Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (29): 4734-4740.doi: 10.12307/2024.566
Previous Articles Next Articles
Application and development of bone tissue engineering scaffolds with bone immune regulatory properties in repairing bone defects
Zhou Yuxiang1, Shen Liejun2, Wan Shiyu3, Chai Luyu1, Pang Renqi1, Li Dengshun4, Wang Xin5, Li Zhanzhen1, 2
- 1Jiangxi University of Chinese Medicine, Nanchang 330004, Jiangxi Province, China; 2Zhoushan Dinghai Guanghua Hospital, Zhoushan 316000, Zhejiang Province, China; 3Guangzhou University of Chinese Medicine, Guangzhou 511400, Guangdong Province, China; 4College of Science and Technology, Jiangxi University of Chinese Medicine, Nanchang 330004, Jiangxi Province, China; 5Chinese PLA General Hospital, Beijing 100028, China
-
Received:2023-10-09Accepted:2023-11-27Online:2024-10-18Published:2024-03-23 -
Contact:Li Zhanzhen, Master’s supervisor, Jiangxi University of Chinese Medicine, Nanchang 330004, Jiangxi Province, China; Zhoushan Dinghai Guanghua Hospital, Zhoushan 316000, Zhejiang Province, China Wang Xin, MD, Master’s supervisor, Chinese PLA General Hospital, Beijing 100028, China -
About author:Zhou Yuxiang, Master candidate, Jiangxi University of Chinese Medicine, Nanchang 330004, Jiangxi Province, China -
Supported by:Zhejiang Provincial Health Science and Technology Plan, No. 2022KY1372 (to SLJ); Zhoushan Medical Health Science and Technology Plan, No. 2022YA16 (to SLJ)
CLC Number:
Cite this article
Zhou Yuxiang, Shen Liejun, Wan Shiyu, Chai Luyu, Pang Renqi, Li Dengshun, Wang Xin, Li Zhanzhen. Application and development of bone tissue engineering scaffolds with bone immune regulatory properties in repairing bone defects[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(29): 4734-4740.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
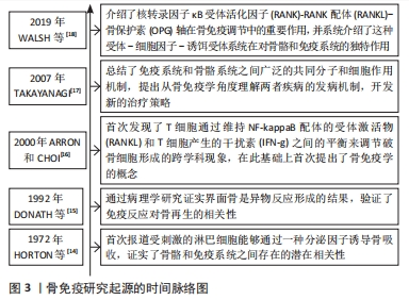
2.1 骨免疫学概述 骨免疫学是一个研究骨骼和免疫系统之间的相互作用的交叉学科,主要强调骨组织与免疫系统之间共享的分子作用机制,及二者间的双向调控体系。在1972年,HORTON等[14]在研究中发现,在体外胎鼠骨骼培养物中,受刺激的淋巴细胞通过分泌破骨细胞激活因子,增加了活性破骨细胞的数量,并诱导骨吸收,这提示骨骼和免疫系统之间可能存在潜在的作用机制。1992年,DONATH等[15]在关于人工假体植入材料的异物反应病理中,将界面骨的形成描述为异物反应的结果,再次证实了免疫反应和骨再生相关性。ARRON和CHOI[16]于2000年首次提出了“骨免疫学”的概念,以描述其发现的T细胞通过维持核转录因子kB配体的受体激活物和T细胞产生的干扰素之间的平衡来调节破骨细胞活性的跨学科现象。TAKAYANAGI[17]总结了免疫系统和骨骼系统之间广泛的共同分子和细胞作用机制,提出从骨免疫学角度理解两者疾病的发病机制,对开发新的治疗策略起到了重要启示作用。WALSH等[18]分析了核转录因子κB受体活化因子(receptor activator of nuclear factor-κB,RANK)-RANK配体(RANK ligand,RANKL)-骨保护素(osteoprotegerin,OPG)轴对骨骼和免疫系统有独特的作用,揭露了RANK-RANKL-OPG信号轴是理解骨细胞-免疫细胞相互作用的基础,并且发现涉及调控骨免疫的信号通路或独立于或协同RANK,分析了相关通路交叉调节模式下如何影响骨稳态或疾病,见图3。"
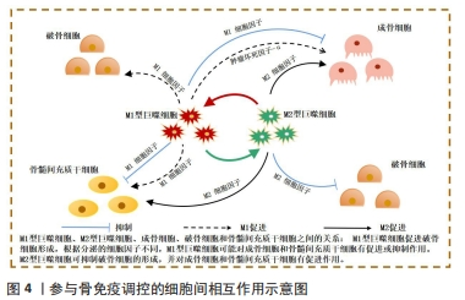
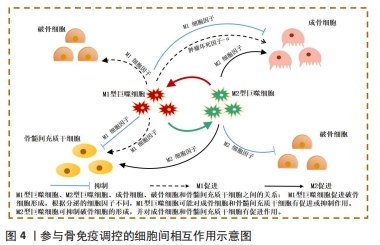
2.2 骨免疫系统中细胞间相互作用 随着骨免疫学的不断发展,众多研究成果已表明骨再生不是一个仅涉及骨形成和骨吸收的简单过程[19]。相反,它是连接了多个系统的复杂体系,如骨骼、血管和免疫系统均在其中起到关键作用。在骨损伤发生时,损伤部位会引发血凝块形成,随后中性粒细胞和多形核白细胞浸润血凝块,并启动骨骼的愈合系统,损伤的骨组织随即进入急性炎症期。随后,巨噬细胞受到多形核白细胞分泌的细胞因子刺激,发挥促成骨和促血管生成的作用[20-21]。最后,淋巴细胞迁移到损伤部位的愈伤组织上,启动适应性免疫反应,从而结束骨愈合进程。既往研究发现,免疫细胞和骨细胞在骨内环境中共享细胞因子和信号分子[22-23]。在生理和病理条件下,骨细胞和免疫细胞协同维持骨内环境的平衡。巨噬细胞作为先天免疫的重要参与者,对控制骨稳态至关重要[24]。 一般来说,巨噬细胞可分为M1(促炎)和M2(抗炎)型,两种巨噬细胞既相互独立,又紧密相连,它们在整个骨再生过程中的不同阶段充当着不同角色[25]。在急性炎症期时,M1巨噬细胞受到炎症分子的吸引,逐渐浸润损伤部位,吞噬外来异物及碎片骨块,进而分泌肿瘤坏死因子α和白细胞介素6等促炎细胞因子影响成骨细胞活性。肿瘤坏死因子α作为M1巨噬细胞释放的代表细胞因子,骨骼系统中,肿瘤坏死因子α通过抑制成骨细胞碱性磷酸酶的活性来抑制骨形成[26-27]。此外,肿瘤坏死因子α还可调控降解Runt相关转录因子2蛋白的Smurf1和Smurf2泛素连接酶来增加Runt相关转录因子2蛋白的表达,从而抑制成骨细胞的形成[28]。并且M1巨噬细胞分泌的肿瘤坏死因子α可促进破骨前体细胞成熟并诱导其分化为破骨细胞,且除直接促进破骨前体细胞成熟外,还可通过激活核转录因子κB和磷脂酰肌醇3激酶/蛋白激酶B(phosphatidylinositol-4,5-bisphosphate 3-kinase/protein kinase B,PI3K/Akt)信号通路,刺激其他细胞表面分泌RANKL,以促进破骨细胞的形成,增强骨吸收[29]。 白细胞介素6作为白细胞介素家族显著的促炎因子,其与白细胞介素6可溶性受体结合后,可激活SHP2/MEK2/ERK和SHP2/PI3K/Akt2通路,通过显著降低成骨细胞的碱性磷酸酶活性、抑制成骨基因的表达和成骨矿化效率,负调控成骨细胞的分化[30]。但是,又有学者提出促炎因子可能在骨重建发展中具有双重作用,同时作为促吸收和骨保护因子,参与到骨稳态的调控[31]。并且受微环境的刺激M1巨噬细胞还通过产生血管内皮生长因子等参与骨内微血管网的重构中,因此M1巨噬细胞对骨再生的影响仍存在争论[32]。 而M2型巨噬细胞则在骨愈合的后期发挥主导作用,通过分泌如转化生长因子刺激成纤维细胞增殖以促进伤口愈合,血小板源性生长因子参与骨内血管的生成,白细胞介素10以参与炎症调节,骨形态发生蛋白2参与骨组织重建[33-35]。因此学者根据功能的不同,将M2型巨噬细胞进一步分出4种亚型:M2a,M2b,M2c,M2d[36-40]。然而,与M1型巨噬细胞相似,M2型巨噬细胞的长期激活可能会增加促纤维化分子的分泌,导致瘢痕组织的过度形成,从而延迟愈合过程[41]。因此,正确调控巨噬细胞向不同表型极化对于成功的骨再生至关重要,如何实现这一目标应该是未来生物材料支架的发展方向。 值得注意的是,目前针对在骨修复过程中的免疫调控研究,多侧重于阐述巨噬细胞对破骨细胞或成骨细胞的单向影响,而忽略了成骨/破骨细胞对巨噬细胞负反馈调节,而对于骨细胞和免疫细胞的双向调控机制更是鲜有涉及。因此为更深入地研究与理解骨免疫学,加强巨噬细胞对破骨细胞或成骨细胞之间正负反馈调节的研究对骨免疫学的发展亦十分关键,见图4。"
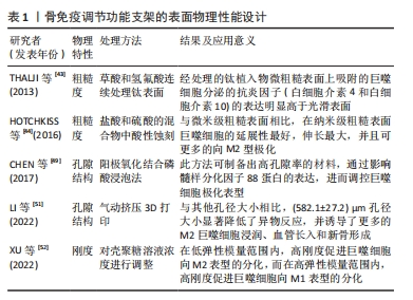
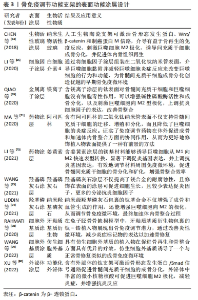
2.3 骨免疫调控功能的骨组织工程支架的设计与应用 既往学者们在设计骨组织工程支架时多侧重增强支架的促成骨和促血管生成等性能,而免疫反应则一度被认为是导致生物材料植入失败的主要原因。然而,随着骨免疫学概念的出现,研究人员发现盲目地减少宿主的免疫反应并不能成功诱导骨再生。而通过各种修饰策略赋予骨组织工程支架以骨免疫调控特性,在宿主体内对骨免疫微环境进行有效干预,精准操纵,更加有利于骨再生。因此,大量的研究开始将目光转向骨组织工程支架的免疫调节特性的研究上,下文是现有关于骨免疫调控特性的设计策略的归纳介绍。 2.3.1 支架物理性能设计 骨组织工程支架作为异物植入机体后,引发机体的炎症反应可分为急性期和慢性期。在急性炎症早期时,机体会募集中性粒细胞至骨损伤部位,与自发黏附于骨支架表面的非特异性蛋白质相互作用,从而激活机体的免疫应答。在这一过程中,细胞的迁移和蛋白质吸附均影响着骨支架与机体的相互作用,因此支架的表面粗糙度、表面孔隙结构和刚度等物理特性会影响细胞迁移和蛋白质黏附,需要在骨免疫特性支架设计时被考虑。 2.3.2 支架表面粗糙度 表面粗糙度已被证实是一种影响支架表面巨噬细胞极化的调节因素。根据观测尺度的不同,可将支架表面分为微米表面或者纳米表面[42]。THALJI等[43]研究发现在骨髓间充质干细胞成骨分化过程中,纳米表面材料比微粗糙表面材料可更快、更强地促进成骨基因表达上调。在HOTCHKISS等[44]在一项体外研究中分析了微米表面对巨噬细胞行为的影响,发现经过粗糙处理的钛植入物,表面吸附的巨噬细胞分泌的抗炎因子(白细胞介素4和白细胞介素10)的表达明显高于光滑表面的钛植入物。而伴随着纳米级生物材料与纳米加工技术的发展,使对人工骨支架进行纳米表面加工来模拟天然骨骼的粗糙度成为可能。还有研究对比了纳米级与微米级钛表面对免疫细胞的调节作用,结果显示,在400-500 nm的钛表面,巨噬细胞的延展性最好,伸长最大,并且可促进巨噬细胞向M2型极化[45]。 2.3.3 支架的孔隙结构 骨组织的孔隙结构与再生密切相关,因此孔隙度和孔隙大小被认为是骨组织工程支架设计的关键物理特性[46-47]。多孔结构对于细胞的氧气与营养输送、代谢废物的清除至关重要,而较高的孔隙率和互联的网络结构又可为细胞迁移和骨组织形成提供便利[48]。另有学者指出多孔结构是巨噬细胞黏附和扩散的重要基础,通过调节自噬途径成分的表达和激活,影响炎症反应、破骨细胞活性和成骨相关细胞因子的分泌[49]。此外研究人员发现更高的孔隙率或更大的孔径的支架,可诱导适度的缺氧环境,促进巨噬细胞M2极化和血管生成,抑制机体的炎症反应[50]。如LI等[51]利用气动挤压3D打印机设计出具有不同孔径的生物活性支架,并通过大鼠下颌骨缺损模型评估了孔径大小对免疫反应的影响。结果表明,相较于与其他尺寸孔径的支架相比,平均孔径为582 μm的支架组显示出明显的异物反应降低趋势,且该组支架上也检测出更多的M2型巨噬细胞、骨整合效果亦是表现为最佳。进一步的研究证实,不同孔径的支架通过影响髓样分化因子88蛋白表达,进而调控巨噬细胞极化。不过在应用设计时,孔隙度和孔径的调整势必会影响生物材料的机械强度,因此在设计过程中应权衡利弊[48]。 2.3.4 支架的刚度 支架表面募集的巨噬细胞极化表型与支架材料的刚度直接相关,通常来说较硬的支架材料可促进巨噬细胞更多的M1极化。XU等[52]采用壳聚糖冻干支架作为研究对象,以研究不同刚度的壳聚糖支架对体外RAW264.7细胞表型的影响,数据显示,支架基质材料刚度对巨噬细胞的极化调节是双向的:在低弹性模量范围内,高刚度促进巨噬细胞向M2表型分化,而在高弹性模量范围内,高刚度促进巨噬细胞向M1表型分化。然而,受到材料特性、刚度范围和实验模型差异等因素的影响,刚度对巨噬细胞极化的影响依旧非常复杂,需要通过进一步的体内外研究来定量分析。 文章总结了骨免疫调节功能支架的表面物理性能设计相关研究进展,见表1。"
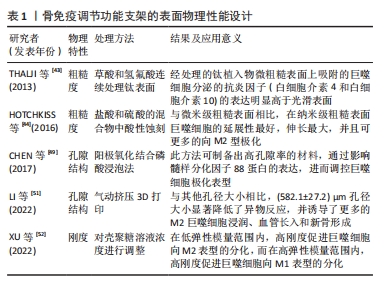
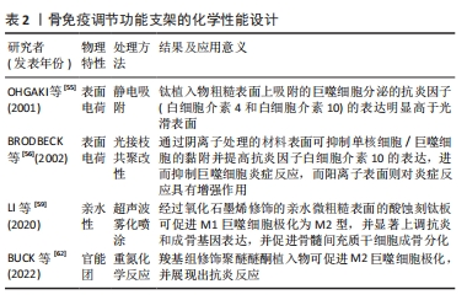
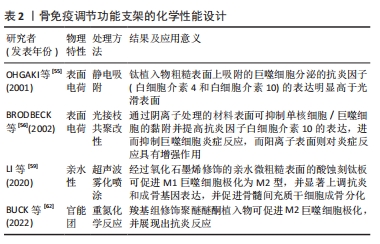
2.4 支架化学性能设计 骨组织工程支架表面化学特性(如亲/疏水性、官能团和电荷等)的变化与蛋白质的吸附密切相关,被吸附在支架表面的蛋白吸附层可与巨噬细胞上表达的相关细胞表面受体(包括整合素、选择素、多配体蛋白聚糖和CD44)之间相互作用,进而影响到巨噬细胞的极化表型[53]。 2.4.1 支架表面电荷 支架的表面电荷因模拟生物电微环境的能力以及其在免疫调节过程中的作用而被广泛研究。既往研究证实,带电表面可以影响支架表面吸附蛋白的浓度和细胞黏附[54]。例如,OHGAKI等[55]发现,钙离子首先通过静电吸附结合在带负电荷的支架表面,然后结合的钙离子吸引细胞黏附蛋白吸附在支架表面,最终影响成骨细胞的黏附和增殖。此外,带电表面也被认为会影响生物材料周围的免疫环境,通常来说阳离子颗粒比阴离子颗粒更易促进炎症反应。BRODBECK等[56]利用体外单核细胞与巨噬细胞培养系统研究了表面电荷对细胞因子产生的影响,结果显示,单核细胞或巨噬细胞中白细胞介素10在阴离子表面的表达显著上调,而在阳离子表面的表达下调。 2.4.2 支架表面亲水性 通过改变支架表面的亲疏水性,可以防止非特异性蛋白质黏附于支架表面,进而减少免疫反应的发生。研究发现,由于疏水相互作用的存在,蛋白质更易结合在疏水性材料表面,被吸附的蛋白通过构象变化引起炎症和异物反应[57-58]。而与疏水性材料表面相比,高亲水性的材料表面可以更好地调节辅助性T细胞和巨噬细胞的募集,促进巨噬细胞向M2型极化并减少白细胞介素类促炎因子的分泌。LI等[59]采用超声波雾化喷涂技术将氧化石墨烯喷涂在经过喷砂和酸蚀刻处理的钛板表面,提高了钛板的亲水性,同时发现相较于未修饰的酸蚀刻钛板,氧化石墨烯修饰的酸蚀刻钛板可刺激巨噬细胞M2极化,并促进表面的骨髓间充质干细胞成骨分化。 2.4.3 支架表面官能团 通过分子接枝方式将氨基、羟基等官能团修饰在支架表面是新兴的一种表面修饰技术,嫁接的官能团可通过影响支架表面蛋白质的吸附,进而影响支架周围的细胞反应和随后的新骨形成[60-61]。例如,BUCK等[62]通过重氮化学反应将羧基组分别附着在聚醚醚酮植入物表面,以解决慢性炎症导致的聚醚醚酮植入物骨修复失败的问题;体外实验表明,由羧基组修饰的表面可促进M2巨噬细胞极化;同时,羧基组修饰的表面能够诱导抗炎反应,且显著促进了骨髓间充质干细胞的成骨分化;并且动物体内实验进一步证实,与未修饰的聚醚醚酮植入物相比,羧基修饰聚醚醚酮植入物都与新生骨的接触更好。 文章总结了骨免疫调节功能支架的化学性能设计相关研究进展,见表2。"
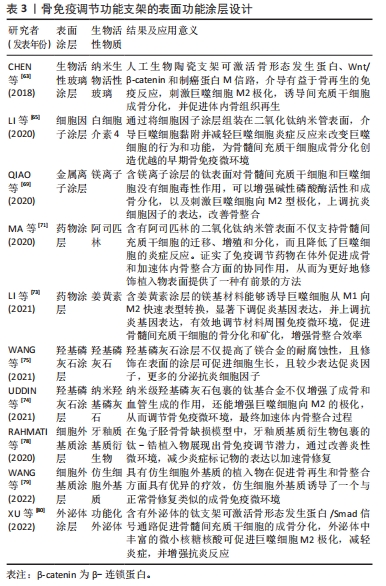
2.5 支架表面功能涂层设计 骨免疫调节特性的骨组织工程支架设计策略众多,除了改变支架表面粗糙度、孔隙结构、刚度、亲/疏水性、表面官能团和表面电荷等理化性能可在一定程度上影响巨噬细胞极化行为,还可考虑通过设计功能涂层的方式,将羟基磷灰石,生物活性玻璃、金属离子、细胞外基质、药物、细胞因子和外泌体等生物活性物质与植入物设计相结合来实现免疫调节,设计具有功能涂层的支架,在支架降解过程中实现缓释免疫调节分子以调节骨免疫反应,亦被视为支架骨免疫调控特性设计的有效策略。 2.5.1 生物活性玻璃涂层 生物活性玻璃是一种可与宿主骨紧密结合的成骨性能优异的骨再生材料。研究表明,将生物活性玻璃涂层修饰在骨植入物表面可明显提高骨整合速率,生物活性玻璃涂层不仅通过释放Na,P,Ca等离子直接促进新骨形成,还可诱导巨噬细胞极化,有利于成骨细胞植入物表面的定植,以增强新骨与植入物的物理结合。例如,CHEN等[63]通过脉冲激光沉淀技术开发了一种纳米生物活性玻璃(Ca2ZnSi2O7)胶原膜,并研究了它对骨免疫调节作用,通过体外和体内实验均证明,生物活性玻璃涂层可激活骨形态发生蛋白、Wnt/β-catenin和制癌蛋白M信号通路,成功介导有益于骨再生的免疫反应,以增强骨髓间充质干细胞的成骨分化。 2.5.2 细胞因子涂层 支架表面修饰细胞因子涂层是常见且有效的骨免疫调节方式[64]。通过在炎症早期爆发释放抗炎相关因子,使巨噬细胞能够及时极化为M2型,缩短炎症反应周期的同时,形成适宜的成骨微环境,最终加速骨重建进程,增强支架的成骨效能。LI等[65]通过在钛植入物表面构建了白细胞介素4/氧化石墨烯涂层,以提高支架的免疫调节性能,增强植入物周围骨组织的骨整合;体外实验发现,白细胞介素4的释放可引导巨噬细胞M2极化,促进骨细胞成骨分化,且体内实验亦证实了该结论。 2.5.3 金属离子涂层 镁、锌、钙等作为直接参与骨组织再生的金属离子,研究已证实众多金属离子对巨噬细胞的M1极化存在抑制作用,可通过降低炎症细胞因子分泌,诱导适宜的骨免疫反应,以促进骨形成[66-68]。因此借助金属离子涂层设计免疫调节特性的骨植入物是一种可行的设计策略。例如,QIAO等[69]通过水热法和阳极化沉积技术将镁离子沉积于氧化钛纳米管表面,体内外实验均显示,镁离子涂层可刺激巨噬细胞向M2型进行极化,增强抗炎细胞因子表达,形成利于骨髓间充质干细胞成骨分化的骨微环境。 2.5.4 药物涂层 药理学研究已证实,许多小分子药物在成骨过程中具有骨免疫调节功能,伴随着生物材料加工技术的发展,使得将小分子药物修饰在骨植入物表面以赋予其骨免疫调节特性成为可能[70]。例如,MA等[71]利用聚乳酸-乙醇酸共聚物将阿司匹林涂层装饰在二氧化钛纳米管表面,与二氧化钛纳米管基材相比,具有阿司匹林涂层的二氧化钛纳米管可促进巨噬细胞更多向M2极化,进而诱导适宜的骨免疫微环境,促进骨髓间充质干细胞成骨分化。此外,部分中药及其活性提取物也被报道具备骨免疫调节的潜力[72]。如LI等[73]通过将姜黄素封装于介孔二氧化硅微球设计具有促成骨和骨免疫调节功能的新型涂层,成功增强了镁植入物的耐腐蚀性和骨整合性。 2.5.5 羟基磷灰石涂层 羟基磷灰石因优异的促成骨性能与促血管生成能力,广泛应用于提高种植体的骨整合能力。最新研究发现,羟基磷灰石具有调节骨免疫的潜力,UDDIN等[74]通过电化学沉积的方式将羟基磷灰石涂层沉积在抛光后的镁合金植入物表面,与未进行涂层处理的镁合金植入物相比,涂层处理的植入物抗炎相关细胞因子的表达水平明显升高。同样,WANG等[75]通过微弧氧化和蒸汽-水热法制备的羟基磷灰石纳米颗粒在体外实验表现出优异的促进巨噬细胞M2极化与促进成骨和血管生成等性能,且体外实验结果表明,羟基磷灰石纳米颗粒能够诱导形成适合成骨的免疫微环境,加速了骨整合的进程。 2.5.6 细胞外基质涂层 细胞外基质因理化性能和机械特性与正常骨组织相似,被认为是骨组织工程中一种极具前途的骨替代材料[76-77]。RAHMATI等[78]通过电化学阴极极化法将牙釉质基质衍生物修饰于钛螺钉表面,并利用家兔股骨缺损模型评估了牙釉质基质衍生物改良的钛螺钉对骨免疫的调节作用;实验结果显示,牙釉质基质衍生物的修饰可显著提高钛螺钉周围成骨细胞的碱性磷酸酶活性,降低了促炎细胞因子的表达。而WANG等[79]通过模拟天然细胞外基质的糖蛋白组成和纤维结构,设计了一种仿生的水凝胶,并将其涂覆于聚己内酯支架上以评估材料的骨修复性能。体外实验表明,仿生水凝胶涂层可增强支架对骨髓间充质干细胞增殖和诱导成骨分化作用,并且通过诱导巨噬细胞M2极化,进一步增强了支架的成骨效能;且体内实验已证实了这种仿生细胞外基质水凝胶涂层支架对骨免疫的调节作用可加速成骨。 2.5.7 外泌体涂层 外泌体是一种包含细胞DNA、RNA、脂质、代谢物以及胞质和细胞表面蛋白的小膜泡。细胞外泌体具有清除细胞内过量和/或不必要成分的作用,以维持细胞内稳态生理功能。最新研究发现外泌体可通过影响受体细胞中的基因表达和信号通路来调节免疫反应,在骨植入物表面设计外泌体涂层是一种调控骨免疫效应的新兴策略。例如,XU等[80]通过将功能化外泌体固定在微弧氧化物钛植入物表面,固定的功能化外泌体可均匀释放并进入骨微环境中,通过激活骨形态发生蛋白/Smad信号通路促进骨髓间充质干细胞的成骨分化和诱导巨噬细胞M2极化,以促进植入假体与宿主骨骨整合。 文章总结了骨免疫调节功能支架的表面功能涂层设计研究进展,见表3。"
| [1] MIGLIORINI F, MAFFULLI N, BARONCINI A, et al. Allograft versus autograft osteochondral transplant for chondral defects of the talus: systematic review and meta-analysis. Am J Sports Med. 2022;50(12):3447-3455. [2] MATIĆ S, VUČKOVIĆ Č, LEŠIĆ A, et al. Pedicled vascularized bone grafts compared with xenografts in the treatment of scaphoid nonunion. Int Orthop. 2021;45(4): 1017-1023. [3] MAUFFREY C, BARLOW BT, SMITH W. Management of segmental bone defects. J Am Acad Orthop Surg. 2015;23(3):143-153. [4] ZHANG Y, LIU X, ZENG L, et al. Tissue engineering: polymer fiber scaffolds for bone and cartilage tissue engineering. Adv Funct Mater. 2019;29(36):1970246. [5] WANG C, HUANG W, ZHOU Y, et al. 3D printing of bone tissue engineering scaffolds. Bioact Mater. 2020;5(1):82-91. [6] LEPPIK L, GEMPP A, KUÇI Z, et al. A new perspective for bone tissue engineering: human mesenchymal stromal cells well-survive cryopreservation on β-TCP scaffold and show increased ability for osteogenic differentiation. Int J Mol Sci. 2022;23(3):1425. [7] HE J, CHEN G, LIU M, et al. Scaffold strategies for modulating immune microenvironment during bone regeneration. Mater Sci Eng C Mater Biol Appl. 2020;108:110411. [8] ZHANG J, TONG D, SONG H, et al. Osteoimmunity-regulating biomimetically hierarchical scaffold for augmented bone regeneration. Adv Mater. 2022;34(36): e2202044. [9] 孔祥宇,王兴,裴志伟,等.生物支架材料及打印技术修复骨缺损[J].中国组织工程研究,2024,28(3):479-485. [10] LU T, YUAN X, ZHANG L, et al. Enhancing osteoinduction and bone regeneration of biphasic calcium phosphate scaffold thought modulating the balance between pro-osteogenesis and anti-osteoclastogenesis by zinc doping. Mater Today Chem. 2023;29:101410. [11] 赵豆豆,林开利.多细胞构建血管化组织工程骨在骨修复中的应用[J].中国组织工程研究,2022,26(27):4386-4392. [12] CHEN R, HAO Z, WANG Y, et al. Mesenchymal stem cell-immune cell interaction and related modulations for bone tissue engineering. Stem Cells Int. 2022; 2022:7153584. [13] CHEN Z, XING F, ZHOU Y, et al. Integrated osteoimmunomodulatory strategies based on designing scaffold surface properties in bone regeneration. J Mater Chem B. 2023;11(29):6718-6745. [14] HORTON JE, RAISZ LG, SIMMONS HA, et al. Bone resorbing activity in supernatant fluid from cultured human peripheral blood leukocytes. Science. 1972;177(4051): 793-795. [15] DONATH K, LAASS M, GüNZL HJ.The histopathology of different foreign-body reactions in oral soft tissue and bone tissue. Virchows Arch A Pathol Anat Histopathol. 1992;420(2):131-137. [16] ARRON JR, CHOI Y. Bone versus immune system. Nature. 2000;408(6812):535-536. [17] TAKAYANAGI H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7(4):292-304. [18] WALSH MC, TAKEGAHARA N, KIM H, et al. Updating osteoimmunology: regulation of bone cells by innate and adaptive immunity. Nat Rev Rheumatol. 2018;14(3):146-156. [19] CHEN Z, KLEIN T, MURRAY RZ, et al. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater Today. 2016;19(6):304-321. [20] TRINDADE R, ALBREKTSSON T, GALLI S, et al. Bone immune response to materials, Part II: copper and polyetheretherketone (PEEK) compared to titanium at 10 and 28 days in rabbit tibia. J Clin Med. 2019;8(6):814. [21] CAI B, LIN D, LI Y, et al. N2-polarized neutrophils guide bone mesenchymal stem cell recruitment and initiate bone regeneration: a missing piece of the bone regeneration puzzle. Adv Sci (Weinh). 2021;8(19):e2100584. [22] KOVTUN A, MESSERER DAC, SCHARFFETTER-KOCHANEK K, et al. Neutrophils in tissue trauma of the skin, bone, and lung: two sides of the same coin. J Immunol Res. 2018;2018:8173983. [23] ONO T, OKAMOTO K, NAKASHIMA T, et al. IL-17-producing γδ T cells enhance bone regeneration. Nat Commun. 2016;7:10928. [24] NIU Y, WANG Z, SHI Y, et al. Modulating macrophage activities to promote endogenous bone regeneration: biological mechanisms and engineering approaches. Bioact Mater. 2021;6(1):244-261. [25] MUñOZ J, AKHAVAN NS, MULLINS AP, et al. Macrophage polarization and osteoporosis: a review. Nutrients. 2020;12(10):2999. [26] ZHAO M, DAI W, WANG H, et al. Periodontal ligament fibroblasts regulate osteoblasts by exosome secretion induced by inflammatory stimuli. Arch Oral Biol. 2019;105:27-34. [27] GLASS GE, CHAN JK, FREIDIN A, et al. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108(4):1585-1590. [28] OSTA B, BENEDETTI G, MIOSSEC P. Classical and paradoxical effects of TNF-α on bone homeostasis. Front Immunol. 2014;5:48. [29] ZHA L, HE L, LIANG Y, et al. TNF-α contributes to postmenopausal osteoporosis by synergistically promoting RANKL-induced osteoclast formation. Biomed Pharmacother. 2018;102:369-374. [30] KANESHIRO S, EBINA K, SHI K, et al. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J Bone Miner Metab. 2014;32(4):378-392. [31] WANG F, KONG L, WANG W, et al. Adrenomedullin 2 improves bone regeneration in type 1 diabetic rats by restoring imbalanced macrophage polarization and impaired osteogenesis. Stem Cell Res Ther. 2021;12(1):288. [32] LI T, PENG M, YANG Z, et al. 3D-printed IFN-γ-loading calcium silicate-β-tricalcium phosphate scaffold sequentially activates M1 and M2 polarization of macrophages to promote vascularization of tissue engineering bone. Acta Biomater. 2018;71:96-107. [33] SPILLER KL, NASSIRI S, WITHEREL CE, et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194-207. [34] SCHLUNDT C, EL KHASSAWNA T, SERRA A, et al. Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone. 2018;106:78-89. [35] PAJARINEN J, LIN T, GIBON E, et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80-89. [36] WANG Y, SMITH W, HAO D, et al. M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int Immunopharmacol. 2019;70:459-466. [37] GORDON S.Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1): 23-35. [38] FERRANTE CJ, LEIBOVICH SJ. Regulation of macrophage polarization and wound healing. Adv Wound Care (New Rochelle). 2012;1(1):10-16. [39] RŐSZER T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015:816460. [40] KRZYSZCZYK P, SCHLOSS R, PALMER A, et al. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol. 2018;9:419. [41] SINDRILARU A, SCHARFFETTER-KOCHANEK K. Disclosure of the culprits: macrophages-versatile regulators of wound healing. Adv Wound Care (New Rochelle). 2013;2(7):357-368. [42] LI X, HUANG Q, ELKHOOLY TA, et al. Effects of titanium surface roughness on the mediation of osteogenesis via modulating the immune response of macrophages. Biomed Mater. 2018;13(4):045013. [43] THALJI G, GRETZER C, COOPER LF. Comparative molecular assessment of early osseointegration in implant-adherent cells. Bone. 2013;52(1):444-453. [44] HOTCHKISS KM, REDDY GB, HYZY SL, et al. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016;31:425-434. [45] LUU TU, GOTT SC, WOO BW, et al. Micro- and nanopatterned topographical cues for regulating macrophage cell shape and phenotype. ACS Appl Mater Interfaces. 2015;7(51):28665-28672. [46] KARAGEORGIOU V, KAPLAN D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474-5491. [47] HANNINK G, ARTS JJ. Bioresorbability, porosity and mechanical strength of bone substitutes: what is optimal for bone regeneration? Injury. 2011;42 Suppl 2: S22-S25. [48] LOH QL, CHOONG C.Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev. 2013;19(6): 485-502. [49] CHEN Z, NI S, HAN S, et al. Nanoporous microstructures mediate osteogenesis by modulating the osteo-immune response of macrophages. Nanoscale. 2017; 9(2):706-718. [50] GARG K, PULLEN NA, OSKERITZIAN CA, et al. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials. 2013;34(18):4439-4451. [51] LI W, DAI F, ZHANG S, et al. Pore size of 3D-printed polycaprolactone/polyethylene glycol/hydroxyapatite scaffolds affects bone regeneration by modulating macrophage polarization and the foreign body response. ACS Appl Mater Interfaces. 2022;14(18):20693-20707. [52] XU J, GUAN W, KONG Y, et al. Regulation of macrophage behavior by chitosan scaffolds with different elastic modulus. Coatings. 2022;12(11):1742. [53] FIRKOWSKA-BODEN I, ZHANG X, JANDT KD. Controlling protein adsorption through nanostructured polymeric surfaces. Adv Healthc Mater. 2018;7(1):1700995. [54] BATOOL F, ÖZçELIK H, STUTZ C, et al. Modulation of immune-inflammatory responses through surface modifications of biomaterials to promote bone healing and regeneration. J Tissue Eng. 2021;12:20417314211041428. [55] OHGAKI M, KIZUKI T, KATSURA M, et al. Manipulation of selective cell adhesion and growth by surface charges of electrically polarized hydroxyapatite. J Biomed Mater Res. 2001;57(3):366-373. [56] BRODBECK WG, NAKAYAMA Y, MATSUDA T, et al. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine. 2002;18(6):311-319. [57] ZHANG K, HUANG H, HUNG HC, et al. Strong hydration at the poly(ethylene glycol) brush/albumin solution interface. Langmuir. 2020;36(8):2030-2036. [58] LI N, XU Z, ZHENG S, et al. Superamphiphilic TiO(2) composite surface for protein antifouling. Adv Mater. 2021;33(25):e2003559. [59] LI Q, SHEN A, WANG Z.Enhanced osteogenic differentiation of BMSCs and M2-phenotype polarization of macrophages on a titanium surface modified with graphene oxide for potential implant applications. RSC Adv. 2020;10(28):16537-16550 [60] HASAN A, PATTANAYEK SK, PANDEY LM. Effect of functional groups of self-assembled monolayers on protein adsorption and initial cell adhesion. ACS Biomater Sci Eng. 2018;4(9):3224-3233 [61] ARIMA Y, IWATA H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials. 2007;28(20):3074-3082. [62] BUCK E, LEE S, GAO Q, et al. The Role of Surface Chemistry in the Osseointegration of PEEK Implants. ACS Biomater Sci Eng. 2022;8(4):1506-1521. [63] CHEN Z, CHEN L, LIU R, et al. The osteoimmunomodulatory property of a barrier collagen membrane and its manipulation via coating nanometer-sized bioactive glass to improve guided bone regeneration. Biomater Sci. 2018;6(5):1007-1019. [64] GONG L, LI J, ZHANG J, et al. An interleukin-4-loaded bi-layer 3D printed scaffold promotes osteochondral regeneration. Acta Biomater. 2020;117:246-260. [65] LI M, WEI F, YIN X, et al. Synergistic regulation of osteoimmune microenvironment by IL-4 and RGD to accelerate osteogenesis. Mater Sci Eng C Mater Biol Appl. 2020;109:110508. [66] LIU W, LI J, CHENG M, et al. Zinc-modified sulfonated polyetheretherketone surface with immunomodulatory function for guiding cell fate and bone regeneration. Adv Sci (Weinh). 2018;5(10):1800749. [67] CHEN Y, GUAN M, REN R, et al. Improved immunoregulation of ultra-low-dose silver nanoparticle-loaded TiO(2) nanotubes via M2 macrophage polarization by regulating GLUT1 and autophagy. Int J Nanomedicine. 2020;15:2011-2026. [68] QI D, SU J, LI S, et al. 3D printed magnesium-doped β-TCP gyroid scaffold with osteogenesis, angiogenesis, immunomodulation properties and bone regeneration capability in vivo. Biomater Adv. 2022;136:212759. [69] QIAO X, YANG J, SHANG Y, et al. Magnesium-doped nanostructured titanium surface modulates macrophage-mediated inflammatory response for ameliorative osseointegration. Int J Nanomedicine. 2020;15:7185-7198. [70] HE M, YANG B, HUO F, et al. A novel coating with universal adhesion and inflammation-responsive drug release functions to manipulate the osteoimmunomodulation of implants. J Mater Chem B. 2021;9(26):5272-5283. [71] MA A, YOU Y, CHEN B, et al. Icariin/aspirin composite coating on TiO2 nanotubes surface induce immunomodulatory effect of macrophage and improve osteoblast activity. Coatings. 2020;10(4):427. [72] 熊伟,袁灵梅,钱国文,等.“补肾壮骨”中药应用于骨组织工程支架修复节段性骨缺损[J]. 中国组织工程研究,2023,27(21):3438-3444. [73] LI B, HUANG R, YE J, et al. A self-healing coating containing curcumin for osteoimmunomodulation to ameliorate osseointegration. Chem Eng J. 2021; 403:126323. [74] UDDIN M, HALL C, SANTOS V, et al. Synergistic effect of deep ball burnishing and HA coating on surface integrity, corrosion and immune response of biodegradable AZ31B Mg alloys. Mater Sci Eng C Mater Biol Appl. 2021;118:111459. [75] WANG X, MEI L, JIANG X, et al. Hydroxyapatite-coated titanium by micro-arc oxidation and steam-hydrothermal treatment promotes osseointegration. Front Bioeng Biotechnol. 2021;9:625877. [76] XING H, LEE H, LUO L, et al. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol Adv. 2020;42:107421. [77] AAMODT JM, GRAINGER DW.Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials. 2016;86:68-82. [78] RAHMATI M, FRANK MJ, WALTER SM, et al. Osteoimmunomodulatory effects of enamel matrix derivate and strontium coating layers: a short- and long-term in vivo study. ACS Appl Bio Mater. 2020;3(8):5169-5181. [79] WANG Y, WANG J, GAO R, et al. Biomimetic glycopeptide hydrogel coated PCL/nHA scaffold for enhanced cranial bone regeneration via macrophage M2 polarization-induced osteo-immunomodulation. Biomaterials. 2022;285:121538. [80] XU H, CHAI Q, XU X, et al. Exosome-functionalized Ti6Al4V scaffolds promoting osseointegration by modulating endogenous osteogenesis and osteoimmunity. ACS Appl Mater Interfaces. 2022;14(41):46161-46175. |
| [1] | Yang Yufang, Yang Zhishan, Duan Mianmian, Liu Yiheng, Tang Zhenglong, Wang Yu. Application and prospects of erythropoietin in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1443-1449. |
| [2] | Wang Weiqing, Zhou Yue. Chronic inflammation regulates adipose tissue fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1307-1312. |
| [3] | Huang Haoran, Fan Yinuo, Wei-Yang Wenxiang, Jiang Mengyu, Fang Hanjun, Wang Haibin, Chen Zhenqiu, Liu Yuhao, Zhou Chi. Urolithin A mediates p38/MAPK pathway to inhibit osteoclast activity [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1149-1154. |
| [4] | Dai Yuexing, Zheng Liqin, Wu Minhui, Li Zhihong, Li Shaobin, Zheng Desheng, Lin Ziling. Effect of vessel number on computational fluid dynamics in vascular networks [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1206-1210. |
| [5] | Liu Jianhong, Liao Shijie, Li Boxiang, Tang Shengping, Wei Zhendi, Ding Xiaofei. Extracellular vesicles carrying non-coding RNA regulate the activation of osteoclasts [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1076-1082. |
| [6] | Wang Shanshan, Shu Qing, Tian Jun. Physical factors promote osteogenic differentiation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1083-1090. |
| [7] | Wang Wen, Zheng Pengpeng, Meng Haohao, Liu Hao, Yuan Changyong. Overexpression of Sema3A promotes osteogenic differentiation of dental pulp stem cells and MC3T3-E1 [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 993-999. |
| [8] | Zeng Fanzhuo, Li Yuxin, Sun Jiachen, Gu Xinyang, Wen Shan, Tian He, Mei Xifan. Efficient strategies for microglia replacement in spinal cord injury models [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1007-1014. |
| [9] | Wang Jianchun, Yang Shuqing, Su Xin, Wang Hongyuan. Different contents of B2O3 affect mechanical properties and bioactivity of bioactive glass scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 712-716. |
| [10] | Lan Weiwei, Yu Yaodong, Huang Di, Chen Weiyi. In vitro degradation behavior of Mg-Zn-Ca alloys [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 717-723. |
| [11] | Zhou Xiaowen, Fu Zuchang, Huang Fei, Ai Jianguo, Zhao Feng. Bone defect blocked by bone cement segmental filling in single-plane tibial bone transport [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 736-740. |
| [12] | Wei Yuanxun, Chen Feng, Lin Zonghan, Zhang Chi, Pan Chengzhen, Wei Zongbo. The mechanism of Notch signaling pathway in osteoporosis and its prevention and treatment with traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 587-593. |
| [13] | Yang Yuqing, Chen Zhiyu. Role and application of early transient presence of M1 macrophages in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 594-601. |
| [14] | Zhou Shibo, Guan Jianbin, Yu Xing, Zhao He, Yang Yongdong, Liu Tao. Animal models of femoral bone defects: preparation status and characteristics [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 633-638. |
| [15] | Liu Chunli, Yan Yujuan, Mo Liwen, Wu Zhijie, Zhang Li. Puerarin inhibits the differentiation of Raw264.7 cells into osteoclasts through the Notch signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5636-5641. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||