Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (13): 2020-2026.doi: 10.3969/j.issn.2095-4344.0496
Previous Articles Next Articles
A comparative study on serum-free and serum culture methods of human umbilical cord mecenchymal stem cells
Zhang Xue-juan1, 2, Liu Gao-mi-yang2, Liu Ju-fen2, Song Yi-jia1, 2, Lin Qing-keng1, 2, Bai Ying-ying1, 2, Pan Xing-hua2
- 1Kunming General Hospital Clinical College of Kunming Medical University, Kunming 650032, Yunnan Province, China; 2Cell Biological Therapy Center of Kunming General Hospital of PLA, Cell Biological Medicine Integrated Engineering Laboratory of State and Region of Yunnan Province, the Stem Cell Therapy Key Laboratory of Yunnan Province, Kunming 650032, Yunnan Province, China
-
Revised:2018-02-02Online:2018-05-08Published:2018-05-08 -
Contact:Pan Xing-hua, M.D., Chief physician, Professor, Cell Biological Therapy Center of Kunming General Hospital of PLA, Cell Biological Medicine Integrated Engineering Laboratory of State and Region of Yunnan Province, the Stem Cell Therapy Key Laboratory of Yunnan Province, Kunming 650032, Yunnan Province, China -
About author:Zhang Xue-juan, Master candidate, Kunming General Hospital Clinical College of Kunming Medical University, Kunming 650032, Yunnan Province, China; Cell Biological Therapy Center of Kunming General Hospital of PLA, Cell Biological Medicine Integrated Engineering Laboratory of State and Region of Yunnan Province, the Stem Cell Therapy Key Laboratory of Yunnan Province, Kunming 650032, Yunnan Province, China. Liu Gao-mi-yang, Master, Physician, Cell Biological Therapy Center of Kunming General Hospital of PLA, Cell Biological Medicine Integrated Engineering Laboratory of State and Region of Yunnan Province, the Stem Cell Therapy Key Laboratory of Yunnan Province, Kunming 650032, Yunnan Province, China. Zhang Xue-juan and Liu Gao-mi-yang contributed equally to this work. -
Supported by:the Project of the Stem Cell Therapy Key Laboratory of Yunnan Province, No. 2015DG034; the Experimental Animal Specific Fund of PLA, No. SYDW(2016)004
CLC Number:
Cite this article
Zhang Xue-juan, Liu Gao-mi-yang, Liu Ju-fen, Song Yi-jia, Lin Qing-keng, Bai Ying-ying, Pan Xing-hua. A comparative study on serum-free and serum culture methods of human umbilical cord mecenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(13): 2020-2026.
share this article
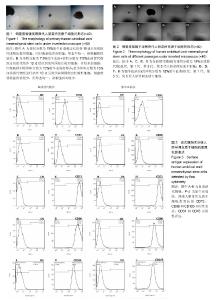
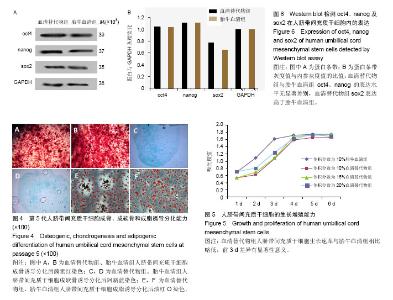
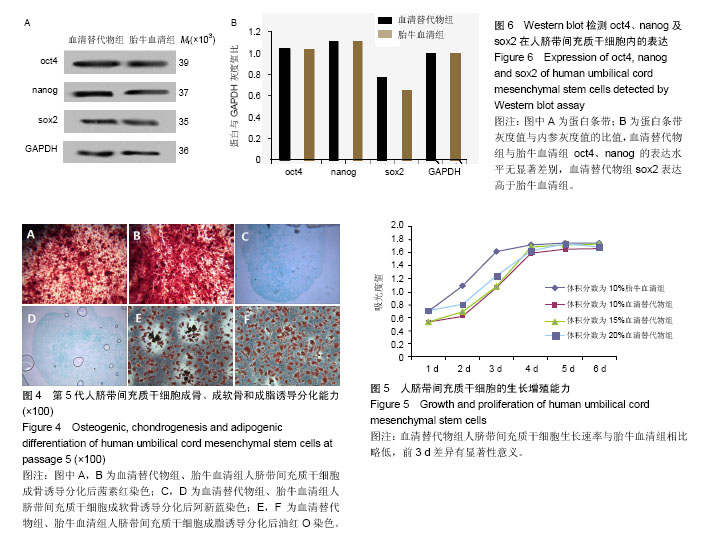
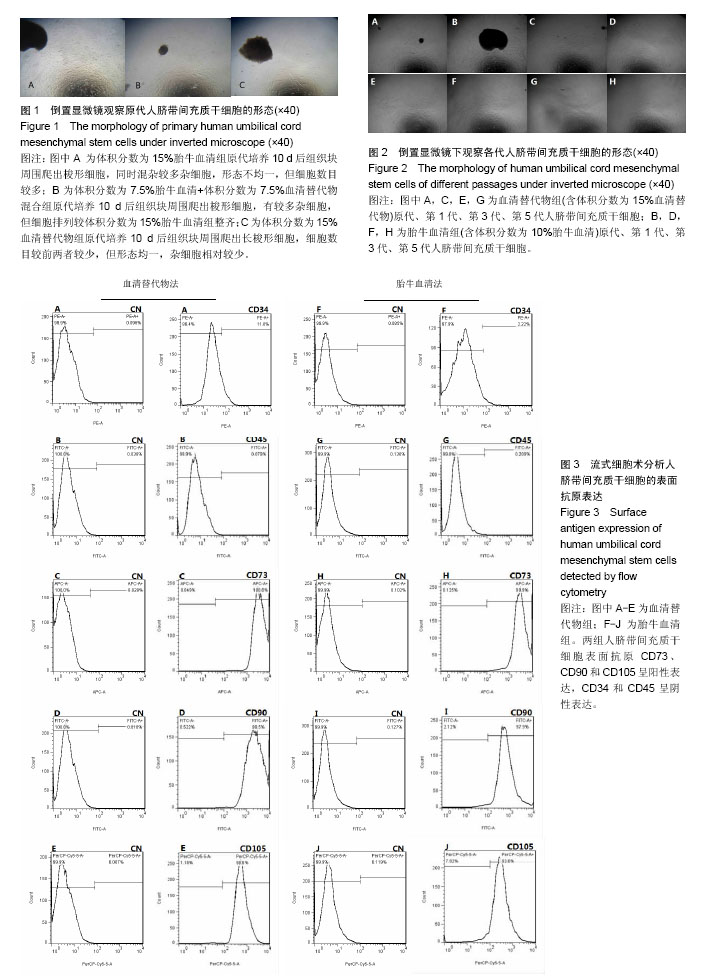
2.1 人脐带间充质干细胞的分离及培养结果 采用组织块贴壁法分离培养人脐带间充质干细胞,原代培养10 d后,血清替代物法形态更均一(图1);随着继续传代培养,血清替代物组细胞呈现较均一的涡漩状生长,而传统胎牛血清组则随着细胞代数的增加逐渐出现细胞分化或者老化现象(图2)。 2.2 人脐带间充质干细胞的表面抗原表达 流式细胞仪检测血清替代物组人脐带间充质干细胞表面抗原CD73、CD90和CD105阳性率为100%、99.5%和98.8%,CD34和CD45阳性率为11.6%和0.079%,胎牛血清组人脐带间充质干细胞表面抗原CD73、CD90和CD105阳性率为99.9%、97.9%和93.5%,CD34和CD45阳性率为2.22%和0.209%(图3),符合人脐带间充质干细胞的表型特征,两种方法培养的人脐带间充质干细胞表面抗原无明显差异。 2.3 人脐带间充质干细胞的诱导分化能力 用第5代人脐带间充质干细胞进行体外诱导分化实验(图4),诱导分化实验用时达14-21 d,阴性对照组细胞100%融合后继续培养细胞逐渐死亡,因此阴性对照的图片未列出。成骨诱导后经茜素红染色,出现红染的钙结节,胎牛血清组和血清替代物组的阳性率分别为90%和80%。成软骨诱导后经阿新蓝染色,出现蓝染的软骨细胞合成的蛋白多糖,胎牛血清组和血清替代物组的阳性率均为90%。成脂诱导后经油红O染色,出现红染的脂滴,胎牛血清组和血清替代物组的阳性率分别为90%和80%。两种培养法无显著差异。 2.4 人脐带间充质干细胞的增殖能力 将人脐带间充质干细胞接种于96孔板,条件培养基分别为体积分数为10%胎牛血清、10%血清替代物、15%血清替代物和20%血清替代物,连续培养6 d,绘制生长曲线,见图5。第1-3天体积分数为10%胎牛血清组增殖速率明显高于3个血清替代物组(P < 0.05),第4天后增殖速率无明显差异(P > 0.05),血清替代物组随着体积分数增高,增殖速率增快。 2.5 人脐带间充质干细胞oct4、nanog及sox2蛋白的表达 Western blot实验结果显示,血清替代物组与胎牛血清组oct4、nanog的表达水平无显著差异,血清替代物组sox2表达水平高于胎牛血清组(P < 0.05),见图6。"
| [1] Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5):267-274.[2] Castrechini NM, Murthi P, Gude NM, et al. Mesenchymal stem cells in human placental chorionic villi reside in a vascular Niche. Placenta. 2010;31(3):203-212.[3] Corotchi MC, Popa MA, Remes A, et al. Isolation method and xeno-free culture conditions influence multipotent differentiation capacity of human Wharton's jelly-derived mesenchymal stem cells. Stem Cell Res Ther. 2013;4(4):81.[4] Jin ES, Min J, Jeon SR, et al. Analysis of molecular expression in adipose tissue-derived mesenchymal stem cells : prospects for use in the treatment of intervertebral disc degeneration. J Korean Neurosurg Soc. 2013;53(4):207-212.[5] Yang LM, Liu Y, Zhao J, et al. Characterization of human umbilical cord mesenchymal stem cells following tissue mass culture. Cell Mol Biol (Noisy-le-grand). 2014;60(1):12-18.[6] Gu F, Wang D, Zhang H, et al. Allogeneic mesenchymal stem cell transplantation for lupus nephritis patients refractory to conventional therapy. Clin Rheumatol. 2014;33(11): 1611-1619.[7] Wang D, Li J, Zhang Y, et al. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther. 2014;16(2):R79.[8] Wang D, Zhang H, Liang J, et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013;22(12):2267-2277.[9] Woodworth TG, Furst DE. Safety and feasibility of umbilical cord mesenchymal stem cells in treatment-refractory systemic lupus erythematosus nephritis: time for a double-blind placebo-controlled trial to determine efficacy. Arthritis Res Ther. 2014;16(4):113.[10] Sun L, Wang D, Liang J, et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62(8):2467-2475. [11] Santos Nascimento D, Mosqueira D, Sousa LM, et al. Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling after myocardial infarction by proangiogenic, antiapoptotic, and endogenous cell-activation mechanisms. Stem Cell Res Ther. 2014;5(1):5.[12] Kong D, Zhuang X, Wang D, et al. Umbilical cord mesenchymal stem cell transfusion ameliorated hyperglycemia in patients with type 2 diabetes mellitus. Clin Lab. 2014;60(12):1969-1976.[13] Liu J, Han D, Wang Z, et al. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy. 2013;15(2):185-191.[14] Wang S, Cheng H, Dai G, et al. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013;1532:76-84.[15] Jiang Y, Zhu W, Zhu J, et al. Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of the lesion artery in patients with stroke in the territory of the middle cerebral artery. Cell Transplant. 2013;22(12):2291-2298.[16] Lv YT, Zhang Y, Liu M, et al. Transplantation of human cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. J Transl Med. 2013;11: 196.[17] Buyl K, Vanhaecke T, Desmae T, et al. Evaluation of a new standardized enzymatic isolation protocol for human umbilical cord-derived stem cells. Toxicol In Vitro. 2015;29(6):1254-1262.[18] Mori Y, Ohshimo J, Shimazu T, et al. Improved explant method to isolate umbilical cord-derived mesenchymal stem cells and their immunosuppressive properties. Tissue Eng Part C Methods. 2015;21(4):367-372.[19] Iftimia-Mander A, Hourd P, Dainty R, et al. Mesenchymal stem cell isolation from human umbilical cord tissue: understanding and minimizing variability in cell yield for process optimization. Biopreserv Biobank. 2013;11(5):291-298.[20] Medicinal and other products and human and animal transmissible spongiform encephalopathies: memorandum from a WHO meeting. Bull World Health Organ. 1997;75(6): 505-513.[21] Selvaggi TA, Walker RE, Fleisher TA. Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood. 1997;89(3):776-779.[22] Spees JL, Gregory CA, Singh H, et al. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9(5):747-756.[23] Tuschong L, Soenen SL, Blaese RM, et al. Immune response to fetal calf serum by two adenosine deaminase-deficient patients after T cell gene therapy. Hum Gene Ther. 2002; 13(13):1605-1610.[24] Drach G, Maret A, Richard MF, et al. Transfer and induction of delayed hypersensitivity to methylated bovine serum albumin in the absence of adjuvant. C R Acad Sci Hebd Seances Acad Sci D. 1977;284(23):2435-2437.[25] Kocaoemer A, Kern S, Klüter H, et al. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 2007;25(5):1270-1278. [26] Ding Y, Yang H, Feng JB, et al. Human umbilical cord-derived MSC culture: the replacement of animal sera with human cord blood plasma. In Vitro Cell Dev Biol Anim. 2013;49(10):771-777.[27] Ma HY, Yao L, Yu YQ, et al. An effective and safe supplement for stem cells expansion ex vivo: cord blood serum. Cell Transplant. 2012;21(5):857-869.[28] Shetty P, Bharucha K, Tanavde V. Human umbilical cord blood serum can replace fetal bovine serum in the culture of mesenchymal stem cells. Cell Biol Int. 2007;31(3):293-298. [29] Murphy MB, Blashki D, Buchanan RM, et al. Adult and umbilical cord blood-derived platelet-rich plasma for mesenchymal stem cell proliferation, chemotaxis, and cryo-preservation. Biomaterials. 2012;33(21):5308-5316.[30] Schallmoser K, Bartmann C, Rohde E, et al. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47(8):1436-1446.[31] Mohammadi S, Nikbakht M, Malek Mohammadi A, et al. Human Platelet Lysate as a Xeno Free Alternative of Fetal Bovine Serum for the In Vitro Expansion of Human Mesenchymal Stromal Cells. Int J Hematol Oncol Stem Cell Res. 2016;10(3): 161-171.[32] Bieback K, Hecker A, Kocaömer A, et al. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27(9):2331-2341.[33] Ng F, Boucher S, Koh S, et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112(2):295-307.[34] Liu ZZ, Chen P, Lu ZD, et al. Enrichment of breast cancer stem cells using a keratinocyte serum-free medium. Chin Med J (Engl). 2011;124(18):2934-2936.[35] Huang D, Peng WJ, Ye Q, et al. Serum-Free Suspension Culture of MDCK Cells for Production of Influenza H1N1 Vaccines. PLoS One. 2015;10(11):e0141686.[36] O'Loughlin AJ, Woffindale CA, Wood MJ. Exosomes and the emerging field of exosome-based gene therapy. Curr Gene Ther. 2012;12(4):262-274.[37] Qin J, Xu Q. Functions and application of exosomes. Acta Pol Pharm. 2014;71(4):537-543.[38] Wahlgren J, Statello L, Skogberg G, et al. Delivery of Small Interfering RNAs to Cells via Exosomes. Methods Mol Biol. 2016; 1364:105-125.[39] Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6(4):481-492.[40] Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007; 25(11):2896-2902.[41] Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15(3):4142-4157.[42] Zhang B, Wu X, Zhang X, et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl Med. 2015;4(5):513-522.[43] Zhang B, Shen L, Shi H, et al. Exosomes from Human Umbilical Cord Mesenchymal Stem Cells: Identification, Purification, and Biological Characteristics. Stem Cells Int. 2016;2016:1929536.[44] Li T, Yan Y, Wang B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22(6):845-854.[45] Zhou Y, Xu H, Xu W, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4(2):34.[46] Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.[47] Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663-676. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [5] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [6] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [7] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [8] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [9] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [10] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [11] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [12] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [13] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [14] | Zhao Min, Feng Liuxiang, Chen Yao, Gu Xia, Wang Pingyi, Li Yimei, Li Wenhua. Exosomes as a disease marker under hypoxic conditions [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1104-1108. |
| [15] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||