,例如Msx与Dlx2的相互作用将影响破骨细胞的形成,进而影响骨的形态及形成[14];Msx也可与Dlx5相互作用而促进骨的形成[42]。而Shh作为上皮信号分子,它能与外胚间充质反应,促进其分化形成软骨[43]。这些细胞因子与Dlx基因是否有直接或间接协调作用,以及其如何协调仍有待进一步研究。"
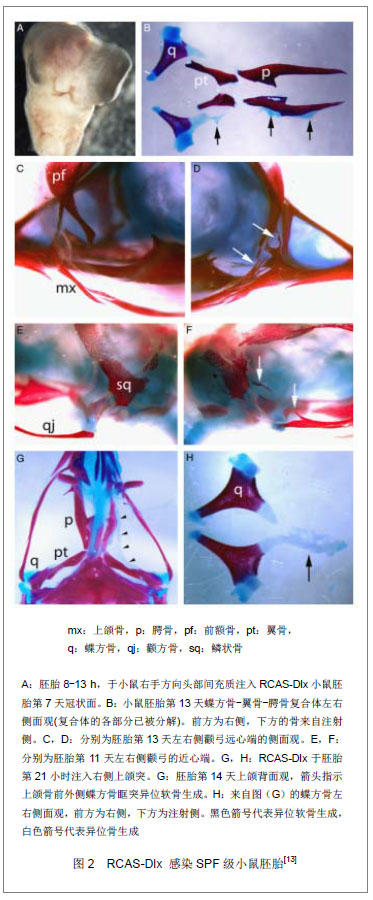
| [1]Gross JB, Hanken J. Review of fate-mapping studies of osteogenic cranial neural crest in vertebrates.Dev Biol. 2008; 317(2):389-400.[2]Chai Y, Maxson RE Jr. Recent advances in craniofacial morphogenesis. Dev Dyn. 2006;235(9):2353-2375.[3]Merrill AE, Eames BF, Weston SJ,et al. Mesenchyme- dependent BMP signaling directs the timing of mandibular osteogenesis. Development. 2008;135(7):1223-1234.[4]Eames BF, Schneider RA.The genesis of cartilage size and shape during development and evolution. Development. 2008;135(23):3947-3958.[5]Stadler HS, Higgins KM, Capecchi MR. Loss of Eph-receptor expression correlates with loss of cell adhesion and chondrogenic capacity in Hoxa13 mutant limbs. Development. 2001;128(21):4177-4188.[6]Cobb J, Duboule D.Comparative analysis of genes downstream of the Hoxd cluster in developing digits and external genitalia. Development. 2005;132(13):3055-3067.[7]代杰文. Dlx基因在哺乳动物颌面部发育中的调控作用研究进展[J]. 口腔颌面外科杂志, 2010, 20(6): 438-442.[8]Depew MJ, Lufkin T, Rubenstein JL.Specification of jaw subdivisions by Dlx genes.Science. 2002;298(5592):381-385.[9]McKeown SJ, Newgreen DF, Farlie PG. Dlx2 over-expression regulates cell adhesion and mesenchymal condensation in ectomesenchyme. Dev Biol. 2005;281(1):22-37.[10]Panganiban G, Rubenstein JL. Developmental functions of the Distal-less/Dlx homeobox genes. Development. 2002; 129(19):4371-4386.[11]Bulfone A, Kim HJ, Puelles L,et al.The mouse Dlx-2 (Tes-1) gene is expressed in spatially restricted domains of the forebrain, face and limbs in midgestation mouse embryos. Mech Dev. 1993;40(3):129-140.[12]Francis-West P, Ladher R, Barlow A,et al. Signalling interactions during facial development. Mech Dev. 1998;75(1-2):3-28.[13]Gordon CT, Brinas IM, Rodda FA,et al. ole of Dlx genes in craniofacial morphogenesis: Dlx2 influences skeletal patterning by inducing ectomesenchymal aggregation in ovo. Evol Dev. 2010;12(5):459-473.[14]Lézot F, Thomas BL, Blin-Wakkach C,et al. Dlx homeobox gene family expression in osteoclasts. J Cell Physiol. 2010; 223(3):779-787.[15]卢境婷,王旭东,代杰文,等.颅神经嵴细胞的迁移及特性[J].中华口腔医学研究杂志:电子版, 2011,5(6):652-657.[16]Kulesa PM, Bailey CM, Kasemeier-Kulesa JC,et al. Cranial neural crest migration: new rules for an old road. Dev Biol. 2010;344(2):543-554.[17]Kulesa PM, Fraser SE. In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches. Development. 2000;127(6):1161-1172.[18]Clay MR, Halloran MC. Regulation of cell adhesions and motility during initiation of neural crest migration. Curr Opin Neurobiol. 2011;21(1):17-22.[19]McKeown SJ, Wallace AS, Anderson RB. Expression and function of cell adhesion molecules during neural crest migration. Dev Biol. 2013;373(2):244-257.[20]Alfandari D, Cousin H, Marsden M. Mechanism of Xenopus cranial neural crest cell migration. Cell Adh Migr. 2010;4(4): 553-560.[21]Takechi M, Adachi N, Hirai T,et al.The Dlx genes as clues to vertebrate genomics and craniofacial evolution.Semin Cell Dev Biol. 2013. pii: S1084-9521(12)00234-0.[22]Coles EG, Taneyhill LA, Bronner-Fraser M.A critical role for Cadherin6B in regulating avian neural crest emigration. Dev Biol. 2007;312(2):533-544.[23]Shimamoto T, Ohyashiki K, Takeshita K. Overexpression of the homeobox gene DLX-7 inhibits apoptosis by induced expression of intercellular adhesion molecule-1.Exp Hematol. 2000;28(4):433-441.[24]Diamond E, Amen M, Hu Q, et al. Functional interactions between Dlx2 and lymphoid enhancer factor regulate Msx2. Nucleic Acids Res. 2006;34(20):5951-5965.[25]Zhang H, Hu G, Wang H, et al. Heterodimerization of Msx and Dlx homeoproteins results in functional antagonism.Mol Cell Biol. 1997;17(5):2920-2932.[26]Woda JM, Pastagia J, Mercola M,et al. Dlx proteins position the neural plate border and determine adjacent cell fates. Development. 2003;130(2):331-342.[27]Depew MJ, Simpson CA, Morasso M,et al. Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. J Anat. 2005;207(5):501-561.[28]Wang CY, Chen LL, Kuo PY,et al. Apoptosis in chondrogenesis of human mesenchymal stem cells: effect of serum and medium supplements. Apoptosis. 2010;15(4): 439-449.[29]Dai J, Kuang Y, Fang B,et al.The effect of overexpression of Dlx2 on the migration, proliferation and osteogenic differentiation of cranial neural crest stem cells. Biomaterials. 2013;34(8):1898-1910.[30]孙昊,王旭东,戴杰文,等. Dlx2基因过表达与前成骨细胞系MC3T3-E1成骨分化过程中的细胞凋亡和周期调控[J].中国组织工程研究, 2012,16(10): 1808-1812.[31]Wang B, Long JE, Flandin P,et al. Loss of Gsx1 and Gsx2 function rescues distinct phenotypes in Dlx1/2 mutants. J Comp Neurol. 2012. [Epub ahead of print][32]Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000; 22(2):138-147.[33]Tavella S, Raffo P, Tacchetti C,et al. N-CAM and N-cadherin expression during in vitro chondrogenesis. Exp Cell Res. 1994;215(2):354-362.[34]Delise AM, Tuan RS. Analysis of N-cadherin function in limb mesenchymal chondrogenesis in vitro. Dev Dyn. 2002;225(2): 195-204.[35]Xu SC, Harris MA, Rubenstein JL,et al. Bone morphogenetic protein-2 (BMP-2) signaling to the Col2alpha1 gene in chondroblasts requires the homeobox gene Dlx-2. DNA Cell Biol. 2001;20(6):359-365.[36]孙昊,王旭东,沈国芳,等.Dlx2在MC3T3-E1细胞成骨分化过程中的作用[J]. 中国口腔颌面外科杂志, 2011,9(3): 189-194.[37]Burns JS, Rasmussen PL, Larsen KH,et al. Parameters in three-dimensional osteospheroids of telomerized human mesenchymal (stromal) stem cells grown on osteoconductive scaffolds that predict in vivo bone-forming potential.Tissue Eng Part A. 2010;16(7):2331-2342.[38]David NB, Saint-Etienne L, Tsang M,et al. Requirement for endoderm and FGF3 in ventral head skeleton formation. Development. 2002;129(19):4457-4468.[39]Yilmaz M, Maass D, Tiwari N,et al.Transcription factor Dlx2 protects from TGFβ-induced cell-cycle arrest and apoptosis. EMBO J. 2011;30(21):4489-4499.[40]Wang Y, Wu XL, Wei DQ,et al. Autoinhibitory mechanism for the mutation-induced impaired FGF9 signaling. J Chem Inf Model. 2012;52(9):2422-2429.[41]Dai J, Wang J, Lu J,et al.The effect of co-culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials. 2012;33(31): 7699-7711.[42]Chung IH, Han J, Iwata J,et al. Msx1 and Dlx5 function synergistically to regulate frontal bone development.Genesis. 2010;48(11):645-655.[43]Benouaiche L, Gitton Y, Vincent C,et al. Sonic hedgehog signalling from foregut endoderm patterns the avian nasal capsule. Development. 2008;135(13):2221-2225. |
| [1] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| [2] | Chen Junyi, Wang Ning, Peng Chengfei, Zhu Lunjing, Duan Jiangtao, Wang Ye, Bei Chaoyong. Decalcified bone matrix and lentivirus-mediated silencing of P75 neurotrophin receptor transfected bone marrow mesenchymal stem cells to construct tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 510-515. |
| [3] | Ma Zhijie, Li Jingyu, Cao Fang, Liu Rong, Zhao Dewei. Influencing factors and biological property of novel biomedical materials: porous silicon carbide coated with bioactive tantalum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 558-563. |
| [4] | Shi Xiaoxiu, Mao Shilong, Liu Yang, Ma Xingshuang, Luo Yanfeng. Comparison of tantalum and titanium (alloy) as orthopedic materials: physical and chemical indexes, antibacterial and osteogenic ability [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 593-599. |
| [5] | Li Xinping, Cui Qiuju, Zeng Shuguang, Ran Gaoying, Zhang Zhaoqiang, Liu Xianwen, Fang Wei, Xu Shuaimei. Effect of modification of β-tricalcium phosphate/chitosan hydrogel on growth and mineralization of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3493-3499. |
| [6] | Zhou Anqi, Tang Yufei, Wu Bingfeng, Xiang Lin. Designing of periosteum tissue engineering: combination of generality and individuality [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3551-3557. |
| [7] | Chen Song, He Yuanli, Xie Wenjia, Zhong Linna, Wang Jian. Advantages of calcium phosphate nanoparticles for drug delivery in bone tissue engineering research and application [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3565-3570. |
| [8] | Ailimaierdan·Ainiwaer, Wang Ling, Gu Li, Dilidaer•Taxifulati, Wang Shan, Yin Hongbin. Effect of transforming growth factor-beta3 on the proliferation and osteogenic capability of osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(17): 2664-2669. |
| [9] | Huang Na, Liu Jiayue, Huang Yingjie, Wen Junmao, Wang Haibin, Zhang Qingwen, Zhou Chi . Bibliometric and visualized analysis of research on osteonecrosis of the femoral head from the Web of Science in the last 5 years [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(17): 2711-2718. |
| [10] | Zhang Xianjun, Zhao Xijiang. In vivo osteogenic properties of silicon-incorporated titanium dioxide nanotubes on titanium screw surface [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2461-2465. |
| [11] | Chen Jufang, Tian Yulou, Hao Xin. Role and potential of adipose-derived stem cells in cranio-maxillofacial bone regeneration [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(13): 2087-2096. |
| [12] | Zhuang Chuanji, Chen Wenzhao, Jiang Xinmin. Osteogenesis in vitro and bone defect repair in vivo of bone morphogenetic protein 9 composite collagen based bone repair material [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1489-1494. |
| [13] | Wu Ming, Zhang Yan. Related factors regulating osteogenic differentiation of bone marrow mesenchymal stem cells through Wnt/β-catenin signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(1): 116-122. |
| [14] | Zhao Chuntao, Qing Mingsong, Yu Langbo, Peng Jiachen . Meta-analysis of total knee arthroplasty guided by kinematic alignment and mechanical alignment [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(9): 1435-1442. |
| [15] |
Zhang Cong, Zhao Yan, Du Xiaoyu, Du Xinrui, Pang Tingjuan, Fu Yining, Zhang Hao, Zhang Buzhou, Li Xiaohe, Wang Lidong.
Biomechanical analysis of the lumbar spine and pelvis in adolescent
idiopathic scoliosis with lumbar major curve |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||