Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (23): 3715-3721.doi: 10.12307/2024.412
Previous Articles Next Articles
Mendelian randomization study on the association between rheumatoid arthritis and osteoporosis and bone mineral density
Wu Ruiqi1, 2, Zhou Yi1, Xia Tian1, Zhang Chi1, Yang Qipei1, 2, Zhang Xuan2, Zhang Yazhong3, Cui Wei1
- 1Ruikang Hospital, Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China; 2Guangxi University of Chinese Medicine, Nanning 530299, Guangxi Zhuang Autonomous Region, China; 3Department of Orthopedics, Wuzhou Hospital of Traditional Chinese Medicine, Wuzhou 543000, Guangxi Zhuang Autonomous Region, China
-
Received:2023-05-30Accepted:2023-07-13Online:2024-08-18Published:2023-09-13 -
Contact:Zhang Yazhong, Associate chief physician, Master’s supervisor, Department of Orthopedics, Wuzhou Hospital of Traditional Chinese Medicine, Wuzhou 543000, Guangxi Zhuang Autonomous Region, China Cui Wei, Chief physician, Professor, Master’s supervisor, Ruikang Hospital, Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China -
About author:Wu Ruiqi, Master candidate, Ruikang Hospital, Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China; Guangxi University of Chinese Medicine, Nanning 530299, Guangxi Zhuang Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 81960803 (to ZC [project participant]); Guangxi Zhuang Autonomous Region Clinical Key Specialty (Trauma Surgery) Construction Project, No. BMYSS(2021) (to ZC [project participant]); Guangxi Natural Science Foundation Project, No. 2023GXNSFAA026075 (to ZC [project participant]); Guangxi Natural Science Foundation for the Youth, No. 2020GXNSFBA159053 (to ZC [project participant]); Guangxi University of Chinese Medicine School-level Doctoral Research and Innovation Project, No. YCBXJ2021019 (to XT); Guangxi University of Chinese Medicine Youth Innovation Research Team Project, No. 2021TD001; Guangxi University of Chinese Medicine Gui School TCM Inheritance and Innovation Team, No. 2022A004 (to XT [project participant]); Guangxi Chinese Medicine Appropriate Technology Development and Promotion Project, Nos. GZSY22-39 (to XT) and GZSY22-36 (to ZC [project participant])
CLC Number:
Cite this article
Wu Ruiqi, Zhou Yi, Xia Tian, Zhang Chi, Yang Qipei, Zhang Xuan, Zhang Yazhong, Cui Wei. Mendelian randomization study on the association between rheumatoid arthritis and osteoporosis and bone mineral density[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(23): 3715-3721.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
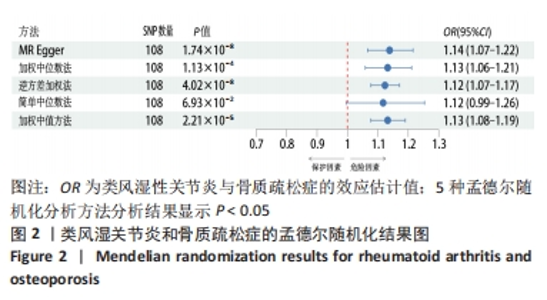
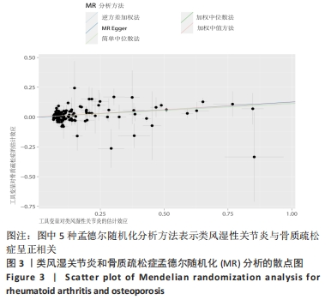
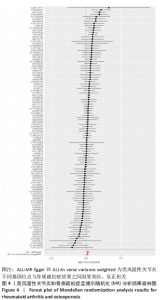
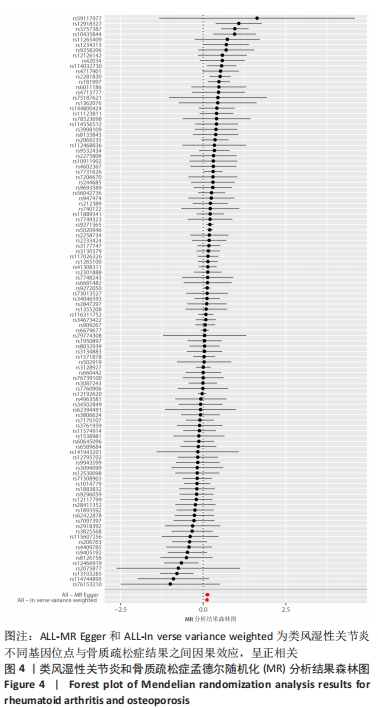
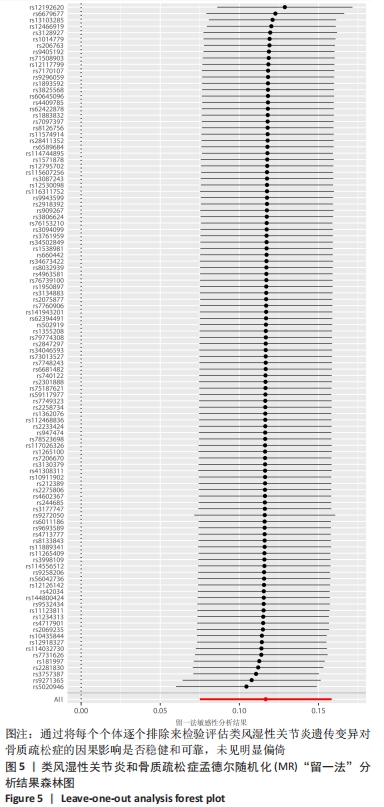
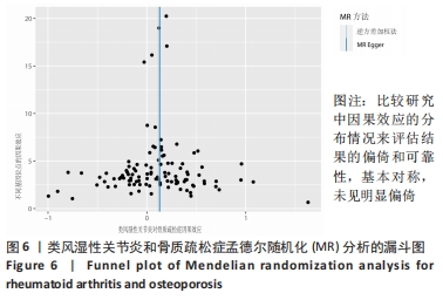
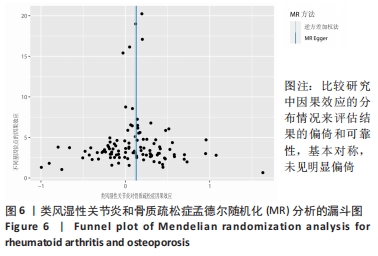
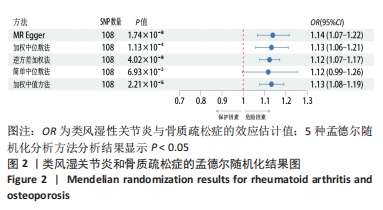
2.1 类风湿性关节炎与骨质疏松症关系的因果关系 首先筛选与类风湿性关节炎紧密相关的单核苷酸多态性(P < 5×10-8),设置连锁不平衡参数(r2=0.001,kb=5 000),即筛选出同时满足假设1,2,3的单核苷酸多态性,剩余139个,然后通过GWAS提取结局骨质疏松症的数据,进行合并暴露与结局数据集,并删除存在回文序列单核苷酸多态性(n=9)。利用PhenoScanner数据库,主要排除其对应表型与骨质疏松症相关潜在的混杂因素的单核苷酸多态性,分别为饮酒:rs12466919;甲状腺功能减退:rs13103285;人体免疫系统疾病:rs3757387与rs12918327。在该研究中,每个单核苷酸多态性的F值都大于10,表明没有弱的工具变量。最终选择了108个单核苷酸多态性作为工具变量来评估类风湿性关节炎和骨质疏松症之间的关联。逆方差加权法方法用于孟德尔随机化分析,而其他4种方法(WM,SM,WME和MR-Egger)用于补充说明,见图2;描述主要结果的散点图见图3。类风湿性关节炎对骨质疏松症(OR=1.123,95%CI:1.077-1.171,P=4.02×10-8)。Cochran’s Q检验结果得出P=0.388 > 0.05,单核苷酸多态性不存在异质性。MR-Egger-intercept未检测到类风湿性关节炎潜在的水平多效性(P=0.571),说明工具变量并不显著通过暴露以外的途径影响结局,此外,MR-PRESSO方法未发现异常的单核苷酸多态性。留一法检验分析显示没有个别单核苷酸多态性对整体因果估计产生影响。工具变量森林图及留一法检验结果的森林图见图4,5。为了进一步检验上述结果的稳定性,绘制的漏斗图中显示的因果效应分布基本对称,未见明显偏倚,见图6。"
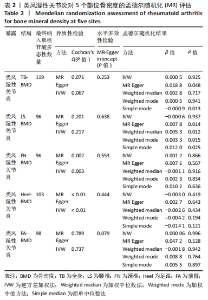
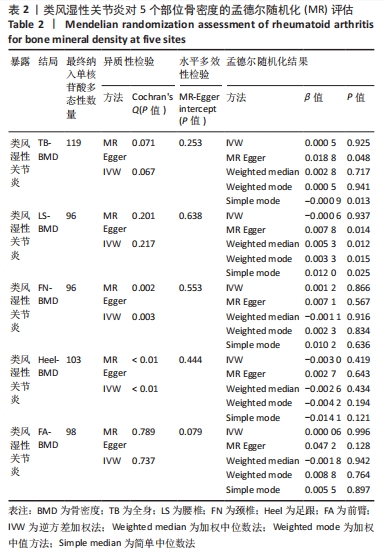
2.2 类风湿性关节炎与骨密度因果关系 类风湿性关节炎的GWAS研究中TB-BMD,LS-BMD,FN-BMD,Heel-BMD及FA-BMD达到显著性差异的工具变量分别为134,109,108,112和110个。根据工具变量之间的连锁不平衡情况,筛选出无连锁不平衡关联的独立工具变量,并删除存在回文序列单核苷酸多态性。使用Phenoscanner V2查找并去除影响骨质疏松症相关潜在混杂因素的单核苷酸多态性,分别是甲状腺功能减退或亢进:rs12117799,rs6679677,rs1950897,rs3128927,rs3757387,rs71508903;糖尿病:rs3130379,rs3087243;免疫系统疾病:rs7731626,rs7749323;吸收不良:rs56042736;基础代谢率:rs42034,rs4713777,rs42034;其他影响骨代谢疾病:rs10435844,rs12918327,rs12918327;最终512个独立的单核苷酸多态性被纳入为类风湿性关节炎的工具变量,其中TB-BMD,LS-BMD,FN-BMD,Heel-BMD以及FA-BMD分别119,96,96,103和98个工具变量。 逆方差加权法分析结果显示,类风湿性关节炎与5个部位的骨密度之间不存在关联因果关系:TB-BMD(OR=1.000,95%CI:0.988-1.012,P=0.925)、LS-BMD(OR=0.999,95%CI:0.982-1.016,P=0.937)、FN-BMD(OR=1.001,95%CI:0.986-1.016,P=0.866)、Heel BMD(OR=0.996,95%CI:0.989-1.004,P=0.419)、FA-BMD(OR=1.063,95%CI:0.970-1.031,P = 0.996)。 在异质性检测方面,文章发现FN-BMD(P=0.002)和Heel BMD (P < 0.001)的单核苷酸多态性之间存在潜在异质性,但随机效应逆方差加权法分析方法的结果表明类风湿性关节炎与骨质疏松症确实存在因果关系,并且类风湿性关节炎升高后对骨质疏松症发病风险也相应增加。 此外,经过MR-Egger-intercept分析未检测到潜在的水平多效性(TB-BMD:P=0.253;LS-BMD:P=0.638;FN-BMD:P=0.553;Heel BMD:P=0.444;FA-BMD:P=0.079),表明类风湿性关节炎的单核苷酸多态性除了通过类风湿性关节炎外不影响5个部位骨密度结果,不违背孟德尔随机化核心假设,以上随机模型显示其与TB-BMD,LS-BMD,FN-BMD,Heel-BMD和FA-BMD之间风险无显著性关联,详见表2。"
| [1] BLACK DM, EASTELL R, ADAMS AL. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. Reply. N Engl J Med. 2020;383(22): 2189-2190. [2] SALARI N, DARVISHI N, BARTINA Y, et al. Global prevalence of osteoporosis among the world older adults: a comprehensive systematic review and meta-analysis.J Orthop Surg Res. 2021;16(1):669. [3] CHE L, WANG Y, SHA D, et al. A biomimetic and bioactive scaffold with intelligently pulsatile teriparatide delivery for local and systemic osteoporosis regeneration. Bioact Mater. 2022;19:75-87. [4] VAN DER WOUDE D, VAN DER HELM-VAN MIL AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018;32(2):174-187. [5] MALOCHET-GUINAMAND S, LAMBERT C, GOSSEC L, et al. Evaluation of the implementation of guidelines on the treatment of osteoporosis in patients with rheumatoid arthritis. J Rheumatol. 2020;47(1):6-14. [6] TONG JJ, XU SQ, ZONG HX, et al. Prevalence and risk factors associated with vertebral osteoporotic fractures in patients with rheumatoid arthritis. Clin Rheumatol. 2020;39(2):357-364. [7] CHOI ST, KWON SR, JUNG JY, et al. Prevalence and fracture risk of osteoporosis in patients with rheumatoid arthritis: a multicenter comparative study of the FRAX and WHO criteria. J Clin Med. 2018;7(12):507. [8] SKRIVANKOVA VW, RICHMOND RC, WOOLF BAR, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375:n2233. [9] HEMANI G, ZHENG J, ELSWORTH B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. [10] GOTO A, YAMAJI T, SAWADA N, et al. Diabetes and cancer risk: a Mendelian randomization study. Int J Cancer. 2020;146(3):712-719. [11] HARTWIG FP, BORGES MC, HORTA BL, et al. Inflammatory biomarkers and risk of schizophrenia: a 2-sample mendelian randomization study. JAMA Psychiatry. 2017;74(12):1226-1233. [12] LEE K, LIM CY. Mendelian randomization analysis in observational epidemiology. J Lipid Atheroscler. 2019;8(2):67-77. [13] HA E, BAE SC, KIM K. Large-scale meta-analysis across East Asian and European populations updated genetic architecture and variant-driven biology of rheumatoid arthritis, identifying 11 novel susceptibility loci. Ann Rheum Dis. 2021;80(5):558-565. [14] PIERCE BL, AHSAN H, VANDERWEELE TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740-752. [15] VERBANCK M, CHEN CY, NEALE B, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693-698. [16] BURGESS S, DUDBRIDGE F, THOMPSON SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880-906. [17] MENSAH-KANE J, SCHMIDT AF, HINGORANI AD, et al. No clinically relevant effect of heart rate increase and heart rate recovery during exercise on cardiovascular disease: a mendelian randomization analysis. Front Genet. 2021;12:569323. [18] ZHENG J, FRYSZ M, KEMP JP, et al. Use of Mendelian randomization to examine causal inference in osteoporosis. Front Endocrinol (Lausanne). 2019;10:807. [19] BOWDEN J, DAVEY SMITH G, HAYCOCK PC, et al. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304-314. [20] YUAN S, CARTER P, MASON AM, et al. Genetic liability to rheumatoid arthritis in relation to coronary artery disease and stroke risk. Arthritis Rheumatol. 2022; 74(10):1638-1647. [21] BOWDEN J, DEL GRECO MF, MINELLI C, et al. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36(11):1783-1802. [22] BURGESS S, THOMPSON SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377-389. [23] FARDELLONE P, SALAWATI E, LE MONNIER L, et al. Bone loss, osteoporosis, and fractures in patients with rheumatoid arthritis: a review. J Clin Med. 2020; 9(10):3361. [24] POURESMAEILI F, KAMALIDEHGHAN B, KAMAREHEI M, et al. A comprehensive overview on osteoporosis and its risk factors. Ther Clin Risk Manag. 2018;14: 2029-2049. [25] ADAMI G, SAAG KG. Osteoporosis pathophysiology, epidemiology, and screening in rheumatoid arthritis. Curr Rheumatol Rep. 2019;21(7):34. [26] HEINLEN L, HUMPHREY MB. Skeletal complications of rheumatoid arthritis. Osteoporos Int. 2017;28(10):2801-2812. [27] SANTOS AM, SALDARRIAGA EL, GIRALDO-BUSTOS R, et al. Dickkopf 1 protein circulating levels as a possible biomarker of functional disability and chronic damage in patients with rheumatoid arthritis. Clin Rheumatol. 2018;37(3):795-801. [28] GARNERO P, THOMPSON E, WOODWORTH T, et al. Rapid and sustained improvement in bone and cartilage turnover markers with the anti-interleukin-6 receptor inhibitor tocilizumab plus methotrexate in rheumatoid arthritis patients with an inadequate response to methotrexate: results from a substudy of the multicenter double-blind, placebo-controlled trial of tocilizumab in inadequate responders to methotrexate alone. Arthritis Rheum. 2010;62(1):33-43. [29] BUCKLEY L, HUMPHREY MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379(26):2547-2556. [30] OHLSSON C, NILSSON KH, HENNING P, et al. WNT16 overexpression partly protects against glucocorticoid-induced bone loss. Am J Physiol Endocrinol Metab. 2018;314(6):E597-E604. [31] ZANOTTI S, YU J, Adhikari S, et al. Glucocorticoids inhibit notch target gene expression in osteoblasts. J Cell Biochem. 2018;119(7):6016-6023. [32] HUANG X, JIE S, LI W, et al. miR-122-5p targets GREM2 to protect against glucocorticoid-induced endothelial damage through the BMP signaling pathway. Mol Cell Endocrinol. 2022;544:111541. [33] GROSSE J, ALLADO E, ROUX C, et al. ACPA-positive versus ACPA-negative rheumatoid arthritis: two distinct erosive disease entities on radiography and ultrasonography. Rheumatol Int. 2020;40(4):615-624. |
| [1] | Guo Sutong, Feng Dehong, Guo Yu, Wang Ling, Ding Yujian, Liu Yi, Qian Zhengying, Li Mingyang. Construction and finite element analysis of normal and osteoporotic hip models [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1342-1346. |
| [2] | Yang Cekai, Cai Zhuoyan, Chen Ming, Liu Hao, Weng Rui, Cui Jianchao, Zhang Shuncong, Yao Zhensong. Relationship between degeneration of paraspinal muscle and refractures in postmenopausal women treated by percutaneous vertebroplasty [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1414-1419. |
| [3] | Xiaheida·Yilaerjiang, Nijiati·Tuerxun, Reyila·Kuerban, Baibujiafu·Yelisi, Chen Xin. Three-dimensional finite element analysis of the distribution pattern of stress in bone tissues with different characteristics [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1277-1282. |
| [4] | Zhang Xiaoyun, Liu Hua, Chai Yuan, Chen Feng, Zeng Hao, Gao Zhengang, Huang Yourong. Effect of Yishen Gushu Formula on bone metabolic markers and clinical efficacyn in patients with osteoporosis of kidney deficiency and blood stasis type [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1155-1160. |
| [5] | Dai Yuexing, Zheng Liqin, Wu Minhui, Li Zhihong, Li Shaobin, Zheng Desheng, Lin Ziling. Effect of vessel number on computational fluid dynamics in vascular networks [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1206-1210. |
| [6] | Zhang Min, Peng Jing, Zhang Qiang, Chen Dewang. Mechanical properties of L3/4 laminar decompression and intervertebral fusion in elderly osteoporosis patients analyzed by finite element method [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 847-851. |
| [7] | Xue Xiaofeng, Wei Yongkang, Qiao Xiaohong, Du Yuyong, Niu Jianjun, Ren Lixin, Yang Huifeng, Zhang Zhimin, Guo Yuan, Chen Weiyi. Finite element analysis of osteoporosis in proximal femur after cannulated screw fixation for femoral neck fracture [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 862-867. |
| [8] | Kaiyisaier•Abudukelimu, Maimaitimin•Abulimiti, Li Lei, Yang Xiaokai, Zhang Yukun, Liu Shuai. Effect of lumbar CT values in the diagnosis of osteoporosis in women patients with lumbar degenerative diseases [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 945-949. |
| [9] | Wang Liping, Lian Tianxing, Hu Yongrong, Yang Hongsheng, Zeng Zhimou, Liu Hao, Qu Bo. HU value of chest CT vertebral body in the opportunistic screening of type 2 diabetes mellitus osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 950-954. |
| [10] | Yu Zhaoyu, Tan Lixin, Sun Kai, Lu Yao, Li Yong. Meta-analysis of cement-augmented pedicle screw for thoracolumbar degenerative diseases with osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 813-820. |
| [11] | Wei Yuanxun, Chen Feng, Lin Zonghan, Zhang Chi, Pan Chengzhen, Wei Zongbo. The mechanism of Notch signaling pathway in osteoporosis and its prevention and treatment with traditional Chinese medicine [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 587-593. |
| [12] | Abuduwupuer·Haibier, Alimujiang·Yusufu, Maihemuti·Yakufu, Maimaitimin·Abulimiti, Tuerhongjiang·Abudurexiti. Meta-analysis of efficacy and safety of terlipatide and bisphosphate in the treatment of postmenopausal osteoporosis fractures [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 639-645. |
| [13] | Xie Ting, Liu Tingting, Zeng Xuehui, Li Yamin, Zhou Panghu, Yi Nianhua. Fucoxanthin alleviates glucocorticoid-induced osteoblast apoptosis by activating nuclear factor erythroid-2-related factor 2 [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(23): 3609-3614. |
| [14] | Lin Chubin, He Xingpeng, Qiu Yuhui, Wu Wenjin, Chang Yu, Ye Tao, Li Pengfei, Yang Jian. Biomechanical analysis of the bones in a rat model of osteoporosis based on the combination of disease and syndrome [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(23): 3636-3641. |
| [15] | Yao Ting, Guan Rongwei, Gao Yuan. Overexpression of SMAD4 interferes with the expression of iron metabolism related proteins in osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(23): 3648-3653. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||