Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (26): 4106-4112.doi: 10.12307/2024.440
Previous Articles Next Articles
Two-sample Mendelian randomization analysis of the relationship between statins and the risk of osteoarthritis
Wu Ruiqi1, Zhang Xuan1, Zhou Yi1, Meng Lin2, 3, Li Hongyu2
- 1Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China; 2Guangxi Orthopedic Hospital, Nanning 530000, Guangxi Zhuang Autonomous Region, China; 3Chongqing Medical University, Chongqing 400016, China
-
Received:2023-06-27Accepted:2023-08-09Online:2024-09-18Published:2023-09-28 -
Contact:Meng Lin, Chief physician, Master’s supervisor, Guangxi Orthopedic Hospital, Nanning 530000, Guangxi Zhuang Autonomous Region, China; Chongqing Medical University, Chongqing 400016, China Li Hongyu, Chief physician, Professor, Master’s supervisor, Guangxi Orthopedic Hospital, Nanning 530000, Guangxi Zhuang Autonomous Region, China -
About author:Wu Ruiqi, Master candidate, Guangxi University of Chinese Medicine, Nanning 530000, Guangxi Zhuang Autonomous Region, China -
Supported by:Guangxi Natural Science Foundation, No. 2021GXNSFAA196033 (to ZX); Guangxi Zhuang Autonomous Region Young Qihuang Scholar Training Project, No. [2022]13; Guangxi Traditional Chinese Medicine Ethnic Medicine Appropriate Technology Promotion and Application Project, No. GZSY23-13 (to ML); Chongqing Municipal Natural Science Foundation (General Project), No. CSTB2022NSCQ-MSX0076 (to ML)
CLC Number:
Cite this article
Wu Ruiqi, Zhang Xuan, Zhou Yi, Meng Lin, Li Hongyu. Two-sample Mendelian randomization analysis of the relationship between statins and the risk of osteoarthritis[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(26): 4106-4112.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
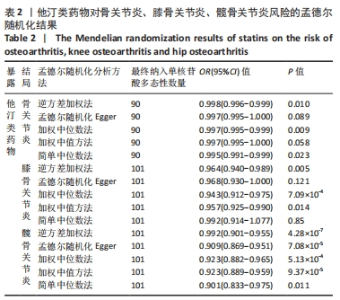
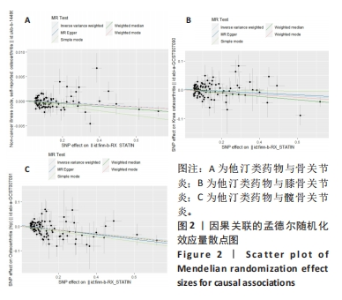
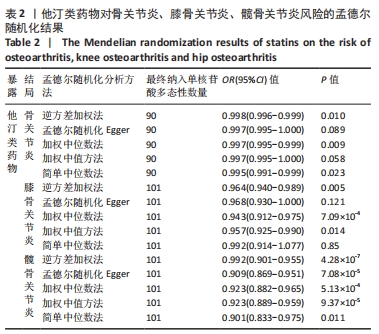
2.1 工具变量 根据此次研究工具变量筛选标准,首先从他汀类药物暴露数据集中共筛选出128个密切相关且无连锁不平衡的单核苷酸多态性,经过几次筛选,骨关节炎结局数据集获得115个单核苷酸多态性,利用PhenoScanner数据库检索有关的表型,剔除其对应表型与骨关节炎具有相关意义的1个单核苷酸多态性(rs1415991),并删除回文单核苷酸多态性(n=23),同时经MR-PRESSO分析后剔除1个离群值(rs117814720),单一单核苷酸多态性对应的F统计量分布范围22.10-257.72,表明在MR分析中没有弱的工具变量,最终纳入90个单核苷酸多态性作为工具变量来评估他汀类药物和骨关节炎之间的关联。 膝骨关节炎结局数据集获得128个单核苷酸多态性,剔除其对应表型与膝骨关节炎具有相关意义的2个单核苷酸多态性(rs78087637、rs998584),并删除回文单核苷酸多态性单核苷酸多态性(n=22),同时经MR-PRESSO分析后剔除3个离群值(rs12112877、rs115478735、rs9849171),单一单核苷酸多态性单核苷酸多态性对应的F统计量分布范围25.50-366.85,表明在MR分析中没有弱的工具变量,最终纳入101个单核苷酸多态性作为工具变量来评估他汀类药物和膝骨关节炎之间的关联。 髋骨关节炎结局数据集获得128个单核苷酸多态性,剔除其对应表型与髋骨关节炎具有相关意义的1个单核苷酸多态性(rs1415991),并删除回文单核苷酸多态性(n=23),同时经MR-PRESSO分析后剔除3个离群值(rs112108223、rs213484、rs217181),单一单核苷酸多态性对应的F统计量分布范围25.67.10-378.92,表明在MR分析中没有弱的工具变量,最终纳入101个单核苷酸多态性作为工具变量来评估他汀类药物和髋骨关节炎之间的关联。 2.2 他汀类药物对骨关节炎疾病的影响 IVW法分析结果显示,遗传预测的他汀类药物与骨关节炎(OR=0.998,95%CI:0.996-0.999,P=0.01)、膝骨关节炎(OR=0.964,95%CI:0.940-0.989,P=0.005)和髋骨关节炎(OR=0.928,95%CI:0.901-0.955,P=4.28×10-7)之间可能呈负向因果效应,且具有统计学意义,同时应用其他4种分析方法(MR-Egger、WM、WME和SM)来确认结果的稳健性,其中,WM、SM和WME方法分析的结果与IVW相似,MR-Egger分析虽不支持他汀类药物与骨关节炎(OR=0.997,95%CI:0.995-1.000,P=0.089)和膝骨关节炎(OR=0.968,95%CI:0.930-1.008,P=0.121)之间具有因果关系,但IVW、MR-Egger、WM、WME和SM 5种分析方法的结果方向一致(OR值均 < 1),详见表2。此外,MR-PRESSO方法在异常值校正前后的因果估计是一致的,散点图同样显示了遗传预测的他汀类药物对骨关节炎、膝骨关节炎、髋骨关节炎风险得到的回归线基本一致,见图2。以上表明MR分析的结果是可靠的。"
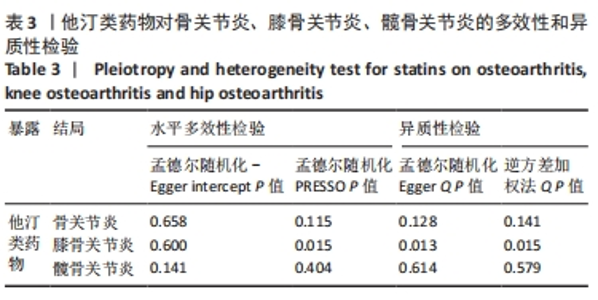
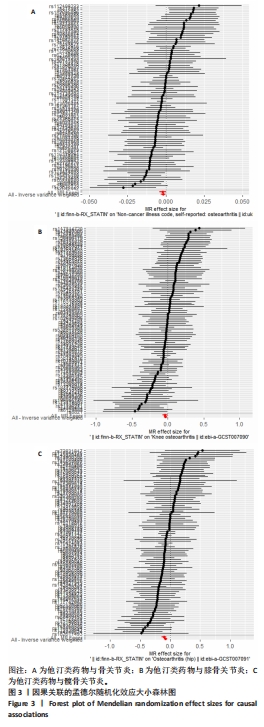
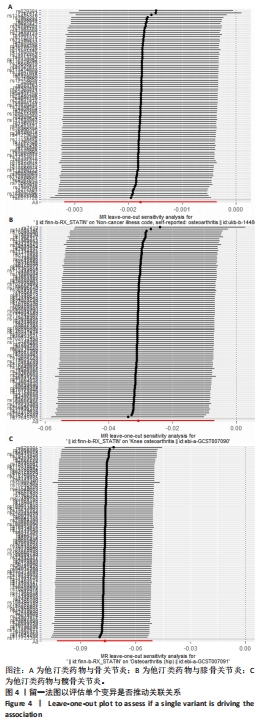
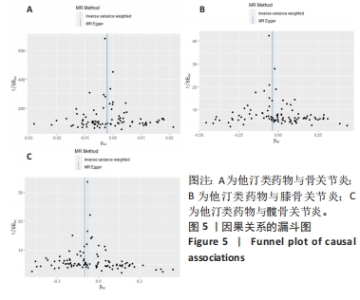
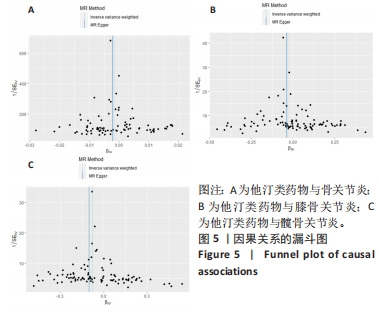
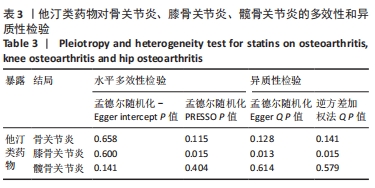
2.3 敏感性分析 MR-Egger-intercept分析未检测到潜在的水平多效性(骨关节炎:P=0.658;膝骨关节炎:P=0.600;髋骨关节炎:P=0.141),说明工具变量并不显著通过暴露以外的途径影响结局。在Cochran’s Q异质性检验中,膝骨关节炎的结果存在潜在的异质性(P=0.015),而在骨关节炎(P=0.141)和髋骨关节炎(P=0.579)的单核苷酸多态性中没有观察到显著的异质性,见表3。虽然发现存在异质性,但IVW分析方法的结果表明,他汀类药物与膝骨关节炎(P=0.005)确实存在因果关系,且未违背核心假设2中的水平多效性检验。留一法检验分析显示没有个别单核苷酸多态性对整体因果估计产生影响。因果关联的MR效应大小的森林图及留一法检验结果见图3,4。为了进一步检验上述结果的稳定性,绘制的漏斗图中显示的因果效应分布基本对称,不受潜在因素影响而发生偏倚,见图5。"
| [1] HUNTER DJ, BIERMA-ZEINSTRA S. Osteoarthritis. Lancet. 2019;393(10182):1745-1759. [2] HUNTER DJ, MARCH L, CHEW M. Osteoarthritis in 2020 and beyond: a Lancet Commission. Lancet. 2020;396(10264):1711-1712. [3] FARNAGHI S, CRAWFORD R, XIAO Y, et al. Cholesterol metabolism in pathogenesis of osteoarthritis disease. Int J Rheum Dis. 2017;20(2):131-140. [4] ZHAO L, HUANG J, FAN Y, et al. Exploration of CRISPR/Cas9-based gene editing as therapy for osteoarthritis. Ann Rheum Dis. 2019;78(5):676-682. [5] SELLAM J, COURTIES A, EYMARD F, et al. Recommendations of the French Society of Rheumatology on pharmacological treatment of knee osteoarthritis. Joint Bone Spine. 2020;87(6):548-555. [6] BI L, YI J, WU C, et al. Atherosclerotic Cardiovascular Disease Risk and Lipid-Lowering Therapy Requirement in China. Front Cardiovasc Med. 2022;9:839571. [7] KOSKINAS KC, SIONTIS GCM, Piccolo R, et al. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur Heart J. 2018;39(14):1172-1180. [8] FULCHER J, O’CONNELL R, VOYSEY M, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397-1405. [9] KADAM UT, BLAGOJEVIC M, BELCHER J. Statin use and clinical osteoarthritis in the general population: a longitudinal study. J Gen Intern Med. 2013;28(7):943-949. [10] CHENG YY, KAO CL, LIN SY, et al. Effect of an increased dosage of statins on spinal degenerative joint disease: a retrospective cohort study. BMJ Open. 2018;8(2):e017442. [11] WANG J, DONG J, YANG J, et al. Association between statin use and incidence or progression of osteoarthritis: meta-analysis of observational studies. Osteoarthritis Cartilage. 2020;28(9):1170-1179. [12] MANSI IA, MORTENSEN EM, PUGH MJ, et al. Incidence of musculoskeletal and neoplastic diseases in patients on statin therapy: results of a retrospective cohort analysis. Am J Med Sci. 2013;345(5):343-348. [13] HEMANI G, ZHENG J, ELSWORTH B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. [14] EMDIN CA, KHERA AV, KATHIRESAN S. Mendelian Randomization. JAMA. 2017; 318(19):1925-1926. [15] HARTWIG FP, BORGES MC, HORTA BL, et al. Inflammatory Biomarkers and Risk of Schizophrenia: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry. 2017;74(12):1226-1233. [16] VON ELM E, ALTMABN DG, EGGER M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806-808. [17] HAWKER GA. Osteoarthritis is a serious disease. Clin Exp Rheumatol. 2019;37 Suppl 120(5):3-6. [18] BURGESS S, DUDBRIDGE F, THOMPSON SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880-1906. [19] YANG J, FERREIRA T, MORRIS AP, et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44(4):369-375, S1-3. [20] BOWDEN J, DAVEY SMITH G, HAYCOCK PC, et al. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40(4):304-314. [21] BOWDEN J, DAVEY SMITH G, BURGESS S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525. [22] BOWDEN J, DEL GRECO MF, MINELLI C, et al. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36(11):1783-1802. [23] BURGESS S, THOMPSON SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377-389. [24] XU J, LI M, GAO Y, et al. Using Mendelian randomization as the cornerstone for causal inference in epidemiology. Environ Sci Pollut Res Int. 2022;29(4):5827-5839. [25] 刘明,高亚,杨珂璐,等.孟德尔随机化研究的报告规范(STROBE-MR)解读[J].中国循证医学杂志,2022,22(8):978-987. [26] CLOCKAERTS S, VAN OSCH GJ, BASTIAANSEN-JENNISKENS YM, et al. Statin use is associated with reduced incidence and progression of knee osteoarthritis in the Rotterdam study. Ann Rheum Dis. 2012;71(5):642-647. [27] HAJ-MIRZAIAN A, MOHAJER B, GUERMAZI A, et al. Statin Use and Knee Osteoarthritis Outcome Measures according to the Presence of Heberden Nodes: Results from the Osteoarthritis Initiative. Radiology. 2019;293(2):396-404. [28] GOTO N, OKAZAKI K, AKASAKI Y, et al. Single intra-articular injection of fluvastatin-PLGA microspheres reduces cartilage degradation in rabbits with experimental osteoarthritis. J Orthop Res. 2017;35(11):2465-2475. [29] BEATTIE MS, LANE NE, HUNG YY, et al. Association of statin use and development and progression of hip osteoarthritis in elderly women. J Rheumatol. 2005;32(1):106-110. [30] MICHAELSSON K, LOHMANDER LS, TRKIEWICZ A, et al. Association between statin use and consultation or surgery for osteoarthritis of the hip or knee: a pooled analysis of four cohort studies. Osteoarthritis Cartilage. 2017;25(11):1804-1813. [31] SHAKIBAEI M, JOHN T, SCHULZE-TANZIL G, et al. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73(9):1434-1445. [32] TANAKA T, MATSUSHITA T, NISHIDA K, et al. Attenuation of osteoarthritis progression in mice following intra-articular administration of simvastatin-conjugated gelatin hydrogel. J Tissue Eng Regen Med. 2019;13(3):423-432. [33] KLEMENT M, DREXEL H, SAELY CH. Impact of ezetimibe on markers of inflammation in patients treated with statins: a systematic review. Inflammopharmacology. 2023;31(4):1647-1656. [34] WU Y, LI X, GUO Y, et al. Pravastatin Reduces Matrix Metalloproteinases Expression and Promotes Cholesterol Efflux in Osteoarthritis Chondrocytes. Evid Based Complement Alternat Med. 2022;2022:9666963. [35] ABELES AM, PILLINGER MH. Statins as antiinflammatory and immunomodulatory agents: a future in rheumatologic therapy? Arthritis Rheum. 2006;54(2):393-407. [36] PATHAK NN, BALAGANUR V, LINGARAJU MC, et al. Effect of atorvastatin, a HMG-CoA reductase inhibitor in monosodium iodoacetate-induced osteoarthritic pain: implication for osteoarthritis therapy. Pharmacol Rep. 2015;67(3):513-519. [37] ZHANG K, JI Y, DAI H, et al. High-Density Lipoprotein Cholesterol and Apolipoprotein A1 in Synovial Fluid: Potential Predictors of Disease Severity of Primary Knee Osteoarthritis. Cartilage. 2021;13:1465S-1473S. [38] GIERMAN LM, KUHNAST S, KOUDIJS A, et al. Osteoarthritis development is induced by increased dietary cholesterol and can be inhibited by atorvastatin in APOE*3Leiden.CETP mice--a translational model for atherosclerosis. Ann Rheum Dis. 2014;73(5):921-927. |
| [1] | Li Yongjie, Fu Shenyu, Xia Yuan, Zhang Dakuan, Liu Hongju. Correlation of knee extensor muscle strength and spatiotemporal gait parameters with peak knee flexion/adduction moment in female patients with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1354-1358. |
| [2] | Qi Haodong, Lu Chao, Xu Hanbo, Wang Mengfei, Hao Yangquan. Effect of diabetes mellitus on perioperative blood loss and pain after primary total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1383-1387. |
| [3] | Du Changling, Shi Hui, Zhang Shoutao, Meng Tao, Liu Dong, Li Jian, Cao Heng, Xu Chuang. Efficacy and safety of different applications of tranexamic acid in high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1409-1413. |
| [4] | Huang Xiarong, Hu Lizhi, Sun Guanghua, Peng Xinke, Liao Ying, Liao Yuan, Liu Jing, Yin Linwei, Zhong Peirui, Peng Ting, Zhou Jun, Qu Mengjian. Effect of electroacupuncture on the expression of P53 and P21 in articular cartilage and subchondral bone of aged rats with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1174-1179. |
| [5] | Zhao Garida, Ren Yizhong, Han Changxu, Kong Lingyue, Jia Yanbo. Mechanism of Mongolian Medicine Erden-uril on osteoarthritis in rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1193-1199. |
| [6] | Li Rui, Zhang Guihong, Wang Tao, Fan Ping. Effect of ginseng polysaccharide on the expression of prostaglandin E2/6-keto-prostaglandin 1alpha in traumatic osteoarthritis model rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1235-1240. |
| [7] | Zhang Kefan, Shi Hui. Research status and application prospect of cytokine therapy for osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 961-967. |
| [8] | Zhang Zeyi, Yang Yimin, Li Wenyan, Zhang Meizhen. Effect of foot progression angle on lower extremity kinetics of knee osteoarthritis patients of different ages: a systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(6): 968-975. |
| [9] | Shen Feiyan, Yao Jixiang, Su Shanshan, Zhao Zhongmin, Tang Weidong. Knockdown of circRNA WD repeat containing protein 1 inhibits proliferation and induces apoptosis of chondrocytes in knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 499-504. |
| [10] | Maisituremu·Heilili, Zhang Wanxia, Nijiati·Nuermuhanmode, Maimaitituxun·Tuerdi. Effect of intraarticular injection of different concentrations of ozone on condylar histology of rats with early temporomandibular joint osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 505-509. |
| [11] | Qiao Hujun, Wang Guoxiang. Evaluation of rat osteoarthritis chondrocyte models induced by interleukin-1beta [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 516-521. |
| [12] | Liu Yuhan, Fan Yujiang, Wang Qiguang. Comparison of protocols for constructing animal models of early traumatic knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 542-549. |
| [13] | Zhang Yaru, Chen Yanjun, Zhang Xiaodong, Chen Shenghua, Huang Wenhua. Effect of ferroptosis mediated by glutathione peroxidase 4 in the occurrence and progression of synovitis in knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 550-555. |
| [14] | Liu Luxing, Di Mingyuan, Yang Qiang. Signaling pathways in the mechanism underlying active ingredients of Chinese medicine in the treatment of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 609-614. |
| [15] | Yan Binghan, Li Zhichao, Su Hui, Xue Haipeng, Xu Zhanwang, Tan Guoqing. Mechanisms of traditional Chinese medicine monomers in the treatment of osteoarthritis by targeting autophagy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 627-632. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||