Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (24): 3814-3820.doi: 10.12307/2022.559
Previous Articles Next Articles
A new method for extracting human bone marrow mesenchymal stem cells and the comparison with traditional methods
Li Shidan1, Xing Wei2, Xie Xiaoyu1, Li Youbin1, Wang Shaochuan1, Fei Jun3
- 1Department of Orthopedics, 2Research Institute of Battle Surgery, 3Department of Emergency, Army Specialty Medical Center, Army Medical University, Chongqing 400002, China
-
Received:2021-07-21Accepted:2021-09-06Online:2022-08-28Published:2022-01-22 -
Contact:Fei Jun, MD, Associate chief physician, Department of Emergency, Army Specialty Medical Center, Army Medical University, Chongqing 400002, China -
About author:Li Shidan, Master candidate, Attending physician, Department of Orthopedics, Army Specialty Medical Center, Army Medical University, Chongqing 400002, China -
Supported by:the Special Fund for Improving Scientific and Technological Innovation Capacity of Army Medical University, No. 2019XLC2023 (to FJ); Chongqing Technology Innovation and Application Development Special General Project, No. cstc2019jscx-msxmX0210 (to LYB and WSC)
CLC Number:
Cite this article
Li Shidan, Xing Wei, Xie Xiaoyu, Li Youbin, Wang Shaochuan, Fei Jun. A new method for extracting human bone marrow mesenchymal stem cells and the comparison with traditional methods[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3814-3820.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
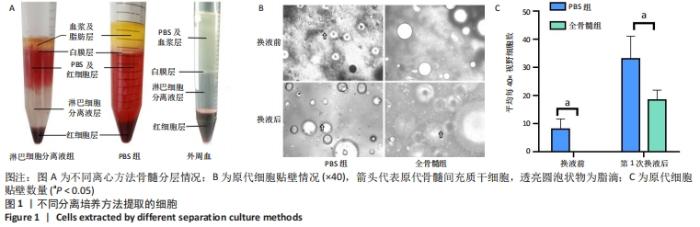
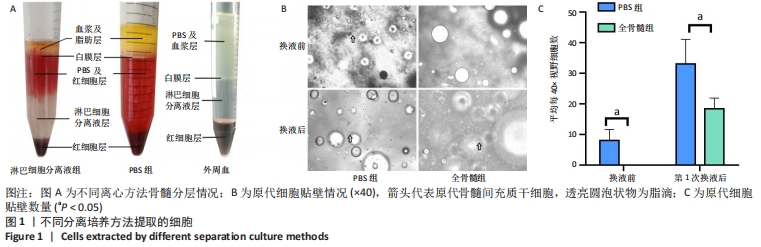
2.1 不同分离培养方法所提取细胞情况 2.1.1 不同离心方法骨髓分层情况 PBS组骨髓离心后分为4层,从下至上分别为:红细胞层、PBS及红细胞混合层、白膜层、血浆及脂肪层;而往PBS组中加入Ficoll分离液后,离心可分为5层,从下至上分别为:红细胞层、淋巴细胞分离液层、PBS及红细胞层、白膜层、血浆及脂肪层;外周血用PBS稀释混匀后加入Ficoll分离液中离心可分为4层:从下至上分别为:红细胞层、淋巴细胞分离液层、白膜层、PBS及血浆层,见图1A。从图中可见,对于骨髓标本,PBS浮于Ficoll分离液上层,而白膜层浮于PBS上层。若要获取骨髓中白膜层细胞,则直接吸取PBS上层细胞即可。而在外周血中,用PBS离心后白膜层介于PBS与Ficoll分离液之间,PBS与血浆均匀混合,不出现白膜层浮于PBS上层的情况。 2.1.2 原代细胞贴壁情况 将PBS组白膜层细胞接种至培养基后,培养基中可见中等量悬浮红细胞及少量脂肪泡。第3天镜下观察,有少量贴壁的梭形细胞,首次换液后红细胞显著减少,仍有少量脂肪泡残留。全骨髓组将骨髓接种至培养基后,培养基中可见大量悬浮红细胞及较多脂肪泡,换液前看不到贴壁细胞,第7天首次换液后仍可见少量红细胞及较多脂肪泡,可见部分贴壁梭形细胞,见图1B。首次换液前后,对可见的梭形细胞计数,PBS组细胞明显多于全骨髓组,见图1C。"
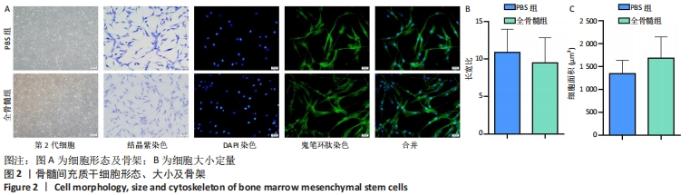
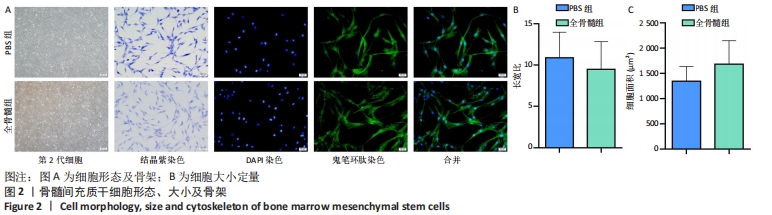
2.2 细胞形态、大小及细胞骨架 相差显微镜下可见两组细胞形态、大小较为均一;结晶紫染色后可见大量长梭形细胞,细胞较为细长,中间粗壮,两端纤细,触角较少;少部分细胞呈扁平状,触角稍多。鬼笔环肽及DAPI染色见两组细胞均为单核细胞,细胞骨架完整,胞核及蛋白纤维网架体系清晰可见,见图2A。PBS组所提取细胞长宽比为10.876±3.093,全骨髓组所提取细胞长宽比为9.485±3.349,两组相比差异无显著性意义(t=0.915 5,P=0.373 5);PBS组所提取细胞平均面积为(1 342.665±290.731) μm2,全骨髓组所提取细胞平均面积为(1 679.665±466.343) μm2,两组相比差异无显著性意义(t=1.840,P=0.084 4),见图2B。"
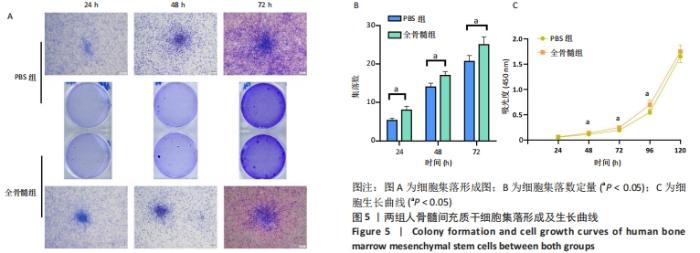
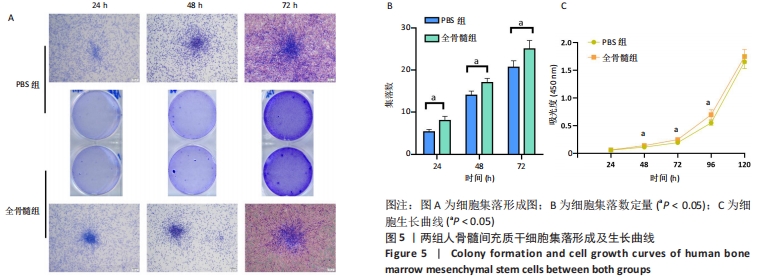
2.5 细胞集落形成及细胞生长曲线 2.5.1 集落形成 细胞接种于6孔板后24 h行结晶紫染色即可看到集落形成。相对于24 h,在48 h及72 h可见细胞集落增大,集落数量增多,见图5A。每一个6孔板空格中,24 h PBS组细胞集落数为(5.333±0.577)个,全骨髓组为(8.000±1.000)个,两组相比差异有显著性意义(t=4.000,P=0.016 1);48 h PBS组平均集落数为(14.000±1.000)个,全骨髓组为(17.000±1.000)个,两组相比差异有显著性意义(t=3.674,P=0.021 3);72 h PBS组平均集落数为(20.667±1.528)个,全骨髓组为(25.000±2.000)个,两组相比差异有显著性意义(t=2.982, P=0.040 6),见图5B。 2.5.2 细胞生长曲线 在72 h之前,两组细胞吸光度值低,生长曲线平缓上升,细胞呈缓慢生长状态。在72 h之后,细胞生长曲线斜率增大,细胞快速生长,于120 h细胞长满96孔板。全骨髓组细胞生长速度快于PBS组:24 h时PBS组细胞吸光度值为0.058±0.010,全骨髓组为0.061±0.004,两组相比差异无显著性意义(t=0.715 8,P=0.494 5);48 h时PBS组细胞吸光度值为0.113±0.010,全骨髓组为0.139±0.010,全骨髓组高于PBS组(t=4.079,P=0.003 5);72 h时PBS组细胞吸光度值为0.192±0.031,全骨髓组为0.247±0.020,全骨髓组高于PBS组(t=3.325,P=0.010 5);96 h时PBS组细胞吸光度值为0.549±0.041,全骨髓组为0.696±0.089,全骨髓组高于PBS组(t=3.353,P=0.010 0);120 h时PBS组细胞吸光度值为1.653±0.123,全骨髓组为1.748±0.127,两组相比差异无显著性意义(t=1.199,P=0.256 0),见图5C。"
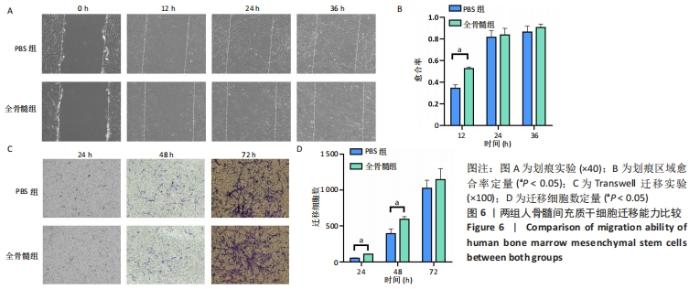
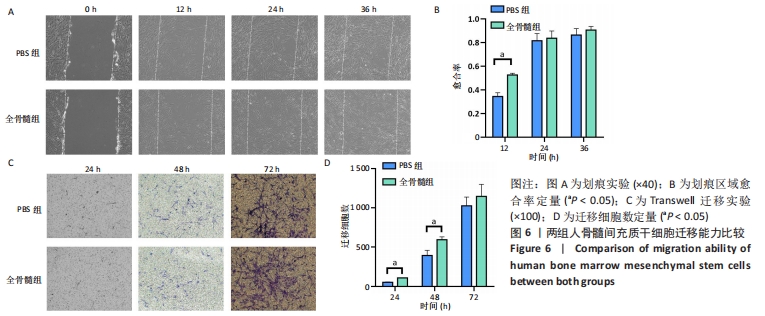
2.6 细胞迁移能力比较 2.6.1 细胞划痕实验 随着时间推移,两组划痕区域周边细胞逐渐向划痕区域生长。24 h之前细胞迁移速率较快,划痕区域有80%左右细胞长入,24 h之后细胞长入速率减缓,见图6A。在12 h时,PBS组细胞愈合率为0.344±0.032,全骨髓组愈合率为0.525±0.015,全骨髓组愈合率大于PBS组(t=8.980,P=0.000 9);在24 h时,PBS组细胞愈合率为0.814±0.061,全骨髓组愈合率为0.836±0.061,两组相比差异无显著性意义(t=0.440 7,P=0.682 2);36 h时,PBS组细胞愈合率为0.863±0.055,全骨髓组愈合率为0.904±0.031,两组相比差异无显著性意义(t=1.157,P=0.311 7),见图6B。 2.6.2 Transwell实验 24 h时,两组均有少量细胞穿过Transwell 小格,从上室迁移至下室。随着时间变化,迁移细胞逐渐增多,72 h有大量细胞迁移至小室下层,见图6C。24 h时,PBS组平均每个100×视野,有(51.33±6.658)个迁移细胞,全骨髓组有(108.667±3.512)个迁移细胞,全骨髓组迁移细胞数多于PBS组(t=13.19,P=0.000 2);48 h时,PBS组迁移细胞数为(393.667±65.310)个,全骨髓组迁移细胞数为(594.667±35.388)个,全骨髓组迁移细胞数多于PBS组(t=4.687,P=0.009 4);72 h时,PBS组迁移细胞数为(1 025.333±110.961)个,全骨髓组迁移细胞数为 (1 145.333±152.753)个,两组相比差异无显著性意义(t=1.101,P=0.332 7),见图6D。"
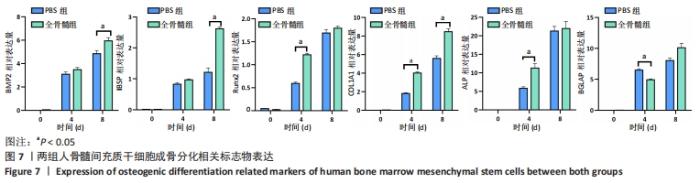
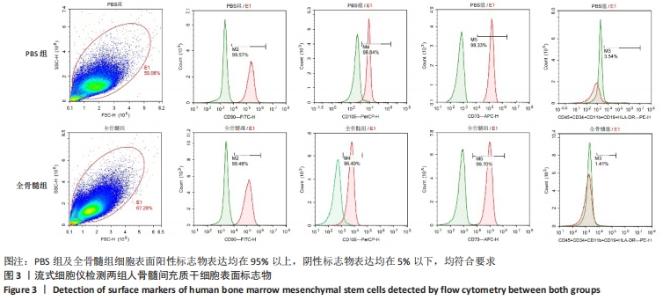
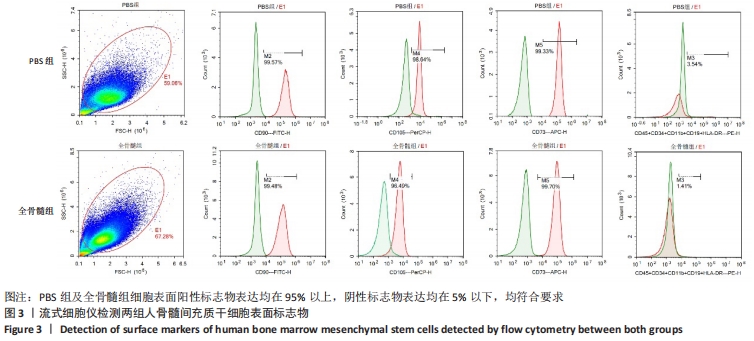
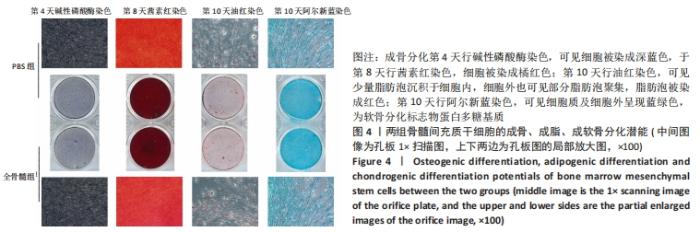
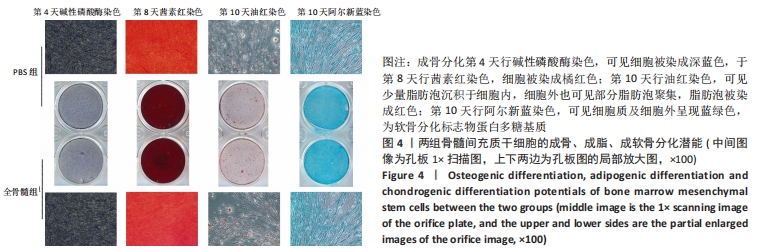
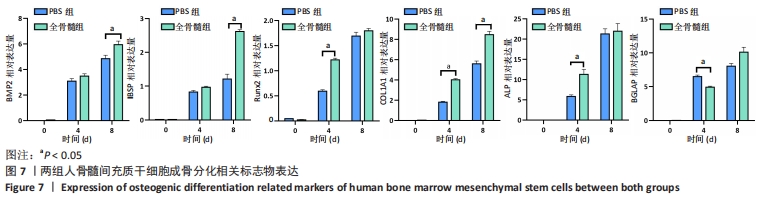
2.7 成骨分化过程中成骨标志物表达 未分化的两组骨髓间充质干细胞基本不表达成骨相关标志物BMP2、IBSP、Runx2、COL1A1、ALP、BGLAP。成骨分化诱导分化过程中,随着时间推移,相应的成骨标志物表达增高。第4天时,全骨髓组表达量高于PBS组的基因有Runx2( t=13.89,P=0.000 2)、COL1A1( t=15.88,P=0.000 1)、ALP( t=4.250,P=0.013 2);PBS组高于全骨髓组的基因为BGLAP(t=5.791,P=0.004 4);第8天时,全骨髓组表达量高于PBS组的基因有BMP2( t=2.852,P=0.046 3)、IBSP( t=9.118,P=0.000 8)、COL1A1( t=6.710,P=0.002 6)。其余时间,两组相比基因表达量差异无显著性意义,见图7。"
| [1] FU X, LIU G, HALIM A, et al. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019; 8(8):784. [2] ZHANG CP, FU XB. Therapeutic potential of stem cells in skin repair and regeneration. Chin J Traumatol. 2008;11(4):209-221. [3] ZHANG R, MA J, HAN J, et al. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am J Transl Res. 2019;11(10):6275-6289. [4] LIU S, LIU D, CHEN C, et al. MSC Transplantation Improves Osteopenia via Epigenetic Regulation of Notch Signaling in Lupus. Cell Metab. 2015;22(4):606-618. [5] JOHNSON CL, SOEDER Y, DAHLKE MH. Concise Review: Mesenchymal Stromal Cell-Based Approaches for the Treatment of Acute Respiratory Distress and Sepsis Syndromes. Stem Cells Transl Med. 2017;6(4):1141-1151. [6] CHENG KT, XIONG S, YE Z, et al. Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J Clin Invest. 2017;127(11):4124-4135. [7] PROCKOP DJ, BRENNER M, FIBBE WE, et al. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010;12(5):576-578. [8] SCHNEIDER RK, PÜLLEN A, KRAMANN R, et al. Long-term survival and characterisation of human umbilical cord-derived mesenchymal stem cells on dermal equivalents. Differentiation. 2010;79(3):182-193. [9] CHAMBERLAIN G, FOX J, ASHTON B, et al. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739-2749. [10] 韩振霞,时庆,汪大琨,等.骨髓源与脐带源间充质干细胞的基本生物学特征比较[J].中国实验血液学杂志,2013,21(5):1248-1255. [11] ALDAHMASH A, ZAHER W, AL-NBAHEEN M, et al. Human stromal (mesenchymal) stem cells: basic biology and current clinical use for tissue regeneration. Ann Saudi Med. 2012;32(1):68-77. [12] NEUHUBER B, SWANGER SA, HOWARD L, et al. Effects of plating density and culture time on bone marrow stromal cell characteristics. Exp Hematol. 2008;36(9):1176-1185. [13] PLOUFFE BD, MURTHY SK, LEWIS LH. Fundamentals and application of magnetic particles in cell isolation and enrichment: a review. Rep Prog Phys. 2015;78(1):016601. [14] 段长伟,柴彦杰,赵疆东,等.骨髓间充质干细胞分离与培养技术[J].宁夏医学杂志, 2021,43(6):573-576. [15] 程斌,方驰华,范应方,等.骨髓间充质干细胞提取方法的改良[J].中国组织工程研究与临床康复,2008,12(47):9293-9296. [16] FRIEDENSTEIN AJ, CHAILAKHYAN RK, GERASIMOV UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20(3):263-272. [17] 李宪哲,谢明颢,练磊.间充质干细胞治疗炎症性肠病的应用及前景[J].中华细胞与干细胞杂志(电子版),2019,9(4):236-241. [18] JIN HJ, BAE YK, KIM M, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14(9):17986-18001. [19] KIM M, KIM C, CHOI YS, et al. Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: implication to age-associated bone diseases and defects. Mech Ageing Dev. 2012;133(5):215-225. [20] BORNES TD, JOMHA NM, MULET-SIERRA A, et al. Porous Scaffold Seeding and Chondrogenic Differentiation of BMSC-seeded Scaffolds. Bio Protoc. 2015;5(24):e1693. [21] FERRIN I, BELOQUI I, ZABALETA L, et al. Isolation, Culture, and Expansion of Mesenchymal Stem Cells. Methods Mol Biol. 2017;1590:177-190. [22] MUSHAHARY D, SPITTLER A, KASPER C, et al. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 2018;93(1):19-31. [23] MEPPELINK AM, WANG XH, BRADICA G, et al. Rapid isolation of bone marrow mesenchymal stromal cells using integrated centrifuge-based technology. Cytotherapy. 2016;18(6):729-739. [24] WULF GG, JACKSON KA, GOODELL MA. Somatic stem cell plasticity: current evidence and emerging concepts. Exp Hematol. 2001;29(12): 1361-1370. [25] 汪健芳,莫春阳,许艳华,等.骨髓间充质干细胞的临床应用研究[J].中国细胞生物学学报,2018,40(S1):2145-2155. [26] DOMINICI M, LE BLANC K, MUELLER I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. [27] 于雷,高俊玲.骨髓间充质干细胞研究与应用概况[J].华北理工大学学报(医学版),2018, 20(2):164-168. [28] LI H, GHAZANFARI R, ZACHARAKI D, et al. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann N Y Acad Sci. 2016;1370(1):109-118. [29] PELIZZO G, VESCHI V, MANTELLI M, et al. Microenvironment in neuroblastoma: isolation and characterization of tumor-derived mesenchymal stromal cells. BMC Cancer. 2018;18(1):1176. |
| [1] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [2] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [3] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [4] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [5] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [6] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [7] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [8] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [9] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [10] | Huang Chuanjun, Zou Yu, Zhou Xiaoting, Zhu Yangqing, Qian Wei, Zhang Wei, Liu Xing. Transplantation of umbilical cord mesenchymal stem cells encapsulated in RADA16-BDNF hydrogel promotes neurological recovery in an intracerebral hemorrhage rat model [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 510-515. |
| [11] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [12] | Chen Chichi, Zhang Yu, He Jiachen, Shi Qin. Osteogenic differentiation of bone marrow mesenchymal stem cells in obese mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3846-3851. |
| [13] | You Wulin, Huang Guicheng, Wang Jianwei. Osteogenic differentiation potential of microencapsulated transgenic bone marrow mesenchymal stem cells cocultured with osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3852-3857. |
| [14] | Cui Yinpeng, Guo Ai, Ma Lifeng, Liu Zhenjiang. Ouabain induces in vitro differentiation of human bone marrow mesenchymal stem cells into osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3796-3801. |
| [15] | Yan Nan, Wu Yanlong, Tang Xiaohui, Zhang Xiaoyan, Wang Hui, Yang Tianze, Zhou Maochun, Wang Zhengdong, Yang Xiaoxia. Bone marrow mesenchymal stem cells may alleviate brain damage caused by the microglial overactivation in the cortex around ischemic site of stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(24): 3790-3795. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||