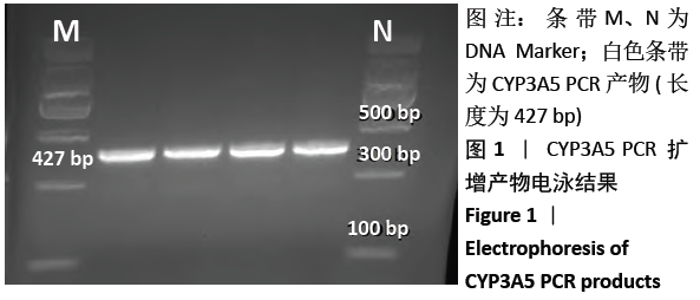
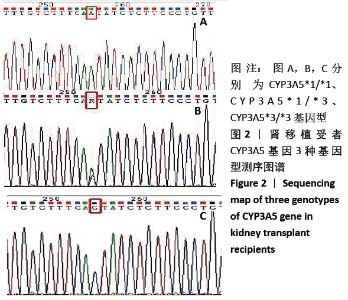
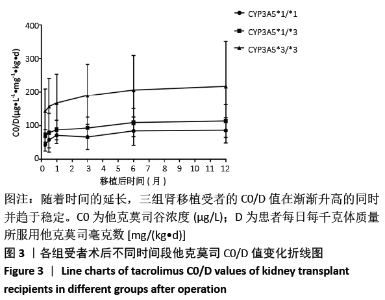
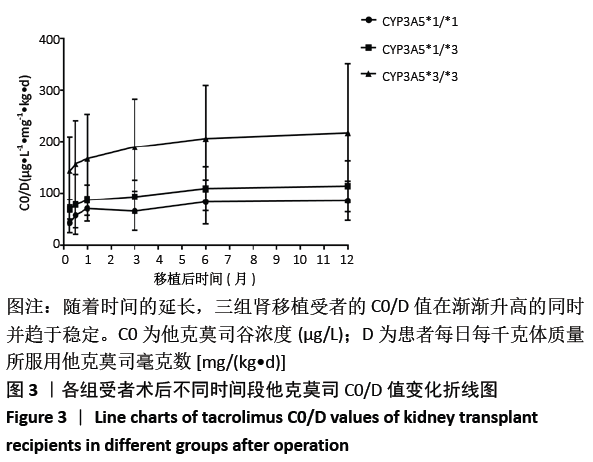
[1] Anglicheau D, Legendre C, Beaune P, et al. Cytochrome P450 3A polymorphisms and immunosuppressive drugs: an update.Pharmacogenomics. 2007;8(7):835-849.
[2] Shuker N, Shuker L, van Rosmalen J, et al. A high intrapatient variability in tacrolimus exposure is associated with poor longterm outcome of kidney transplantation. Transpl Int. 2016; 29(11): 1158-1167.
[3] Sablik KA, Clahsen-van Groningen MC, Hesselink DA, et al. Tacrolimus intra-patient variability is not associated with chronic active antibody mediated rejection. Plos One. 2018;13(5):e0196552.
[4] 付生军,刘静,李涛,等. CYP3A5基因型与肾移植患者他克莫司血药浓度关系的系统评价[J].中国循证医学杂志,2013,13(12): 1440-1445.
[5] Rojas L, Neumann I, Herrero MJ, et al. Effect of CYP3A5*3 on kidney transplant recipients treated with tacrolimus: a systematic review and meta-analysis of observational studies. Pharmacogenomics J. 2015;15(1):38-48.
[6] 赵富磊,李骞,张峻.基因多态性对肾移植患者他克莫司药代动力学影响的研究现状[J].中国临床药理学杂志,2017,33(23):2489-2491.
[7] Birdwell KA, Decker B, Barbarino JM, et al. Clinical pharmacogenetics implementation consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin Pharmacol Ther. 2015; 98(1):19-24.
[8] 李敏,李峰,高鑫. 基因多态性对他克莫司血药浓度影响的研究进展[J].实用医药杂志,2017,34(2):162-164.
[9] Largeau B, Guellec CB, Longuet H, et al. Comparison of Tacrolimus Starting Doses Based on CYP3A5 Phenotype or Genotype in Kidney Transplant Recipients. Prog Transplant. 2019;29(4):300-308.
[10] Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation:mecharlisms of action and therapeutic efficacy.Cfit Rev Oncol Hematol. 2005;56(1):23-46.
[11] Li JL, Liu S, Fu Q, et al. Interactive effects of CYP3A4,CYP3A5, MDR1 and NR1I2 polymorphisms on tracrolimus trough concentrations in early postrenal transplant recipients. Pharmacogenomics. 2015; 16(12):1355-1365.
[12] 陈晨,张晏洁,贺小露,等.他克莫司个体化用药指南解读[J].医学研究生学报,2017,30(4):342-347.
[13] Zhang X, Liu ZH, Zheng JM, et al. Influence of CYP3A5 and MDR1 polymorphisms on tacrolimus concentration in the early stage after renal transplantation. Clin Transplant. 2005; 19(5):638-643
[14] 李洋,张琪,姜楠,等.供受体CYP3A5基因多态性与肝移植患者术后他克莫司浓度/剂量比的关系[J].器官移植,2011,1(2):4-8.
[15] 张小东,张际青,尹航,等.CYP3A5基因型对肾移植受者术后早期血他克莫司浓度的影响[J].中华器官移植杂志,2009,30(12):717-721.
[16] 林 玲,宋文利,沈中阳,等.肾移植受者CYP3A5*3和CYP3A5*18B对他克莫司药动学的影响[J].中华器官移植杂志,2012,33(4): 220-224.
[17] 王明睿,宋翟秀宇,宋王 钢,宋等. CYP3A5基因多态性对肾移植术后患者他克莫司代谢的影响[J].吉林大学学报(医学版),2018,3(44): 537-542.
[18] 丰贵文,郭涛,李金锋,等.CYP3A5基因多态性对肾移植受者他克莫司个体化用药指导[J].中华器官移植杂志,2013,11(34):647-650.
[19] 张关敏,李 良,陈文倩,等.他克莫司在中国肾移植患者中的群体药物动力学研究[J].药学学报,2008,43(7):695-701.
[20] Hu RH, Lee PH, Tsai MK. Clinical influencing factors for daily dose, trough level, and relative clearance of tacrolimus in renal transplant recipients. Transplant Proc. 2000;32(7):1689- 1692.
[21] Zahir H, McLachlan AJ, Nelson A, et al. Population pharmacokinetic estimation of tacrolimus apparent clearance in adult liver transplant recipients. Ther Drug Monit. 2005,27(4):422- 430.
[22] 张大伟,许亮,徐俊楠,等.肾移植受者术前甘油三酯代谢对移植肾早期功能恢复的影响[J].解放军医学杂志,2017,42(5):427-431. |