Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (27): 4288-4292.doi: 10.12307/2024.572
Previous Articles Next Articles
Causal relationship between blood metabolites and sarcopenia-related traits: a Mendelian randomization study
Chen Tianxin, Dong Tingting, Li Yan, Zhang Sheng, Zhang Lei
- The Forth Department of Bone and Joint, Wangjing Hospital of China Academy of Chinese Medical Sciences, Beijing 100102, China
-
Received:2023-10-09Accepted:2023-11-25Online:2024-09-28Published:2024-01-26 -
Contact:Zhang Lei, Chief physician, Professor, Doctoral supervisor, The Forth Department of Bone and Joint, Wangjing Hospital of China Academy of Chinese Medical Sciences, Beijing 100102, China -
About author:Chen Tianxin, MD candidate, The Forth Department of Bone and Joint, Wangjing Hospital of China Academy of Chinese Medical Sciences, Beijing 100102, China
CLC Number:
Cite this article
Chen Tianxin, Dong Tingting, Li Yan, Zhang Sheng, Zhang Lei. Causal relationship between blood metabolites and sarcopenia-related traits: a Mendelian randomization study[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(27): 4288-4292.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

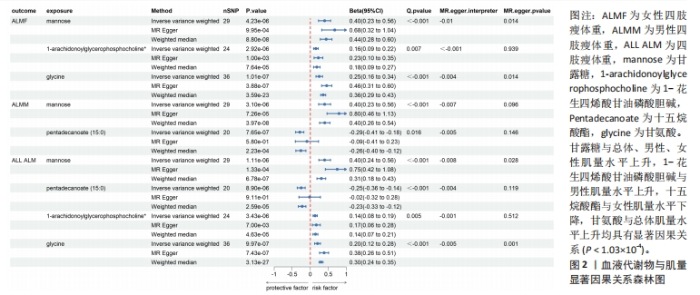
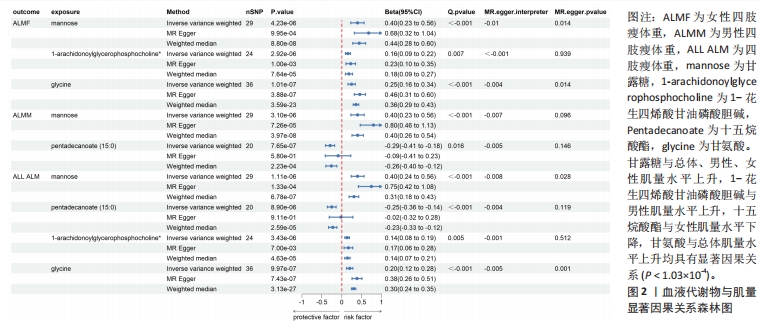
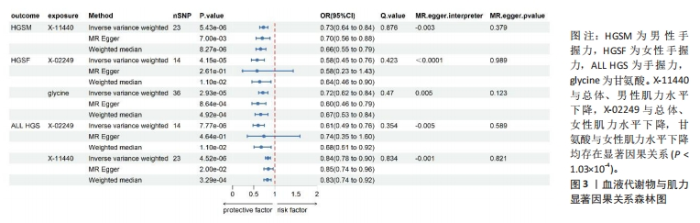
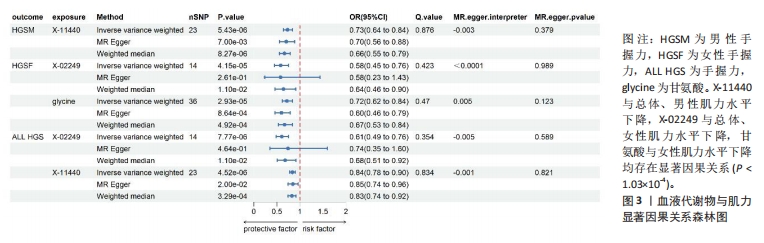
2.1 工具变量筛选 首先根据全基因组显著性、连锁不平衡设定的条件,筛选出486种血液代谢物符合条件的工具变量,每个代谢物得到的工具变量数量从3-500个不等。486种血液代谢物的单核苷酸多态性的最小F值为17.64,最大F值为2 913.70,均符合F > 10的要求,提示研究受弱工具变量影响的可能性较低。 2.2 MR分析结果 2.2.1 血液代谢物与肌量的因果关系 采用逆方差加权法首先评估486种血液代谢物对总体肌量、男性肌量和女性肌量的因果关系。MR分析显示44种代谢物与男性肌量、39种代谢物与女性肌量、40种代谢物与总体肌量存在潜在因果关系(P < 0.05),具体见图1。甘露糖与总体、男性、女性肌量水平上升,1-花生四烯酸甘油磷酸胆碱与男性肌量水平上升,十五烷酸酯与女性肌量水平下降,甘氨酸与总体肌量水平上升均具有显著因果关系(P < 1.03×10-4),经MR-Egger法、加权中位数法Beta值显示因果关系方向一致,具体见图2。"
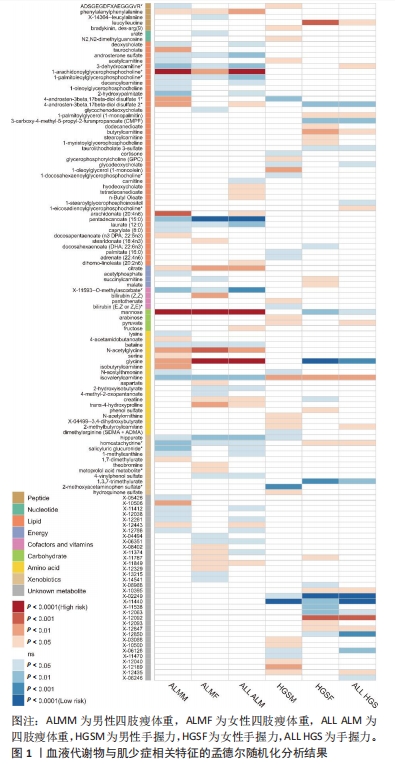
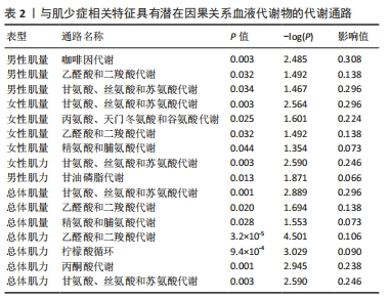
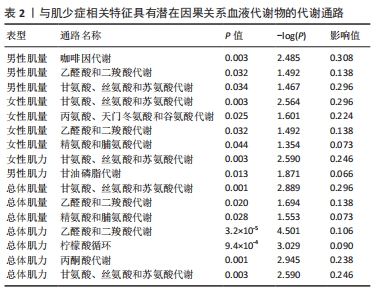
2.3 敏感性分析 将Bonferroni调整后的显著因果关系进一步敏感性分析,Cochran′s Q检验显示血液代谢物与总体肌量存在显著因果关系的结果均存在异质性(P < 0.05),故研究均采用随机效应模型进行分析。MR-Egger回归法检验显示甘露糖与总体肌量、女性肌量的因果关系存在水平多效性(P < 0.05);甘氨酸与总体肌量、女性肌量的因果关系存在水平多效性,提示这些因果关系缺乏稳定性;其余因果关系无显著水平多效性(P > 0.05),见图2,3。留一法显示显著因果关系在逐个剔除单核苷酸多态性后结果稳定。 2.4 代谢通路分析 将与肌少症相关特征具有潜在因果关系的血液代谢物进行代谢通路分析,共得到8条代谢通路,其中“乙醛酸和二羧酸代谢”“甘氨酸、丝氨酸和苏氨酸代谢”是总体肌力、肌量的共同代谢通路。此外,在性别差异上,“精氨酸和脯氨酸代谢”和“丙氨酸、天门冬氨酸和谷氨酸代谢”等与女性肌量水平改变相关,“咖啡因代谢”、“甘油磷脂代谢”分别与男性肌量、肌力水平改变相关。具体见表2。"
| [1] SAYER AA, CRUZ-JENTOFT A. Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing. 2022;51(10):1-5. [2] PETERMANN-ROCHA F, BALNTZI V, GRAY SR, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1): 86-99. [3] MURPHY CH, MCCARTHY SN, MCMORROW AM, et al. Prevalence and determinants of sarcopenia in community-dwelling older adults in Ireland. Aging Clin Exp Res. 2023;35(8):1651-1660. [4] YUAN S, LARSSON SC. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism. 2023;144:155533. [5] SAEDI AA, DEBRUIN DA, HAYES A, et al. Lipid metabolism in sarcopenia. Bone. 2022;164: 116539. [6] XIA Z, CHOLEWA J, ZHAO Y, et al. Targeting Inflammation and Downstream Protein Metabolism in Sarcopenia: A Brief Up-Dated Description of Concurrent Exercise and Leucine-Based Multimodal Intervention. Front Physiol. 2017;8:434. [7] MARTÍNEZ-ARNAU FM, FONFRÍA-VIVAS R, BUIGUES C, et al. Effects of Leucine Administration in Sarcopenia: A Randomized and Placebo-controlled Clinical Trial. Nutrients. 2020;12(4):1-16. [8] LI CW, YU K, SHYH-CHANG N, et al. Sterol metabolism and protein metabolism are differentially correlated with sarcopenia in Asian Chinese men and women. Cell Prolif. 2021;54(4):e12989. [9] 陈天鑫,张智龙,朱瑜琪,等.恶性肿瘤与骨密度因果关系的双向两样本孟德尔随机化研究[J].现代预防医学,2023,50(18):3276-3280+3287. [10] XIAO G, HE H, LIU L, et al. Causality of genetically determined metabolites on anxiety disorders: a two-sample Mendelian randomization study. J Transl Med. 2022;20(1):475. [11] SHIN SY, FAUMAN EB, PETERSEN AK, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. 2014;46(6):543-550. [12] YANG J, LIU P, WANG S, et al. Causal relationship between sarcopenia and osteoarthritis: a bi-directional two-sample mendelian randomized study. Eur J Med Res. 2023;28(1):327. [13] PEI YF, LIU YZ, YANG XL, et al. The genetic architecture of appendicular lean mass characterized by association analysis in the UK Biobank study. Commun Biol. 2020;3(1):608. [14] JONES G, TRAJANOSKA K, SANTANASTO AJ, et al. Genome-wide meta-analysis of muscle weakness identifies 15 susceptibility loci in older men and women. Nat Commun. 2021;12(1): 654. [15] EVANS DM, SMITH GD. Mendelian Randomization: New Applications in the Coming Age of Hypothesis-Free Causality. Annu Rev Genomics Hum Genet. 2015;16:327-350. [16] DIDELEZ V, SHEEHAN N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res. 2007;16(4):309-330. [17] YU XH, CAO RR, YANG YQ, et al. Identification of causal metabolites related to multiple autoimmune diseases. Hum Mol Genet. 2022;31(4):604-613. [18] 彭志华,潘俊曦,冯庆辉,等.两样本孟德尔随机化分析血脂与肌肉减少症的因果关系[J].中国组织工程研究,2024,28(23):3699-3703. [19] SANDERSON E. Multivariable Mendelian Randomization and Mediation. Cold Spring Harb Perspect Med. 2021;11(2):1-13. [20] BURGESS S, THOMPSON SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377-389. [21] HARTLEY AE, POWER GM, SANDERSON E, et al. A Guide for Understanding and Designing Mendelian Randomization Studies in the Musculoskeletal Field. JBMR Plus. 2022;6(10):e10675. [22] LU Y, PANG Z, XIA J. Comprehensive investigation of pathway enrichment methods for functional interpretation of LC-MS global metabolomics data. Brief Bioinform. 2023; 24(1):1-15. [23] CHEN S, HE W. Metabolome-Wide Mendelian Randomization Assessing the Causal Relationship Between Blood Metabolites and Bone Mineral Density. Calcif Tissue Int. 2023;112(5):543-562. [24] DHANALAKSHMI M, SRUTHI D, JINURAJ KR, et al. Mannose: a potential saccharide candidate in disease management. Med Chem Res. 2023;32(3):391-408. [25] ZHANG W, CHENG H, GUI Y, et al. Mannose Treatment: A Promising Novel Strategy to Suppress Inflammation. Front Immunol. 2021;12:756920. [26] BATES SJ, MORROW E, ZHANG AY, et al. Mannose-6-phosphate, an inhibitor of transforming growth factor-beta, improves range of motion after flexor tendon repair. J Bone Joint Surg Am. 2006;88(11):2465-2472. [27] LI CW, YU K, SHYH-CHANG N, et al. Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle. 2022;13(2):781-794. [28] BAUMGARTNER C, WOLF P, BEIGLBÖCK H, et al. Potential role of skeletal muscle glycerophosphocholine in response to altered fluid balance in humans: an in vivo nuclear magnetic resonance study. Am J Physiol Endocrinol Metab. 2023;324(4):E339-E346. [29] LI Z, LEI H, JIANG H, et al. Saturated fatty acid biomarkers and risk of cardiometabolic diseases: A meta-analysis of prospective studies. Front Nutr. 2022;9:963471. [30] KOBAYASHI H. [Amino Acid Nutrition in the Prevention and Treatment of Sarcopenia]. Yakugaku Zasshi. 2018;138(10):1277-1283. [31] GHELLER BJ, BLUM JE, LIM EW, et al. Extracellular serine and glycine are required for mouse and human skeletal muscle stem and progenitor cell function. Mol Metab. 2021;43:101106. [32] STARNES JW, PARRY TL, O’NEAL SK, et al. Exercise-Induced Alterations in Skeletal Muscle, Heart, Liver, and Serum Metabolome Identified by Non-Targeted Metabolomics Analysis. Metabolites. 2017;7(3):1-14. [33] MIKKOLA TM, SALONEN MK, KAJANTIE E, et al. Associations of Fat and Lean Body Mass with Circulating Amino Acids in Older Men and Women. J Gerontol A Biol Sci Med Sci. 2020;75(5): 885-891. [34] BRAS H, LIABEUF S. Differential effects of spinal cord transection on glycinergic and GABAergic synaptic signaling in sub-lesional lumbar motoneurons. J Chem Neuroanat. 2021;113:101847. [35] FALEGAN OS, VOGEL HJ, HITTEL DS, et al. High Aerobic Capacity Mitigates Changes in the Plasma Metabolomic Profile Associated with Aging. J Proteome Res. 2017;16(2):798-805. [36] CHEN G, YE G, ZHANG X, et al. Metabolomics Reveals Protection of Resveratrol in Diet-Induced Metabolic Risk Factors in Abdominal Muscle. Cell Physiol Biochem. 2018;45(3):1136-1148. [37] KUMAR A, KUMAR Y, SEVAK JK, et al. Metabolomic analysis of primary human skeletal muscle cells during myogenic progression. Sci Rep. 2020;10(1):11824. [38] JIANG D, LIU C, CHEN Y, et al. Whole body vibration activates AMPK/CPT1 signaling pathway of skeletal muscle in young and aging mice based on metabolomics study. Endocr J. 2022; 69(5):585-596. [39] UENO S, SEINO Y, HIDAKA S, et al. Blockade of glucagon increases muscle mass and alters fiber type composition in mice deficient in proglucagon-derived peptides. J Diabetes Investig. 2023;14(9):1045-1055. [40] MCMILLIN SL, MINCHEW EC, LOWE DA, et al. Skeletal muscle wasting: the estrogen side of sexual dimorphism. Am J Physiol Cell Physiol. 2022;322(1):C24-C37. [41] KARPPINEN JE, TÖRMÄKANGAS T, KUJALA UM, et al. Menopause modulates the circulating metabolome: evidence from a prospective cohort study. Eur J Prev Cardiol. 2022;29(10):1448-1459. [42] PATIN F, CORCIA P, VOURC’H P, et al. Omics to Explore Amyotrophic Lateral Sclerosis Evolution: the Central Role of Arginine and Proline Metabolism. Mol Neurobiol. 2017;54(7):5361-5374. [43] WEI Z, GE F, CHE Y, et al. Metabolomics Coupled with Pathway Analysis Provides Insights into Sarco-Osteoporosis Metabolic Alterations and Estrogen Therapeutic Effects in Mice. Biomolecules. 2021;12(1):1-20. [44] HOSOI T, YAKABE M, SASAKAWA H, et al. Sarcopenia phenotype and impaired muscle function in male mice with fast-twitch muscle-specific knockout of the androgen receptor. Proc Natl Acad Sci U S A. 2023;120(4):e2218032120. [45] GATINEAU E, SAVARY-AUZELOUX I, MIGNE C, et al. Chronic Intake of Sucrose Accelerates Sarcopenia in Older Male Rats through Alterations in Insulin Sensitivity and Muscle Protein Synthesis. J Nutr. 2015;145(5):923-930. [46] BAGHERI A, HASHEMI R, HESHMAT R, et al. Patterns of Nutrient Intake in Relation to Sarcopenia and Its Components. Front Nutr. 2021;8:645072. [47] XU Z, YOU W, CHEN W, et al. Single-cell RNA sequencing and lipidomics reveal cell and lipid dynamics of fat infiltration in skeletal muscle. J Cachexia Sarcopenia Muscle. 2021;12(1):109-129. |
| [1] | Xu Rui, Li Yanyan, Xu Hong. Effect and mechanism of short-chain fatty acids in aged rats with sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(32): 5172-5176. |
| [2] | Chen Jixin, Yu Weijie, Guo Tianci, Zhou Qinxin, Niu Puyu, Ye Yuntian, Liu Aifeng. Sleep characteristics and risk of osteoarthritis: a two-sample and multivariate Mendelian randomization study [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(32): 5203-5209. |
| [3] | Fan Yidong, Qin Gang, He Kaiyi, Gong Yufang, Li Weicai, Wu Guangtao. Expression of immune-related genes in rheumatoid arthritis and a two-sample Mendelian randomization study of immune cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(27): 4312-4318. |
| [4] | Zhan Qunzhang, Zhang Yuling, Han Yuxin, Lyu Jiazhen, Zheng Xiaoxia, Qu Chongzheng. Association between obesity and osteoporosis: a two-sample Mendelian randomization analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(27): 4319-4324. |
| [5] | Chai Jinlian, Li Shudong, Li Wei, Du Haitao, Dong Limin, Liang Xuezhen, Wang Ping. Gut microbiota and drug-associated osteonecrosis: a two‑sample Mendelian randomization study [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(27): 4325-4331. |
| [6] | Ma Weiwei, Xiong Yong, Chen Honggu, Huang Wenzhuo, Huang Xin, Zhou Xiaohong. Relationship between statin drugs and bone density: a drug target-mediated Mendelian randomization study [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(27): 4340-4345. |
| [7] | Guo Hui, Kong Jianda, Tian Chunlan. The role of mitochondrial autophagy-related receptor proteins and signaling pathways in the prevention and treatment of sarcopenia through exercise [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(27): 4397-4404. |
| [8] | Liu Xu, Chen Bo, Ning Ke, Chen Xiaohong. Mechanism and potential of vitamin C supplementation in sarcopenia prevention and treatment [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(27): 4405-4412. |
| [9] | Wu Ruiqi, Zhang Xuan, Zhou Yi, Meng Lin, Li Hongyu. Two-sample Mendelian randomization analysis of the relationship between statins and the risk of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(26): 4106-4112. |
| [10] | Peng Zhihua, Pan Junxi, Feng Qinghui, Tian Tianzhao, Zhang Sheng, Li An, Cai Yingfeng. The causal relationship between blood lipids and muscle atrophy based on Mendelian randomization analysis of two samples [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(23): 3699-3703. |
| [11] | Wu Ruiqi, Zhou Yi, Xia Tian, Zhang Chi, Yang Qipei, Zhang Xuan, Zhang Yazhong, Cui Wei. Mendelian randomization study on the association between rheumatoid arthritis and osteoporosis and bone mineral density [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(23): 3715-3721. |
| [12] | Kong Jianda, Xie Yingao, Chen Shijuan, Zhu Lei. Blood flow restriction training interventions for sarcopenia in older adults: biological mechanisms and proposed application protocols [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(23): 3743-3750. |
| [13] | Long Yi, Yang Jiaming, Ye Hua, Zhong Yanbiao, Wang Maoyuan. Extracellular vesicles in sarcopenic obesity: roles and mechanisms [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 315-320. |
| [14] | Xie Peng, Zhang Jiang, Deng Xiaolei, Wei Bo, Hou Decai. A systematic review of mouse model construction for sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 263-266. |
| [15] | Zuo Jun, Ma Shaolin. Bioinformatics analysis and validation of differentially expressed genes and small molecule drug prediction in proliferative scar [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(14): 2166-2172. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||