Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (23): 3736-3742.doi: 10.12307/2024.341
Previous Articles Next Articles
Osteoprotegerin/receptor activator of nuclear factor kappa-B/receptor activator of nuclear factor kappa-B ligand and the application of relevant target therapy in oral medicine
Zhou Jing, Zhang Zhao
- Department of Prosthodontics, Hebei Key Laboratory of Stomatology, Hebei Clinical Research Center for Oral Diseases, School and Hospital of Stomatology, Hebei Medical University, Shijiazhuang 050017, Hebei Province, China
-
Received:2023-05-06Accepted:2023-06-05Online:2024-08-18Published:2023-09-14 -
Contact:Zhang Zhao, MD, Associate chief physician, Department of Prosthodontics, Hebei Key Laboratory of Stomatology, Hebei Clinical Research Center for Oral Diseases, School and Hospital of Stomatology, Hebei Medical University, Shijiazhuang 050017, Hebei Province, China -
About author:Zhou Jing, Master candidate, Physician, Department of Prosthodontics, Hebei Key Laboratory of Stomatology, Hebei Clinical Research Center for Oral Diseases, School and Hospital of Stomatology, Hebei Medical University, Shijiazhuang 050017, Hebei Province, China -
Supported by:Hebei Provincial Department of Finance Scientific Research Project on Prevention and Treatment of Geriatric Diseases, No. 361029 (to ZZ); 2020 Government-funded Clinical Medicine Excellent Talent Training Project of Hebei Provincial Department of Finance, No. 2020048149-2 (to ZZ); Hebei Provincial Health and Health Commission Medical Science Research Project Program, No. 20190098 (to ZZ)
CLC Number:
Cite this article
Zhou Jing, Zhang Zhao. Osteoprotegerin/receptor activator of nuclear factor kappa-B/receptor activator of nuclear factor kappa-B ligand and the application of relevant target therapy in oral medicine[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(23): 3736-3742.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

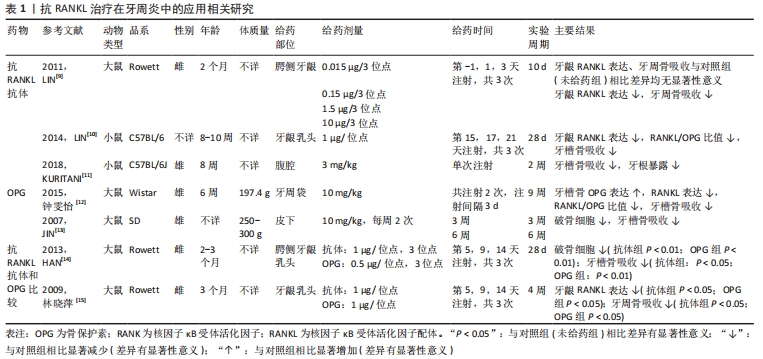
2.1 抗RANKL治疗在口腔领域的应用 抗RANKL抗体和OPG是RANKL的特异性抑制剂[6],其通过与RANKL结合直接靶向调节RANK-RANKL系统,抑制破骨细胞分化,从而达到抑制骨吸收的目的。抗RANKL治疗在口腔领域有广泛应用,近年来研究热点主要集中于牙周炎、正畸牙移动和种植修复等方面。 2.1.1 抗RANKL治疗在牙周炎中的应用 牙周炎是由牙菌斑生物膜所引起的牙周组织感染性疾病,可导致牙周支持组织的破坏(附着丧失、牙周袋形成和牙槽骨吸收),是成人牙齿丧失的首要原因。TEODORESCU等[7]和BELIBASAKIS等[8]研究发现,与健康受试者相比,牙周炎患者牙龈组织、龈沟液、唾液和血液中的RANKL和RANKL/OPG比值均显著升高,且该变化与局部炎症状态相关。BELIBASAKIS等[8]研究还发现牙周炎患者在接受常规牙周治疗前后RANKL/OPG比值无明显变化,推测治疗后该比值居高不下可能是骨吸收的分子机制仍然活跃,有疾病复发的风险。因此,BELIBASAKIS等[8]认为可能需要额外的辅助治疗来降低RANKL/OPG比值,从而抑制牙槽骨破坏。大量研究表明可以通过局部或全身应用抗RANKL抗体和OPG来实现。 (1)抗RANKL抗体在牙周炎中的应用:抗RANKL抗体可以通过阻断和减少牙周组织中的RANKL表达来抑制牙槽骨吸收。 局部应用抗RANKL抗体:LIN等[9]研究发现,在建立大鼠实验性牙周炎模型过程中,牙龈可溶性RANKL浓度和骨丢失之间存在显著的相关性,在牙周炎建模期间于大鼠上颌腭侧牙龈注射抗RANKL抗体后,可显著降低牙龈可溶性RANKL的表达、抑制牙槽骨吸收。LIN等[10]随后在小鼠实验性牙周炎模型中再次证实了这一结论,其通过在小鼠牙龈乳头注射抗RANKL抗体来治疗牙周炎,结果显示牙龈组织中RANKL/OPG比值显著降低,牙槽骨吸收明显减少,差异有显著性意义。 全身应用抗RANKL抗体:KURITANI等[11]在实验性牙周炎小鼠腹腔注射抗RANKL抗体后有效抑制了牙槽骨破坏,同时也避免了牙根暴露。 (2)OPG在牙周炎中的应用:OPG作为RANKL的另一种特异性抑制剂,也可以通过与RANKL直接结合来阻断和减少组织中的RANKL,从而抑制牙周骨吸收。 局部应用OPG:钟雯怡等[12]在实验性牙周炎大鼠牙周袋内注射重组人骨保护素治疗后,牙槽骨中OPG的表达水平明显高于治疗前,同时RANKL的表达水平明显低于治疗前,RANKL/OPG比值显著降低,牙槽骨吸收明显减少,差异有显著性意义(P < 0.05)。 全身应用OPG:JIN等[13]为了研究全身给药OPG对牙槽骨吸收的影响,采用结扎诱导大鼠牙周炎模型,实验组通过皮下注射重组人骨保护素融合蛋白进行治疗,结果表明全身应用OPG可显著抑制破骨细胞形成,从而抑制牙槽骨吸收(P < 0.05)。 (3)抗RANKL抗体和OPG治疗牙周炎的比较:HAN等[14]研究结果显示牙龈卟啉单胞菌感染导致的牙周骨吸收依赖于RANKL,并随RANKL表达的上调而加重,在牙龈卟啉单胞菌感染后的大鼠牙龈乳头分别注射抗RANKL抗体和骨保护素后,均可显著降低牙龈可溶性RANKL的表达,抑制破骨细胞形成,从而抑制牙周骨吸收;与对照组(未给药组)相比,局部注射抗RANKL抗体(1 μg/位点)抑制牙槽骨吸收(P < 0.05),且局部注射OPG(0.5 μg/位点)同样也抑制了牙槽骨吸收(P < 0.01),此结果提示相同剂量的OPG在治疗牙周炎方面可能比抗RANKL抗体有更强的骨吸收抑制作用。 然而,林晓萍等[15]研究显示局部注射相同剂量的抗RANKL抗体(1 μg/位点)和OPG(1 μg/位点)在治疗牙周炎方面有相似的骨吸收抑制作用。由此可见,抗RANKL抗体和OPG在牙周炎的治疗效果上是否存在差异具有一定争议,有待进一步研究。 以上研究表明,局部和全身应用抗RANKL抗体和OPG均可预防和治疗牙周炎相关的牙槽骨破坏,相关研究见表1。"

2.1.2 抗RANKL治疗在正畸牙移动中的应用 正畸治疗主要是通过矫治器对牙齿和颌骨施加一定的力,在力的作用下牙齿周围支持组织与颌骨组织改建、重塑,从而实现牙齿和颌骨的移动。然而,在矫治过程中,任何施加于矫治牙的力,必然引起等力的反作用,往往伴随着不良的牙齿移动。鉴于牙齿移动在细胞水平上受到破骨细胞活性的调控,破骨细胞形成、分化和活化在分子水平上又受到OPG/RANK/RANKL信号通路的调控,因此,基于该信号通路对破骨细胞进行生物调控可能为正畸治疗过程中调节牙齿移动和改善锚固控制提供一个可行的选择[16]。 (1)增强正畸锚固:抗RANKL抗体应用方面,YOSHIMATSU等[17]首先给实验组小鼠腹腔注射抗RANKL抗体,随后进行正畸牙齿移动,加力10 d后,对移动牙齿周围的组织进行组织学评估,结果表明经抗体治疗的小鼠破骨细胞形成及牙齿移动受到了抑制,同时也避免了牙齿移动过程中的牙根吸收。OPG应用方面,KELES等[18]通过构建小鼠正畸牙移动模型来探索皮下注射OPG对牙槽骨吸收和牙齿移动的影响,结果表明全身应用OPG可以减少压力侧破骨细胞形成、抑制牙槽骨吸收,从而抑制牙齿移动,起到增强正畸锚固的作用。国内外大量研究表明局部应用OPG也能有效抑制破骨细胞的生成,从而改善骨量和正畸锚固效果[19-25]。 不同浓度OPG的比较,陈雁南等[23-24]为了研究局部注射不同浓度OPG对大鼠正畸牙移动的影响,实验组于大鼠上颌第一磨牙腭侧黏骨膜下注射不同浓度的OPG,结果表明高浓度OPG比低浓度OPG 在抑制破骨细胞形成及牙齿移动方面的效果更显著。DUNN等[25]研究也表明与低剂量OPG组相比,局部注射高剂量OPG能显著抑制牙齿的移动,然而局部注射OPG不仅抑制了靠近注射部位的牙齿移动,还抑制了远处理想的牙齿移动。 不同给药形式OPG的比较,为精准控制正畸牙移动,研究人员在OPG的给药形式方面进行了探索,SYDORAK等[16]开发了一种局部释放OPG的系统即微球封装OPG,以增强正畸锚固,而不抑制远处理想的牙齿移动,并比较了局部单次注射微球封装OPG、单次注射非封装OPG和多次注射高剂量非封装OPG对正畸力作用下支抗牙的锚固效果,结果表明单次注射微球封装OPG可显著增强正畸锚固,而单次注射非封装OPG则没有。虽然多次注射高剂量非封装OPG也可增强正畸锚固,但与此同时也抑制了远处理想的牙齿移动,并改变了牙槽骨和股骨骨质量参数,而单次注射封装的OPG仅抑制靠近注射部位的牙齿移动,说明OPG的微球包封可以控制药物的释放,并增强了部位特异性的正畸锚固,而没有全身的不良反应。随着进一步的改进,这种药物传递系统可以适用于广泛的正畸生物治疗。 综上所述,OPG在增强正畸锚固过程中的给药途径、给药剂量及给药形式存在一定争议,有待进一步研究证实。 (2)预防正畸后复发:HUDSON等[26]研究首次证明,RANK/RANKL/OPG轴对破骨细胞活性的调节在牙槽骨成熟和正畸后的稳定性中起着关键作用,通过多次局部注射OPG可显著减少正畸后复发,不仅能预防靠近注射部位牙齿的复发,而且能预防远离注射部位牙齿的复发,但多次注射对全身骨代谢有一定影响。 为避免多次注射对全身的影响,SCHNEIDER等[27]通过在大鼠正畸后模型中单次局部注射OPG来预防正畸后复发,结果表明单次局部注射OPG可有效预防正畸后复发,且对全身骨代谢的影响小,但单次局部注射只能预防靠近注射部位牙齿复发,而不能预防远离注射部位牙齿复发。因此,OPG在预防正畸后复发过程中的给药剂量及作用范围有待进一步研究。 这些研究表明,OPG和抗RANKL抗体有潜力作为一种安全有效的药理学手段来控制破骨细胞的分化和活化,用于在正畸牙齿移动过程中保持锚固和防止正畸后复发,相关研究见表2。"

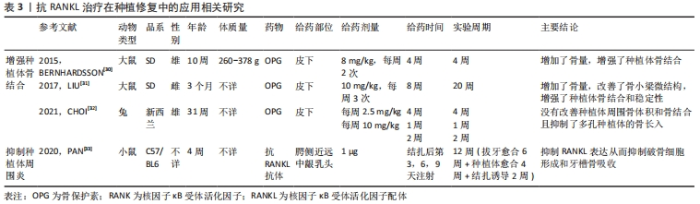
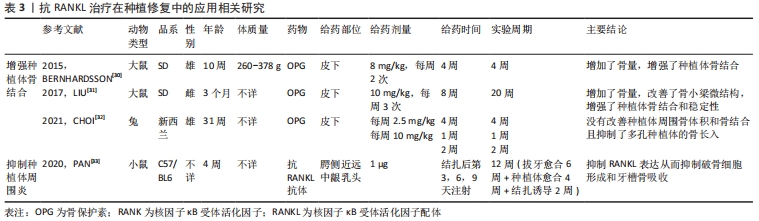
2.1.3 抗RANKL治疗在种植修复中的应用 目前,种植义齿已成为牙列缺损或缺失的主要修复方式之一。种植义齿是将替代天然牙根的种植体植入颌骨,获取类似于牙固位支持的修复体。骨结合是种植体-骨界面的正常愈合,是现代牙种植技术和口腔种植学成功的生物学基础,未达到骨结合的种植体无法行使咀嚼功能[28]。此外,由于种植体缺少天然牙周的防御屏障,一旦种植体周围发生感染,炎症会迅速扩散,导致支持骨吸收,骨整合失败[29]。KAPASA等[5]回顾以往文献总结得出,增加种植体周围OPG表达或OPG/RANKL比值可以降低种植失败率,有效抑制种植后并发症的发生。 (1)增强种植体骨结合:BERNHARDSSON等[30]首先通过手术给大鼠植入种植体,随后实验组皮下注射OPG,4周后经影像学和生物力学分析表明OPG显著改善了种植体周围骨结合,增强了种植体稳定性并增加了骨量,有效改善了骨微结构。LIU等[31]研究发现皮下注射OPG在增强骨质疏松大鼠种植体骨结合、改善骨小梁微结构方面同样有效。然而,CHOI等[32]研究发现皮下注射OPG不但不能改善兔种植体周围骨体积和骨结合,还减少了多孔种植体的骨长入。因此,需要进一步的研究来探索OPG在种植体骨结合方面的具体作用机制。 (2)抑制种植体周围炎:PAN等[33]通过局部牙龈注射抗RANKL抗体来治疗小鼠种植体周围炎,结果表明注射抗体后显著降低了牙龈组织中RANKL mRNA的表达、抑制了牙槽骨丢失。 总而言之,抗RANKL治疗在增强种植体骨结合、预防和治疗种植后并发症等方面具有远大前景,相关研究见表3。"
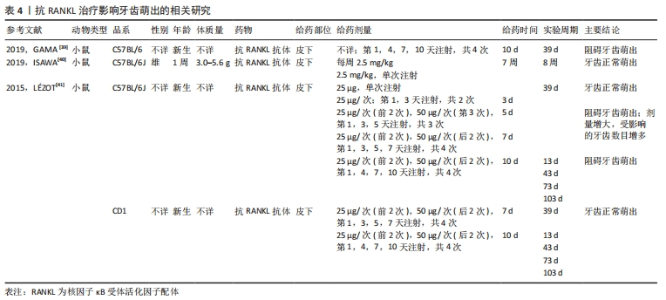
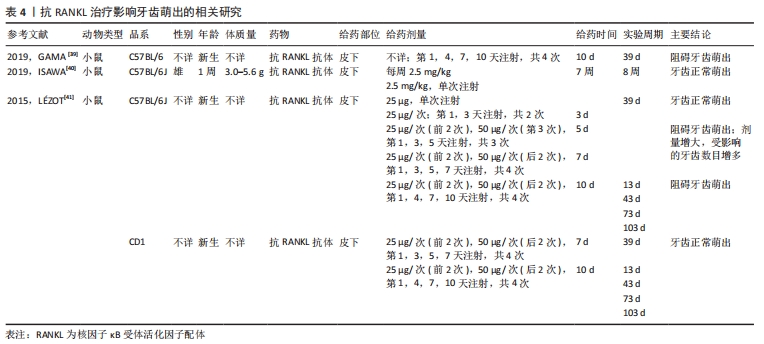
2.1.4 抗RANKL治疗在根尖周病变中的应用 抗RANKL治疗在靶向抑制破骨细胞生成、抑制根尖周骨吸收方面具有潜在优势。LIU等[34]研究提示可以通过降低RANKL/OPG比值来减少破骨细胞的数量和骨吸收面积,从而抑制大鼠根尖周病变的进展。IKEDA等[35]在诱导小鼠根尖周炎过程中,实验组每周于腹腔内注射一次抗RANKL抗体,3周后经显微计算机断层成像和组织学分析表明抗RANKL抗体以剂量依赖的方式抑制根尖周病变的增大,其通过减少根尖周组织中破骨细胞的数量以及炎症细胞的浸润来抑制根尖周骨吸收。该研究表明全身应用RANKL抑制剂可以分布到颌骨的局部炎症区域从而抑制根尖周病变的进展。因此,抗RANKL治疗很有可能在未来作为根尖周病变常规根管治疗的辅助疗法。 2.1.5 抗RANKL治疗在牙本质吸收方面的应用潜力 恒牙在发育、稳态和衰老过程中很少发生牙本质吸收,而在目前的牙齿再植模型中,所有样本都存在牙本质吸收。研究发现RANKL/OPG比值的增加与牙本质和牙根吸收相关[36-37]。CHEN等[38]研究表明牙髓损伤如牙齿再植,会刺激牙髓细胞产生促炎因子,进而激活RANKL,导致牙本质吸收。ZHENG等[37]研究发现牙髓间充质细胞在稳态下高表达OPG、低表达RANKL,从而减弱牙本质吸收,而大鼠脱位牙在逆行牙髓摘除术、牙再植后,牙周膜细胞及牙槽骨细胞可通过根尖孔迁移至根管内,由于其高表达RANKL、低表达OPG,从而导致牙本质吸收。 尽管目前还没有研究将抗RANKL治疗应用于牙本质吸收,但由于其可以靶向调节OPG/RANK/RANKL系统,因此通过抗RANKL治疗来抑制牙本质吸收在未来是一种可能的选择。 2.2 抗RANKL治疗的局限性 2.2.1 阻碍牙根发育、牙齿萌出 牙齿萌出过程实际上是牙槽骨改建的过程,既有冠方破骨细胞骨吸收以形成萌出通道,又有根方成骨细胞骨形成以提供支持和萌出动力,OPG/RANK/RANKL信号通路在这一过程中起到了至关重要的作用。GAMA等[39]研究表明在新生小鼠皮下注射抗RANKL抗体后,可阻断RANK/RANKL之间的相互作用,抑制破骨细胞形成和活化,从而导致磨牙牙根发育和牙齿萌发停滞。然而,ISAWA等[40]研究结果显示,尽管皮下注射抗RANKL抗体通过阻断RANK/RANKL信号通路显著减少了破骨细胞的形成,但并没有影响正常的牙齿萌出。LéZOT等[41]研究发现牙齿萌出受抗RANKL抗体剂量及受试小鼠品系的影响,不受给药间隔长短的影响。研究显示低剂量抗RANKL抗体组表现出正常的牙齿萌出,高剂量抗RANKL抗体组的牙齿萌出受到抑制,且随剂量增大,受抑制的牙齿数目增多。因此,推测可能通过减少抗RANKL抗体的给药剂量来降低对牙齿萌出的影响,相关研究见表4。"

2.2.2 药物相关性颌骨坏死 药物相关性颌骨坏死是一种与骨吸收抑制药物或抗血管生成药物治疗相关的严重不良反应,主要临床表现为颌骨坏死,经常发生在牙槽外科手术如拔牙后,此外,其危险因素还包括牙周炎、吸烟、糖尿病等[42-44]。 大量研究发现RANKL抑制剂如OPG、抗RANKL抗体等通过抑制破骨细胞分化,导致死骨堆积,同时抑制新骨形成,从而增加药物相关性颌骨坏死的发病率,且与给药剂量呈相关性[45-48],有研究显示在停止使用RANKL抑制剂后,破骨细胞的数量可以完全恢复,从而逆转小鼠颌骨坏死的特征[46]。 潘剑等[49]、ALROWIS等[50]和KAWAHARA等[51]结合国内外最新相关研究和近年来对药物相关性颌骨坏死的诊治经验提出,应用RANKL抑制剂前应完善口腔检查,排除局部危险因素如拔牙、龋病等,应用过程中应建立良好的口腔卫生习惯、定期口腔检查与维护、尽量避免创伤性牙槽手术,必要时需采取预防性治疗如牙拔除术前预防性使用抗生素、暂时停用RANKL抑制剂,术中保护骨组织、微创拔牙、及时关闭创口,术后规范护理、保持口腔卫生等措施,这样即可预防药物相关性颌骨坏死的发生。 2.2.3 延迟口腔创口愈合 KUROSHIMA等[52]研究发现每3周皮下注射5 mg/kg抗RANKL抗体,治疗9周后通过手术形成口腔创口,术后3周经组织形态学分析表明抗RANKL抗体可延缓小鼠口腔创口的愈合,提示应用RANKL抑制剂过程中应尽量避免创伤性牙槽手术。 总之,应用RANKL抑制剂前应尽量排除局部和全身危险因素;应用过程中应严格控制给药剂量、定期口腔检查与维护、尽量避免创伤性牙槽手术、必要时需采取预防性治疗或暂时停止用药等措施来降低上述不良反应发生的风险以达到更好的应用效果。 2.3 RANKL治疗在口腔领域的应用 2.3.1 RANKL治疗在正畸牙移动中的应用 正畸治疗的平均时间在20个月左右[53],且随着治疗时间的延长,不良反应的发生和严重程度也会增加,例如龋齿、牙周病、牙根吸收、疼痛和不适等[54-57]。因此,加速正畸牙移动、缩短治疗周期是目前正畸研究的主要方向之一。 CHANG等[58]在构建大鼠正畸牙移动模型中,实验组于大鼠上颌第一磨牙腭侧中央位点注射RANKL缓释剂,2周后经影像学和组织学分析表明局部注射RANKL缓释剂能有效加速正畸牙移动,且没有发现明显的牙根吸收。这与KANZAKI等[59]和IGLESIAS-LINARES等[60]的研究结果一致,与对照组相比,局部RANKL治疗显著增加了牙周组织中RANKL的表达和压力侧破骨细胞的形成,加速了牙齿移动,且对全身没有任何影响。 以上研究表明,局部注射RANKL可以加速正畸牙移动,缩短治疗周期,从而减少正畸并发症的发生。 2.3.2 RANKL治疗在牙齿萌出方面的应用潜力 OPG/RANK/RANKL信号通路的异常调控会导致牙齿萌出障碍或早萌。多项研究发现RANKL基因敲除的小鼠无法激活RANK/RANKL信号通路,表现出破骨细胞的活化障碍[61-62],从而影响生理性牙槽骨吸收,造成牙槽骨肥厚性沉积,引起严重的骨硬化症,最终导致牙齿萌出障碍。相反,在过表达RANKL的转基因小鼠中观察到牙齿早萌[63]。上述研究提示RANKL治疗在调节牙齿萌出方面具有应用前景。"

| [1] AMIN N, BOCCARDI V, TAGHIZADEH M, et al. Probiotics and bone disorders: the role of RANKL/RANK/OPG pathway. Aging Clin Exp Res. 2020;32(3):363-371. [2] TAKEGAHARA N, KIM H, CHOI Y. RANKL biology. Bone. 2022;159:116353. [3] UDAGAWA N, KOIDE M, NAKAMURA M, et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab. 2021;39(1):19-26. [4] YASUDA H. Discovery of the RANKL/RANK/OPG system. J Bone Miner Metab. 2021; 39(1):2-11. [5] KAPASA ER, GIANNOUDIS PV, JIA X, et al. The Effect of RANKL/OPG Balance on Reducing Implant Complications. J Funct Biomater. 2017;8(4):42. [6] YASUDA H. OPG, anti-rANKL antibody. Nihon Rinsho. 2005;63(9):1647-1653. [7] TEODORESCU AC, MARTU I, TESLARU S, et al. Assessment of Salivary Levels of RANKL and OPG in Aggressive versus Chronic Periodontitis. J Immunol Res. 2019;2019:6195258. [8] BELIBASAKIS GN, BOSTANCI N. The RANKL-OPG system in clinical periodontology. J Clin Periodontol. 2012;39(3):239-248. [9] LIN X, HAN X, KAWAI T, et al. Antibody to receptor activator of NF-κB ligand ameliorates T cell-mediated periodontal bone resorption. Infect Immun. 2011;79(2):911-917. [10] LIN J, BI L, YU X, et al. Porphyromonas gingivalis exacerbates ligature-induced, RANKL-dependent alveolar bone resorption via differential regulation of Toll-like receptor 2 (TLR2) and TLR4. Infect Immun. 2014;82(10):4127-4134. [11] KURITANI M, SAKAI N, KARAKAWA A, et al. Anti-mouse RANKL Antibodies Inhibit Alveolar Bone Destruction in Periodontitis Model Mice. Biol Pharm Bull. 2018;41(4):637-643. [12] 钟雯怡,武岐山,高丽,等.重组人骨保护素对牙周炎大鼠牙槽骨RANKL、OPG蛋白表达的影响[J].重庆医学,2015,44(14):1879-1881. [13] JIN Q, CIRELLI JA, PARK CH, et al. RANKL inhibition through osteoprotegerin blocks bone loss in experimental periodontitis. J Periodontol. 2007;78(7):1300-1308. [14] HAN X, LIN X, YU X, et al. Porphyromonas gingivalis infection-associated periodontal bone resorption is dependent on receptor activator of NF-κB ligand. Infect Immun. 2013;81(5):1502-1509. [15] 林晓萍,韩晓哲,魏巍,等.抗RANKL多克隆抗体对P.gingivalis感染的大鼠牙周骨吸收的抑制作用[J].解剖科学进展,2009,15(3):269-272. [16] SYDORAK I, DANG M, BAXTER SJ, et al. Microsphere controlled drug delivery for local control of tooth movement. Eur J Orthod. 2019;41(1):1-8. [17] YOSHIMATSU M, KITAURA H, MORITA Y, et al. Effects of anti-mouse RANKL antibody on orthodontic tooth movement in mice. J Dent Sci. 2022;17(3):1087-1095. [18] KELES A, GRUNES B, DIFURIA C, et al. Inhibition of tooth movement by osteoprotegerin vs. pamidronate under conditions of constant orthodontic force. Eur J Oral Sci. 2007; 115(2):131-136. [19] 娄颖,马欣.骨保护素对大鼠正畸牙齿移动过程中破骨细胞分化和p38-MAPK信号通路的影响[J].吉林大学学报(医学版),2020,46(6):1221-1226,1350. [20] FERNÁNDEZ-GONZÁLEZ FJ, CAÑIGRAL A, LÓPEZ-CABALLO JL, et al. Recombinant osteoprotegerin effects during orthodontic movement in a rat model. Eur J Orthod. 2016;38(4):379-385. [21] 周建萍,陈雁南,任嫒姝,等.局部应用骨保护素对鼠正畸牙移动影响的实验研究[J].重庆医学,2009,38(15):1915-1917,1920. [22] 陈雁南,裴帆,李晓智,等.局部注射骨保护素与二磷酸盐对大鼠正畸牙移动的影响[J].解放军医学杂志,2011,36(11):1200-1202,1206. [23] 陈雁南,裴帆,李晓智,等.局部注射不同浓度骨保护素对大鼠正畸牙移动影响的研究[J].激光杂志,2011,32(5):89-90,92. [24] 陈雁南. OPG对实验大鼠正畸牙移动的影响[D].重庆:重庆医科大学,2012. [25] DUNN MD, PARK CH, KOSTENUIK PJ, et al. Local delivery of osteoprotegerin inhibits mechanically mediated bone modeling in orthodontic tooth movement. Bone. 2007; 41(3):446-455. [26] HUDSON JB, HATCH N, HAYAMI T, et al. Local delivery of recombinant osteoprotegerin enhances postorthodontic tooth stability. Calcif Tissue Int. 2012;90(4):330-342. [27] SCHNEIDER DA, SMITH SM, CAMPBELL C, et al. Locally limited inhibition of bone resorption and orthodontic relapse by recombinant osteoprotegerin protein. Orthod Craniofac Res. 2015;18 Suppl 1:187-195. [28] 李静,陈争晖,凯迪丽娅·亚力坤,等.微渠多孔羟基磷灰石支架修复犬下颌骨大面积缺损后与种植体的骨结合[J].中国组织工程研究,2023,27(12):1920-1926. [29] 高鑫,曾融生.骨保护素在口腔领域的研究进展[J].国际口腔医学杂志,2019,46(3): 316-319. [30] BERNHARDSSON M, SANDBERG O, ASPENBERG P. Anti-RANKL treatment improves screw fixation in cancellous bone in rats. Injury. 2015;46(6):990-995. [31] LIU Y, HU J, LIU B, et al. The effect of osteoprotegerin on implant osseointegration in ovariectomized rats. Arch Med Sci. 2017;13(2):489-495. [32] CHOI JH, WANG Z, ROSS FP, et al. Systemic osteoprotegerin does not improve peri-implant bone volume or osseointegration in rabbits. J Orthop Res. 2021;39(8):1611-1621. [33] PAN K, HU Y, WANG Y, et al. RANKL blockade alleviates peri-implant bone loss and is enhanced by anti-inflammatory microRNA-146a through TLR2/4 signaling. Int J Implant Dent. 2020;6(1):15. [34] LIU L, ZHANG C, HU Y, et al. Protective effect of metformin on periapical lesions in rats by decreasing the ratio of receptor activator of nuclear factor kappa B ligand/osteoprotegerin. J Endod. 2012;38(7):943-947. [35] IKEDA M, KARAKAWA A, TAKIZAWA H, et al. Effects of Anti-Receptor Activator of Nuclear Factor Kappa B Ligand Antibody and Zoledronic Acid on Periapical Lesion Development in Mice. J Endod. 2022;48(5):632-640. [36] FUKUSHIMA H, KAJIYA H, TAKADA K, et al. Expression and role of RANKL in periodontal ligament cells during physiological root-resorption in human deciduous teeth. Eur J Oral Sci. 2003;111(4):346-352. [37] ZHENG Y, CHEN M, HE L, et al. Mesenchymal dental pulp cells attenuate dentin resorption in homeostasis. J Dent Res. 2015;94(6):821-827. [38] CHEN MY, CHEN KL, CHEN CA, et al. Responses of immature permanent teeth with infected necrotic pulp tissue and apical periodontitis/abscess to revascularization procedures. Int Endod J. 2012;45(3):294-305. [39] GAMA A, PEREA L, YEPES C, et al. Effects of post-natal inhibition of RANKL on molar eruption and root formation in C57BL/6 mice. Orthod Fr. 2019;90(1):55-63. [40] ISAWA M, KARAKAWA A, SAKAI N, et al. Biological Effects of Anti-RANKL Antibody and Zoledronic Acid on Growth and Tooth Eruption in Growing Mice. Sci Rep. 2019;9(1): 19895. [41] LÉZOT F, CHESNEAU J, NAVET B, et al. Skeletal consequences of RANKL-blocking antibody (IK22-5) injections during growth: mouse strain disparities and synergic effect with zoledronic acid. Bone. 2015;73:51-59. [42] 冯志强,安金刚,张益等.晚期药物相关性颌骨坏死的手术治疗[J].华西口腔医学杂志,2023,41(1):43-51. [43] 戢晓,朱桂全.维生素D与药物相关性颌骨坏死关系的研究进展[J].国际口腔医学杂志,2022,49(4):441-447. [44] BETH-TASDOGAN NH, MAYER B, HUSSEIN H, et al. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst Rev. 2022;7(7): CD012432. [45] SOUNDIA A, HADAYA D, ESFANDI N, et al. Osteonecrosis of the jaws (ONJ) in mice after extraction of teeth with periradicular disease. Bone. 2016;90:133-141. [46] DE MOLON RS, SHIMAMOTO H, BEZOUGLAIA O, et al. OPG-Fc but Not Zoledronic Acid Discontinuation Reverses Osteonecrosis of the Jaws (ONJ) in Mice. J Bone Miner Res. 2015;30(9):1627-1640. [47] AGHALOO TL, CHEONG S, BEZOUGLAIA O, et al. RANKL inhibitors induce osteonecrosis of the jaw in mice with periapical disease. J Bone Miner Res. 2014;29(4):843-854. [48] WILLIAMS DW, LEE C, KIM T, et al. Impaired bone resorption and woven bone formation are associated with development of osteonecrosis of the jaw-like lesions by bisphosphonate and anti-receptor activator of NF-κB ligand antibody in mice. Am J Pathol. 2014;184(11):3084-3093. [49] 潘剑,刘济远.药物相关性颌骨坏死的发病机制及其防治[J].华西口腔医学杂志, 2021,39(3):245-254. [50] ALROWIS R, ALDAWOOD A, ALOTAIBI M, et al. Medication-Related Osteonecrosis of the Jaw (MRONJ): A Review of Pathophysiology, Risk Factors, Preventive Measures and Treatment Strategies. Saudi Dent J. 2022;34(3):202-210. [51] KAWAHARA M, KUROSHIMA S, SAWASE T. Clinical considerations for medication-related osteonecrosis of the jaw: a comprehensive literature review. Int J Implant Dent. 2021;7(1):47. [52] KUROSHIMA S, AL-SALIHI Z, YAMASHITA J. Mouse anti-RANKL antibody delays oral wound healing and increases TRAP-positive mononuclear cells in bone marrow. Clin Oral Investig. 2016;20(4):727-736. [53] TSICHLAKI A, CHIN SY, PANDIS N, et al. How long does treatment with fixed orthodontic appliances last? A systematic review. Am J Orthod Dentofacial Orthop. 2016;149(3): 308-318. [54] KHLEF HN, HAJEER MY, AJAJ MA, et al. The effectiveness of traditional corticotomy vs flapless corticotomy in miniscrew-supported en-masse retraction of maxillary anterior teeth in patients with Class II Division 1 malocclusion: A single-centered, randomized controlled clinical trial. Am J Orthod Dentofacial Orthop. 2020;158(6):e111-e120. [55] SIRRI MR, BURHAN AS, HAJEER MY, et al. Evaluation of Perceived Pain, Discomfort, Functional Impairments, and Satisfaction When Relieving Crowded Lower Anterior Teeth in Young Adult Patients Using Corticision-Assisted Fixed Orthodontic Treatment: A Randomized Controlled Trial. Cureus. 2022;14(7):e26489. [56] AL-IBRAHIM HM, HAJEER MY, BURHAN AS, et al. Evaluation of Patient-Centered Outcomes Associated With the Acceleration of Upper Incisor Decrowding Using Self-Ligating Brackets With or Without Piezocision in Comparison With Traditional Brackets: A Three-Arm Randomized Controlled Trial. Cureus. 2022;14(6):e26467. [57] ALFAILANY DT, HAJEER MY, ALJABBAN O, et al. The Effectiveness of Repetition or Multiplicity of Different Surgical and Non-Surgical Procedures Compared to a Single Procedure Application in Accelerating Orthodontic Tooth Movement: A Systematic Review and Meta-Analysis. Cureus. 2022;14(3):e23105. [58] CHANG JH, CHEN PJ, ARUL MR, et al. Injectable RANKL sustained release formulations to accelerate orthodontic tooth movement. Eur J Orthod. 2020;42(3):317-325. [59] KANZAKI H, CHIBA M, ARAI K, et al. Local RANKL gene transfer to the periodontal tissue accelerates orthodontic tooth movement. Gene Ther. 2006;13(8):678-685. [60] IGLESIAS-LINARES A, MORENO-FERNANDEZ AM, YAÑEZ-VICO R, et al. The use of gene therapy vs. corticotomy surgery in accelerating orthodontic tooth movement. Orthod Craniofac Res. 2011;14(3):138-148. [61] GAMA A, VARGAS-FRANCO JW, SÁNCHEZ MESA DC, et al. Origins of Alterations to Rankl Null Mutant Mouse Dental Root Development. Int J Mol Sci. 2020;21(6):2201. [62] HUANG H, WANG J, ZHANG Y, et al. Bone resorption deficiency affects tooth root development in RANKL mutant mice due to attenuated IGF-1 signaling in radicular odontoblasts. Bone. 2018;114:161-171. [63] VARGAS-FRANCO JW, CASTANEDA B, GAMA A, et al. Genetically-achieved disturbances to the expression levels of TNFSF11 receptors modulate the effects of zoledronic acid on growing mouse skeletons. Biochem Pharmacol. 2019;168:133-148. |
| [1] | Guo Caopei, Cheng Piaotao, Yang Chengbing, Gong Shouhang, Peng Jiaze, Zhang Lin, Peng Jiachen. Bone immunity and bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(14): 2261-2266. |
| [2] | Sun Jinyi, Wang Qinying, Li Ying, Meng Maohua, Chen Helin, Zeng Xiao, Shu Jiayu, Li Wenjie, Luo Yuncai, Dong Qiang. The role of silent information regulator in periodontitis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(11): 1737-1742. |
| [3] | Feng Hao, Zhang Bin, Wang Jianping. Bone marrow mesenchymal stem cell transplantation can improve bone metabolism in osteoporotic rats [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(1): 72-75. |
| [4] | Han Yu, Li Wenjing, Wu Jie, Cui Zhanqin. Effects of interleukin-10 on alveolar bone remodeling under orthodontic force in a high glucose condition [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(26): 4180-4185. |
| [5] | Yang Ruijuan, Li Yangyang, Cai Ruiyan, Liu Huibin, Guo Chun. Interleukin-1 alpha induces osteoclast activation and bone loss [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(23): 3691-3699. |
| [6] | Liu Qiqi, Liu Min, Yang Jian, Yu Ke. Lipopolysaccharide stimulates the expression of interleukin-6 and nuclear factor kappa B receptor activator ligand in mouse MLO-Y4 osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(20): 3121-3126. |
| [7] | Tian Xinbao, Xu Jianfeng, Huang Yuan, Lai Zheying, Li Xiaolong, Liu Xiaoli, Lin Ruizhu, Zhu Ning. Internal heat-type acupuncture inhibits osteoblast viability and promotes bone formation in a rat model of steroid-induced avascular necrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(17): 2642-2648. |
| [8] | Huang Zhicai, Xie Liuqin, Wang Guangsu, Zhang Guoxing, Tang Zhenglong. Parathyroid hormone promotes bone healing in rabbits after free reduction of mandibular condyle fractures [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(14): 2172-2178. |
| [9] | Wang Dong, Ding Hai, Chang Wenju, Wang Jinzi. Biological changes and mechanism of salvianolic acid B in ovariectomized osteoporosis rat models [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(12): 1927-1930. |
| [10] | Li Ping, Lin Yu, Chen Xiang, Liu Zhentao, Xiao Lili, Lin Xueyi, Hua Peng . Characteristics of bone remodeling in female ovariectomized rat models of osteoporosis undergoing Erzhi Pill extract intervention [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(2): 191-195. |
| [11] | Liu Hong, Wan Zhe, Zhang Zhen, Zhang Qin. Relationship between root resorption and intrusive force during maxillary molar intrusion in Beagle dogs [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(14): 2172-2176. |
| [12] | Zheng Rui, Xie Jing, Lu Shuai, Sun Yong. Beta-tricalcium phosphate combined with advanced platelet-rich fibrin contributes to bone regeneration: X-ray and immunohistochemical analysis [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(10): 1514-1519. |
| [13] | Zhou Yi-fei, Zheng Qian, Mao Jie, Lv Jia-ling, Wu Xiao-ling, Xu Xiao-mei. Combined oral contraceptives facilitate periodontium remodeling during orthodontic tooth movement in rats [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(4): 542-547. |
| [14] | Wang Ping-ting, Zhou Pan, Zheng Guo-ying, Liu Da-yong. Intervention with pyrrolidine dithioearbamate stimulates expression of RANKL and OPG in lipopolysaccharide-stimulated periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(21): 3365-3370. |
| [15] | Wan Lei1, Huang Hong-xing1, Huang Hong2, Wang Fan3, Chai Shuang4, Wang Ji-li4, Liu Shao-jin4, Huang Jia-chun4. Bushen Jianpi Huoxue decoction increases the proliferation of Sost-overexpressed adenovirus transfected osteoblasts and alkaline phosphatase activity [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(16): 2461-2466. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||