Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (13): 1981-1986.doi: 10.3969/j.issn.2095-4344.0495
Previous Articles Next Articles
Gelatin fibrous scaffolds promote fibrogenic differentiation of human dental pulp stem cells
Jiang Li-ming1, 2, Xia Shang1, Song Ge1, Chen Xu1, 2
- 1School of Stomatology, China Medical University, Shenyang 110002, Liaoning Province, China; 2Liaoning Province Key Laboratory of Oral Disease, Shenyang 110002, Liaoning Province, China
-
Revised:2018-01-26Online:2018-05-08Published:2018-05-08 -
Contact:Chen Xu, M.D., Professor, School of Stomatology, China Medical University, Shenyang 110002, Liaoning Province, China; Liaoning Province Key Laboratory of Oral Disease, Shenyang 110002, Liaoning Province, China -
About author:Jiang Li-ming, M.D., Lecturer, Attending physician, School of Stomatology, China Medical University, Shenyang 110002, Liaoning Province, China; Liaoning Province Key Laboratory of Oral Disease, Shenyang 110002, Liaoning Province, China -
Supported by:the Scientific Research Project of Liaoning Province Education Department, No. LS201610; Young Science and Technology Foundation Startup Project of School of Stomatology, China Medical University, No. K101593-15-05
CLC Number:
Cite this article
Jiang Li-ming, Xia Shang, Song Ge, Chen Xu. Gelatin fibrous scaffolds promote fibrogenic differentiation of human dental pulp stem cells[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(13): 1981-1986.
share this article
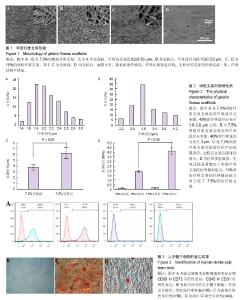
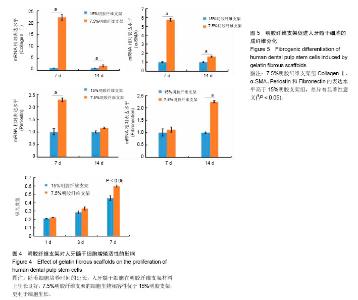
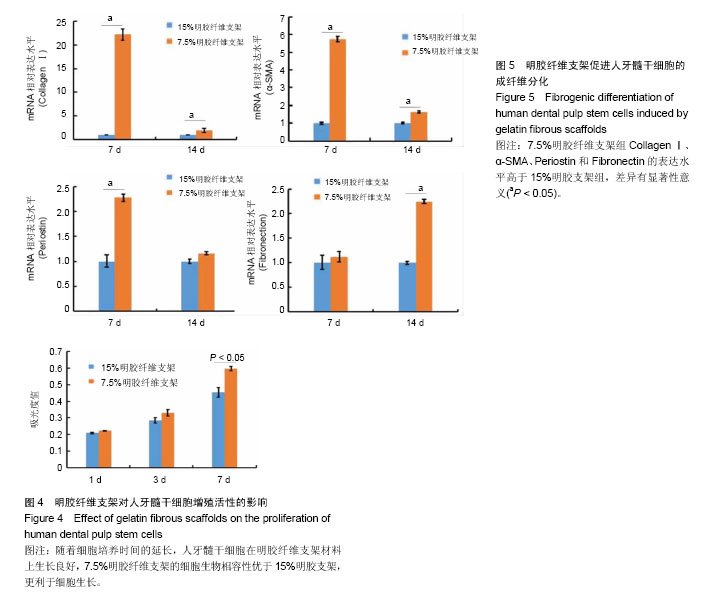
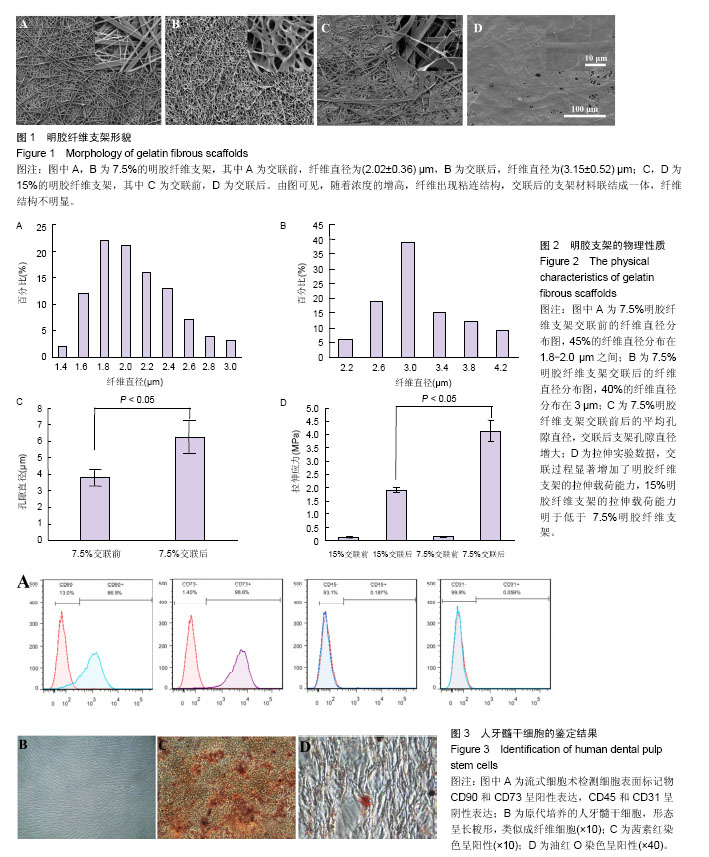
2.1 明胶纤维支架的表面结构特点 扫描电镜观察可见,7.5%明胶支架呈现纵横交错的纤维结构,纤维表面光滑,直径不均一,为(2.02 ±0.36) μm,45%的纤维直径分布于1.8-2.2 μm之间(图1A,图2A)。交联后的7.5%明胶纤维支架直径和孔隙均增大,纤维直径较为均一,为(3.15±0.52) μm,40%的纤维直径分布在3 μm左右(图1B,图2B)。交联前后的孔隙直径分别为(3.78±0.5) μm和(6.26±1.05) μm(图2C)。而15%明胶支架纤维出现纤维粘结,交联后支架表面纤维结构基本消失,几乎无孔隙,呈现平面结构(图1C和1D)。 2.2 明胶纤维支架的拉伸应力 交联前,7.5%和15%明胶纤维支架材料的拉伸应力相似,为(0.143±0.019) MPa和(0.116±0.024) MPa,交联过程使两种支架材料的拉伸应力分别增加至(4.14±0.39) MPa和(1.893±0.096) MPa,7.5%明胶纤维支架的延展性优于15%明胶支架(t =3.899,P < 0.05),见图2D。 2.3 人牙髓干细胞的培养和鉴定结果 流式细胞术检测细胞表面标记物,结果显示CD73(98.8±0.15)%,CD90(91.4±0.35)%呈阳性表达,CD45(0.22±0.03)%、CD31(0.025±0.01)%呈阴性表达,见图3A。牙髓组织经酶消化后,原代培养的人牙髓干细胞贴壁生长,形态呈长梭形,类似成纤维细胞,见图3B。经成骨诱导培养21 d后,细胞可见明显的矿化结节形成,茜素红染色呈阳性,见图3C。经成脂诱导培养21 d后,可见脂滴形成,油红O染色呈阳性,见图3D。 2.4 明胶纤维支架对人牙髓干细胞增殖活性的影响 明胶支架具有良好的细胞生物相容性,人牙髓干细胞可以在明胶纤维支架上黏附和生长。第1天时,两种支架材料上的细胞数量无明显差异,第3天时,细胞数量均增高,第7天时,7.5%明胶纤维支架上的细胞数量明显高于15%明胶支架组(P < 0.05),表明7.5%明胶支架的多孔纤维结构更利于细胞的增殖,见图4。 2.5 明胶纤维支架促进人牙髓干细胞的成纤维分化 Collagen Ⅰ是牙髓组织中重要的细胞外基质,α-SMA、Fibronectin和Periostin均是纤维结缔组织标志性因子。第7天时,7.5%明胶纤维支架组的Collagen Ⅰ水平显著增高,为15%明胶纤维支架组的22.2倍,第14天时,7.5%明胶纤维支架组CollagenⅠ水平约为15%明胶纤维支架组的2倍,证明该支架的纳米纤维结构可以促进人牙髓干细胞向成纤维细胞分化。第7天时,7.5%明胶纤维支架组的α- SMA和Periostin水平分别是15%明胶纤维支架组的5.7倍和2.28倍,两组间Fibronectin的表达差异无显著性意义(P > 0.05);第14天时,7.5%明胶纤维支架组α-SMA水平为15%明胶纤维支架组的1.6倍,Fibronectin的表达增高为15%明胶纤维支架组的2.24倍(P < 0.05),两组Periostin的表达差异无显著性意义(P > 0.05),见图5。"
| [1] Society Of Cariology And Endodontology CS. Guidelines for Root Canal Therapy. Chin J Dent Res. 2015;18(4):213-216. [2] Jordan RA, Holzner AL, Markovic L, et al. Clinical effectiveness of basic root canal treatment after 24 months: a randomized controlled trial. J Endod. 2014;40(4):465-470. [3] Kang SH, Kim BS, Kim Y. Cracked Teeth: Distribution, Characteristics, and Survival after Root Canal Treatment. J Endod. 2016;42(4):557-562.[4] Galler KM, Brandl FP, Kirchhof S, et al. Suitability of Different Natural and Synthetic Biomaterials for Dental Pulp Tissue Engineering. Tissue Eng Part A. 2017 Jul 20. doi: 10.1089/ten.TEA.2016.0555. [Epub ahead of print][5] 杜暘,高秀秋,金鼎. 丝素胶原支架与人牙周膜成纤维细胞相容性研究[J]. 中国实用口腔科杂志, 2009, 2(9):534-536.[6] Wang SD, Ma Q, Wang K, et al. Strong and biocompatible three-dimensional porous silk fibroin/graphene oxide scaffold prepared by phase separation. Int J Biol Macromol. 2018;111: 237-246.[7] Harumi Miyagi SP, Kerkis I, da Costa Maranduba CM, et al. Expression of extracellular matrix proteins in human dental pulp stem cells depends on the donor tooth conditions. J Endod. 2010;36(5): 826-831. [8] Jafary F, Hanachi P, Gorjipour K. Osteoblast Differentiation on Collagen Scaffold with Immobilized Alkaline Phosphatase. Int J Organ Transplant Med. 2017;8(4):195-202.[9] Shekhter AB, Guller AE, Istranov LP, et al. Morphology of collagen matrices for tissue engineering (biocompatibility, biodegradation, tissue response). Arkh Patol. 2015;77(6):29-38. [10] Hou G, Zhou F, Guo Y, et al. In vivo study of a bioactive nanoparticle-gelatin composite scaffold for bone defect repair in rabbits. J Mater Sci Mater Med. 2017;28(11):181.[11] Fayyazbakhsh F, Solati-Hashjin M, Keshtkar A, et al. Release behavior and signaling effect of vitamin D3 in layered double hydroxides-hydroxyapatite/gelatin bone tissue engineering scaffold: An in vitro evaluation. Colloids Surf B Biointerfaces. 2017;158:697-708.[12] Ishihara T, Miyazaki M, Notani N, et al. Locally Applied Simvastatin Promotes Bone Formation in a Rat Model of Spinal Fusion. J Orthop Res. 2017;35(9):1942-1948.[13] Oryan A, Sharifi P, Moshiri A, et al. The role of three-dimensional pure bovine gelatin scaffolds in tendon healing, modeling, and remodeling: an in vivo investigation with potential clinical value. Connect Tissue Res. 2017;58(5):424-437.[14] Duan W, Haque M, Kearney MT, et al. Collagen and Hydroxyapatite Scaffolds Activate Distinct Osteogenesis Signaling Pathways in Adult Adipose-Derived Multipotent Stromal Cells. Tissue Eng Part C Methods. 2017;23(10):592-603.[15] Yang F, Miao Y, Wang Y, et al. Electrospun Zein/Gelatin Scaffold- Enhanced Cell Attachment and Growth of Human Periodontal Ligament Stem Cells. Materials (Basel). 2017;10(10): E1168.[16] Kucinska-Lipka J, Gubanska I, Janik H, et al. Fabrication of polyurethane and polyurethane based composite fibres by the electrospinning technique for soft tissue engineering of cardiovascular system. Mater Sci Eng C Mater Biol Appl. 2015;46: 166-176.[17] Huang L, Zhu L, Shi X, et al. A compound scaffold with uniform longitudinally oriented guidance cues and a porous sheath promotes peripheral nerve regeneration in vivo.Acta Biomater. 2017. pii: S1742-7061(17)30766-3. doi: 10.1016/j.actbio.2017.12.010. [Epub ahead of print][18] Ghaderi Gandomani M, Sahebghadam Lotfi A, Kordi Tamandani D, et al. The enhancement of differentiating adipose derived mesenchymal stem cells toward hepatocyte like cells using gelatin cryogel scaffold. Biochem Biophys Res Commun. 2017;491(4):1000-1006.[19] Hess SC, Stark WJ, Mohn D, et al. Gene expression in human adipose-derived stem cells: comparison of 2D films, 3D electrospun meshes or co-cultured scaffolds with two-way paracrine effects. Eur Cell Mater. 2017;34:232-248.[20] Guo W, Qiu J, Liu J, et al. Graphene microfiber as a scaffold for regulation of neural stem cells differentiation. Sci Rep. 2017;7(1): 5678. [21] Que RA, Arulmoli J, Da Silva NA, et al. Recombinant collagen scaffolds as substrates for human neural stem/progenitor cells. J Biomed Mater Res A. 2018 Jan 17. doi: 10.1002/jbm.a.36343. [Epub ahead of print][22] Lee J, Yoo JJ, Atala A, et al. Controlled heparin conjugation on electrospun poly(ε-caprolactone)/gelatin fibers for morphology- dependent protein delivery and enhanced cellular affinity. Acta Biomater. 2012;8(7):2549-2558.[23] Yang L, Tanabe K, Miura T, et al. Influence of lyophilization factors and gelatin concentration on pore structures of atelocollagen/gelatin sponge biomaterial. Dent Mater J. 2017;36(4):429-437.[24] Zhu J, Yang F, He F, et al. A tubular gelatin scaffold capable of the time-dependent controlled release of epidermal growth factor and mitomycin C. Colloids Surf B Biointerfaces. 2015;135:416-424.[25] Alidadi S, Oryan A, Bigham-Sadegh A, et al. Role of platelet gel embedded within gelatin scaffold on healing of experimentally induced critical-sized radial bone defects in rats. Int Orthop. 2017; 41(4):805-812. [26] Elsayed Y, Lekakou C, Labeed F, et al. Fabrication and characterisation of biomimetic, electrospun gelatin fibre scaffolds for tunica media-equivalent, tissue engineered vascular grafts. Mater Sci Eng C Mater Biol Appl. 2016;61:473-483.[27] Sisson K, Zhang C, Farach-Carson MC, et al. Evaluation of cross-linking methods for electrospun gelatin on cell growth and viability. Biomacromolecules. 2009;10(7):1675-1680. [28] Wissink MJ, Beernink R, Pieper JS, et al. Immobilization of heparin to EDC/NHS-crosslinked collagen. Characterization and in vitro evaluation. Biomaterials. 2001;22(2):151-163.[29] Barnes CP, Pemble CW, Brand DD, et al. Cross-linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Eng. 2007;13(7):1593-1605.[30] Kwon IK, Kidoaki S, Matsuda T. Electrospun nano- to microfiber fabrics made of biodegradable copolyesters: structural characteristics, mechanical properties and cell adhesion potential. Biomaterials. 2005;26(18):3929-3939.[31] Van Nieuwenhove I, Salamon A, Adam S, et al. Gelatin- and starch-based hydrogels. Part B: In vitro mesenchymal stem cell behavior on the hydrogels. Carbohydr Polym. 2017;161:295-305.[32] Menicanin D, Hynes K, Han J, et al. Cementum and Periodontal Ligament Regeneration. Adv Exp Med Biol. 2015;881:207-236.[33] Hilkens P, Bronckaers A, Ratajczak J, et al. The Angiogenic Potential of DPSCs and SCAPs in an In Vivo Model of Dental Pulp Regeneration. Stem Cells Int. 2017;2017:2582080.[34] Lambrichts I, Driesen RB, Dillen Y, et al. Dental Pulp Stem Cells: Their Potential in Reinnervation and Angiogenesis by Using Scaffolds. J Endod. 2017;43(9S):S12-S16..[35] Zhang X, Li H, Sun J, et al. Cell-derived micro-environment helps dental pulp stem cells promote dental pulp regeneration. Cell Prolif. 2017;50(5). doi: 10.1111/cpr.12361. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [5] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [6] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [7] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [8] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [9] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [10] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [11] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [12] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [13] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [14] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [15] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||