Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (16): 2545-2550.doi: 10.12307/2022.255
Previous Articles Next Articles
Selection of conditions for fabricated porous scaffolds in bone tissue engineering by silk fibroin protein
Yang Xinghua1, 2, Zhang Jing3, Chen Daiyun1, Xiong Shijiang2
- 1School of Stomatology, Shangdong First Medical University, Tai’an 271000, Shandong Province, China; 2School of Stomatology, Shandong University, Jinan 250012, Shandong Province, China; 3Department of Stomatology, Tengzhou Center Renmin Hospital, Tengzhou 277500, Shandong Province, China
-
Received:2020-06-03Revised:2020-06-04Accepted:2020-11-09Online:2022-06-08Published:2021-12-23 -
Contact:Xiong Shijiang, Doctoral supervisor, Professor, School of Stomatology, Shandong University, Jinan 250012, Shandong Province, China -
About author:Yang Xinghua, MD, Associate professor, School of Stomatology, Shangdong First Medical University, Tai’an 271000, Shandong Province, China; School of Stomatology, Shandong University, Jinan 250012, Shandong Province, China -
Supported by:the General Project of Shandong Provincial Colleges and Universities Science and Technology Plan, No. J13LL01 (to YXH)
CLC Number:
Cite this article
Yang Xinghua, Zhang Jing, Chen Daiyun, Xiong Shijiang. Selection of conditions for fabricated porous scaffolds in bone tissue engineering by silk fibroin protein[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(16): 2545-2550.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
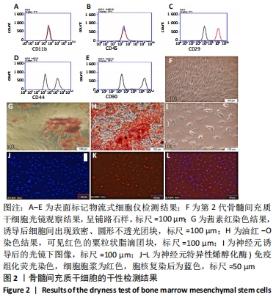
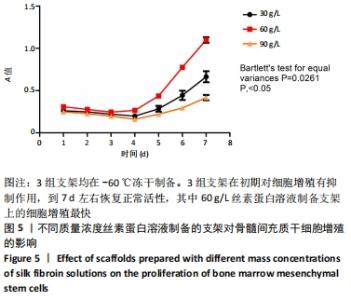
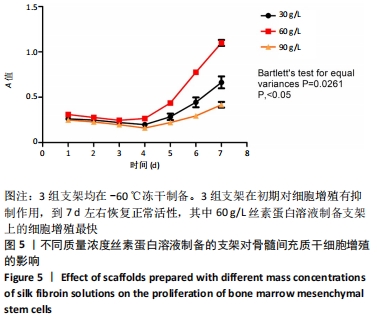
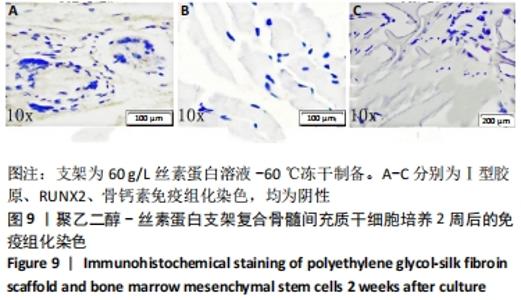
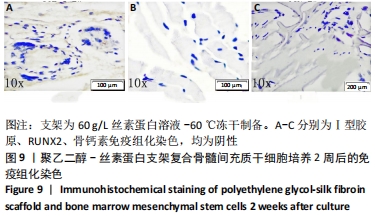
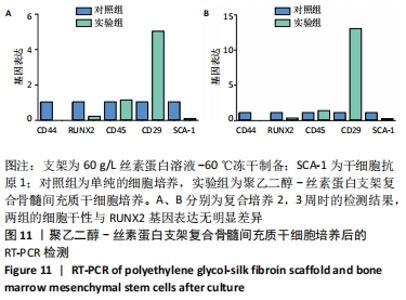
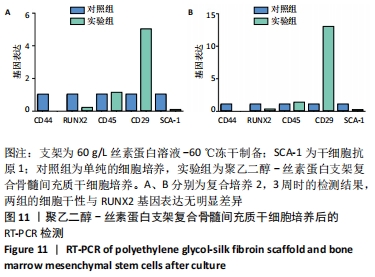
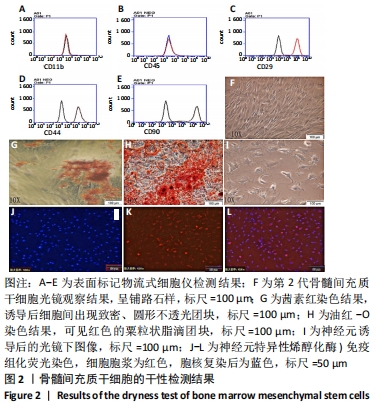
2.1 骨髓间充质干细胞培养与“干”性检测结果 培养基2 d内漂浮大量的血细胞,3 d后首次半换液,5-7 d可见少量的贴壁成纤维细胞样集落形成。待细胞铺满培养皿底70%-60%底面积时,0.25%胰蛋白酶消化后进行传代培养,骨髓间充质干细胞传代后生长迅速,一般4-7 d可传代一次,传代细胞形态仍呈多角形、短梭形。 流式细胞仪检测结果显示,分离培养的细胞CD11b、CD45表达为阴性,CD29、CD44、CD90表达为阳性;见图2A-E。光镜下可见第2代骨髓间充质干细胞呈铺路石样,见图2F,茜素红染色可见诱导后细胞间出现致密、圆形不透光团块,见图2G,油红-O染色可见呈现红色的粟粒状脂滴团块,见图2H,干细胞神经元诱导后的光镜下图像及神经元特异性烯醇化酶免疫组化染色显示可分化为神经元样细胞,见图2I-L。以上结果符合干细胞的特性表现。"
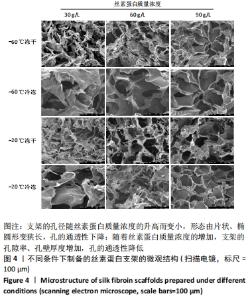
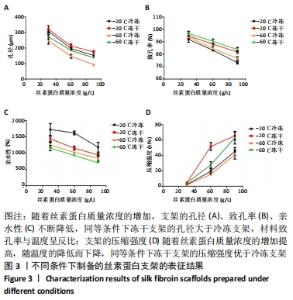
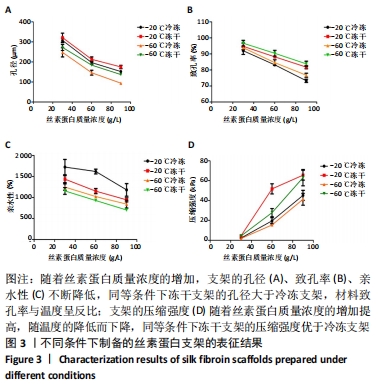
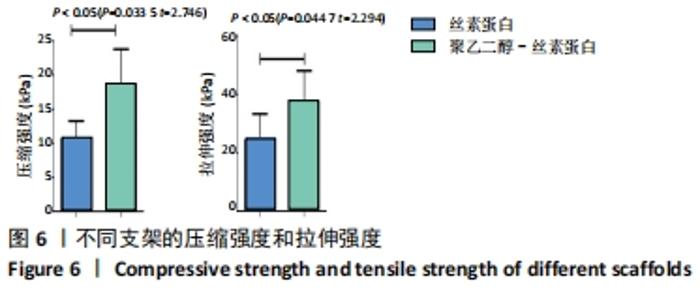
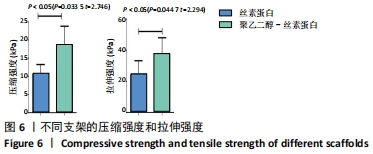
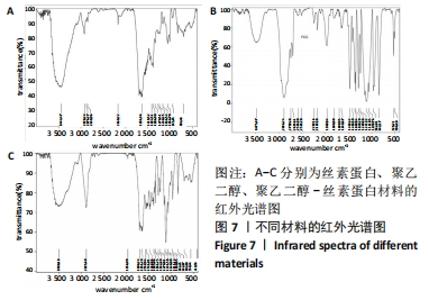
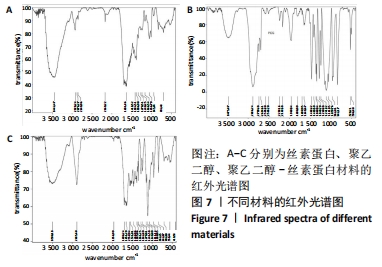
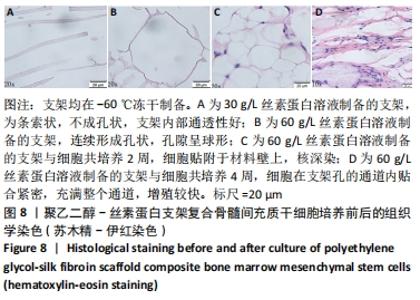
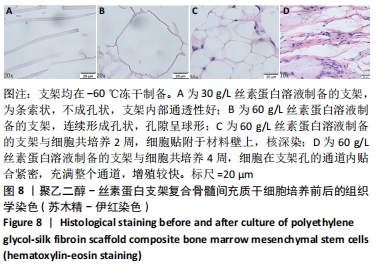
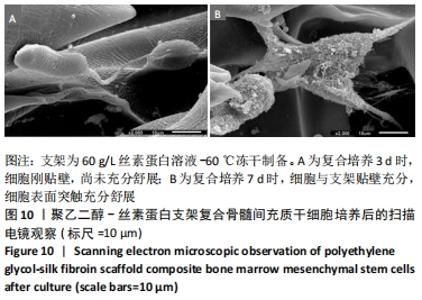
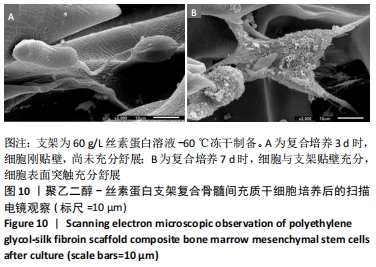
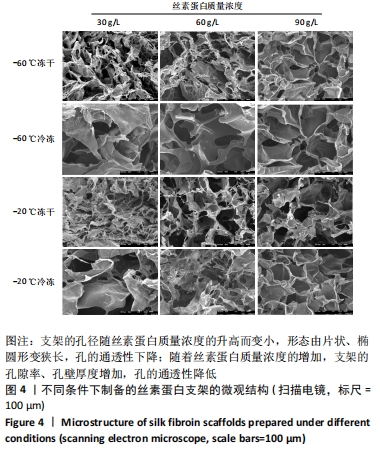
不同质量浓度丝素蛋白支架的孔径在(94.22±18.14)-(321.35±70.99) μm间,孔隙率在(73.25±1.79)%-(95.38±4.55)%之间,亲水性在(469.34±135.10)%-(1722.41±313.10)%之间,压缩强度在(1.77±0.29)-(65.54±12.22) kPa之间,其中60 g/L丝素蛋白溶液制备的支架孔径为(213.07±37.89) μm,更适宜细胞生长。由图3折线图趋势走向可看出,随着丝素蛋白质量浓度的增加,支架的孔径、致孔率、亲水性降低,同等条件下冻干支架的孔径大于冷冻支架,支架致孔率与温度呈反比;支架的压缩强度随着丝素蛋白质量浓度的增加提高,随温度的降低下降,同等条件下冻干支架的压缩强度优于冷冻支架。 2.2.2 支架的微观结构 扫描电镜显示,支架的孔径随丝素蛋白质量浓度的升高而变小,形态由片状、椭圆形变狭长,孔的通透性下降;随着丝素蛋白质量浓度的增加,支架的孔隙率、孔壁厚度增加,孔的通透性降低,见图4。冷冻条件下制备支架的孔径的规律性、通透性低于冻干条件下制备的支架,相同质量浓度丝素蛋白溶液在-20 ℃冻干制备的支架孔径大于-60 ℃冻干制备的支架。"
| [1] 赵群.SF水凝胶的研究进展[J].轻工科技,2016(8):29-32. [2] 肖红利,黄文良,能坤,等.SF壳聚糖和纳米羟基磷灰石制备骨软骨梯度孔径支架初探[J].中华老年医学杂志,2018,37(7):816-820. [3] NASKAR D, GHOSH AK, MANDAL M, et al. Dual growth factor loaded nonmulberry silk fibroin/carbon nanofiber composite 3D scaffolds for in vitro and in vivo bone regeneration. Biomaterials. 2017;136:67-85. [4] MIDHA S, MURAB S, GHOSH S. Osteogenic signaling on silk-based matrices. Biomaterials. 2016;97:133-153. [5] 赵晓阳,邓立志,邓宇斌,等.聚赖氨酸修饰SF膜对神经干细胞生长和分化的影响[J].生物工程学报,2018,34(10):1650-1659. [6] 杨强,丁晓明,徐宝山.含软骨钙化层的仿生支架体内构建组织工程骨软骨[J].中华骨科杂志,2018,38(6):321-329. [7] 牛涵波,陈枉枉,徐义英,等.SF-壳聚糖三维支架的构建及其生物相容性[J].江苏大学学报(医学版),2019,29(3):238-241. [8] KIM UJ, PARK J, KIM HJ, et al. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26:2775-2785. [9] BHARDWAJ N, KUNDU SC. Silk fibroin protein and chitosan polyelectrolyte complex porous scaffolds for tissue engineering applications. Carbohydr Polym. 2011;85:325-233. [10] 廖银琳,王卉,张克勤.医用组织工程多孔丝素支架制备方法的进展[J].印染, 2012,38(10):48-53. [11] ZHANG YF,WEI F, MA ZC, et al. The effects of pore architecture in silk fibroin scaffolds on the growth and differentiation of mesenchymal stem cells expressing BMP7. Acta Biomater. 2010;6:3021-3028. [12] BHUMIRATANA S, GRAYSON WL, CASTANEDA A, et al. Nucleation and growth of mineralized bone matrix on silk-hydroxyaatitencomposite scaffolds. Biomaterials. 2011;32:2812-2820. [13] ISHAUG SL, CRANE GM, MILLER MJ, et al. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36:17-28. [14] HULBERT SF, YOUNG FA, MATHEWS RS, et al. Potential of ceramic materials as permanently implantable skeletal prostheses. J Biomed Mater Res. 1970;4:433-456. [15] ZELTINGER J, SHERWOOD JK, GRAHAM DA, et al. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001;7:557-572. [16] LEE M, WU BM, DUNN JC. Effect of scaffold architecture and pore size on smooth muscle cell growth. J Biomed Mater Res A. 2008;87(4):1010-1016. [17] BHARDWAJ N, CHAKRABORTY S, KUNDU SC. Freeze-gelled silk fibroin protein scaffolds for potential applications in soft tissue engineering. Int J Biol Macromol. 2011;49(3): 260-267. [18] Parinita A, Krishna P, Vishwanath V, et al. Enhanced chondrogenesis of mesenchymal stem cells over silk fibroin/chitosan‐chondroitin sulfate three dimensional scaffold in dynamic culture conditio. J Biomed Mater Res B Appl Biomater. 2018;106:2576-2587. [19] Qi Q, Yao YT, Jia XS. Effects of polyethylene glycol content on the properties of a silk fibroin/nano-hydroxyapatite polyethylene glycol electrospun scaffold. RSC Adv. 2019; 9:33941-33948. [20] 杜曼·吐鲁木汗,库瓦提·绕线,祖帕尔·苏莱曼,等.SF/氧化石墨稀复合膜对长骨骨不连的实验及治疗研究[J].解剖学研究,2018,40(5):430-435. [21] 王芋骁,孔志杰,黄溪,等.交联改性SF材料制备和医学应用[J].现代丝绸科学与技术,2013,28(5):187-190. [22] 赵光元,许燕,周建平,等.组织工程骨支架微宏观模拟分析[J].机械设计与研究, 2018,34(3):158-162. [23] MEINEL L, HOFMANN S, BETZ O, et al. Osteogenesis by human mesenchymal stem cells cultured on silkbiomaterials: Comparison of adenovirus mediated gene transfer and protein delivery of BMP-2. Biomaterials. 2006;27:4993-5002. [24] 孙皎,宁丽,顾国珍.医用SF皮肤再生膜的细胞相容性评价[J].生物医学工程学杂志,2000, 17(4):391-392. [25] 袁清献,高丽兰,李瑞欣,等.3D打印SF-Ⅱ型胶原软骨支架[J].山东大学学报(理学版),2018,53(3):82-87. [26] YAN S, FENG LB, ZHU QY, et al. Controlled release of BMP-2 from a heparin-conjugated strontium-substituted nanohydroxyapatite/silk fibroin scaffold for bone regeneration. ACS Biomater. 2018;4(9):3291-3303. [27] UEBERSAX L, MERKLE HP, MEINEL L. Insulin-like growth factor I releasing silk fibroin scaffolds induce chondrogenic differentiation of human mesenchymal stem cells. J Control Release. 2008;127(1):12-21. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [3] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [4] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [5] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [6] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [7] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [8] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| [9] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [10] | Zhou Ying, Zhang Huan, Liao Song, Hu Fanqi, Yi Jing, Liu Yubin, Jin Jide. Immunomodulatory effects of deferoxamine and interferon gamma on human dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1012-1019. |
| [11] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [12] | Wang Jifang, Bao Zhen, Qiao Yahong. miR-206 regulates EVI1 gene expression and cell biological behavior in stem cells of small cell lung cancer [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1027-1031. |
| [13] | Liu Feng, Peng Yuhuan, Luo Liangping, Wu Benqing. Plant-derived basic fibroblast growth factor maintains the growth and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1032-1037. |
| [14] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [15] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||