Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (11): 1774-1780.doi: 10.3969/j.issn.2095-4344.0178
Previous Articles Next Articles
Classification characteristics and clinical application of lumbar interspinous distraction system
Lu Jun-feng, Wang Bao-dong, Cao Yang
- First Affiliated Hospital, Harbin Medical University, Harbin 150001, Heilongjiang Province, China
-
Online:2018-04-18Published:2018-04-18 -
Contact:Cao Yang, M.D., First Affiliated Hospital, Harbin Medical University, Harbin 150001, Heilongjiang Province, China -
About author:Lu Jun-feng, Master candidate, First Affiliated Hospital, Harbin Medical University, Harbin 150001, Heilongjiang Province, China -
Supported by:a grant from the Science and Technology Bureau of Harbin City, No. 2013AA3BS016
CLC Number:
Cite this article
Lu Jun-feng, Wang Bao-dong, Cao Yang. Classification characteristics and clinical application of lumbar interspinous distraction system [J]. Chinese Journal of Tissue Engineering Research, 2018, 22(11): 1774-1780.
share this article

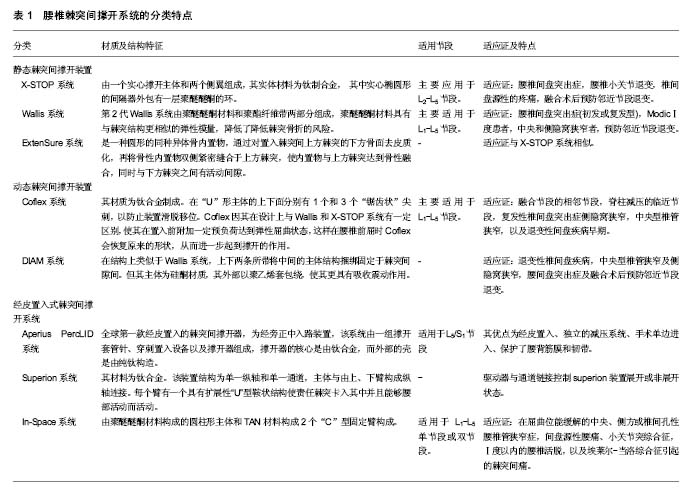
2.1 腰椎棘突间撑开技术的特点、分类及发展史 1950年Knowles尝试将一种圆型钢制的“塞子”置于棘突之间,用于治疗腰椎椎管狭窄,但因其内置物极容易脱落,从而必须手术取出,导致该种方法未能得到认可。近年来随着生物医疗科技、材料科学的进步与发展,设计理念的提升,各种棘突间撑开装置相继问世。1986年,法国学者Sénégas等[4]研制出一种能够将钛制撑开器捆绑固定于棘突的腰椎棘突间撑开装置-Wallis系统。从此之后,陆续有多种新型棘突间撑开装置被研发出来,根据其设计模式分为静态固定系统如X-STOP、Wallis、ExtenSure,动态固定系统如Coflex、椎间辅助运动装置(the device for intervertebral assisted motion, DIAM)等(图1),还有经皮置入的AperiusPercLID、Superion、In-Space等。但其总的设计理念基本为撑开责任节段的棘突间隙并限制相应节段的后伸运动,从而增加相应节段的椎管及椎间孔面积,降低椎间盘及小关节突负荷同时可撑开椎管内褶皱的黄韧带,解除对脊髓及神经根压迫[5],通过替代应用于责任节段或联合应用于融合节段的邻近节段,可以起到改善临床症状并预防邻近节段退变的作用[6],见表1。"


2.2 静态棘突间撑开装置 2.2.1 X-STOP系统 X-Stop系统是于2005年首个被美国FDA认证运用于临床的棘突间撑开装置,属于不可压缩的棘突间内固定动态系统[7]。是由一个实心撑开主体和两个侧翼组成,其实体材料为钛(Ti)制合金, 其中实心椭圆形的间隔器外包有一层聚醚醚酮的环。其实心主体用于撑开上下的棘突,两面的侧翼联合周围的韧带可以防止置入假体发生移位。主要应用于L2-L5节段,目前FDA批准的适应证有:中央型椎管狭窄及侧隐窝狭窄;潜在的临床适应证为腰椎间盘突出症,腰椎小关节退变,椎间盘源性的疼痛,融合术后预防邻近节段退变[8-10],其局麻操作、创伤小、完整保留棘上韧带、术后康复快等优点极大推动了临床应用[11]。 2.2.2 Wallis系统 法国医生Sénégas等[12]在1984年开始对第1代Wallis棘突间动态稳定系统开始研发,于1986年第1代Wallis系统开始应用于临床。第1代其主体部分由钛合金和涤纶带组成,成“H”型,通过涤纶带将Wallis系统固定于棘突间隙内,能有效改善相应椎间盘和小关节突压力,但因其内置物材料刚性过大致棘突局部应力集中,明显增加了棘突骨折等风险,使其仅用于临床试验研究,未进行推广。第2代Wallis系统由聚醚醚酮材料和聚酯纤维带两部分组成,聚醚醚酮材料具有与棘突结构更相似的弹性模量,降低了降低棘突骨折的风险,设计上延续了不固定于椎体上的“漂浮“特点,于2002年经FDA批准进行临床试验[12]。主要适用于L1-L5节段,适应证为:腰椎间盘突出症(初发或复发型),Modic Ⅰ度患者,中央和侧隐窝狭窄者,预防邻近节段退变[8-9]。 2.2.3 ExtenSure系统 ExtenSure系统是一种圆形的同种异体骨内置物,于2005年被FDA批准应用于临床。通过对置入棘突间上方棘突的下方骨面去皮质化,再将骨性内置物双侧紧密缝合于上方棘突,使内置物与上方棘突达到骨性融合,同时与下方棘突之间有活动间隙。其优势在于内置物即可与棘突达到长期生物稳定,又因同种异体骨于棘突的弹性模量相近,可减少术后并发 症[13]。ExtenSure系统适应证与X-STOP系统相似。 2.3 动态棘突间撑开装置 2.3.1 Coflex系统 Coflex系统是由Jacques Samani在1994年发明的,并于2006年被FDA 批准进行研究用器械豁免(IDE)临床试验[14]。Coflex系统由一个“U”形主体,上下两个“夹状”侧翼组成,其材质为钛合金制成。在“U”形主体的上下面分别有1个和3个“锯齿状”尖刺,以防止装置滑脱移位。Coflex因其在设计上与Wallis和X-STOP系统有一定区别,使其在置入前附加一定预负荷达到弹性屈曲状态,这样在腰椎前屈时Coflex会恢复原来的形状,从而进一步起到撑开的作用[15]。Coflex放置于腰椎椎板间而不是棘突间,因此不绝对依赖保留棘突,这也容许对责任节段进行较大的减压,包括部分椎板、关节突切除,神经根管的减压,黄韧带及棘上韧带的切除等。因此适应证广泛,主要适用于L1-L5节段,主要适用于退变性椎管狭窄需行减压的患者[15],潜在适应证为:融合节段的相邻节段,脊柱减压的临近节段,复发性椎间盘突出症侧隐窝狭窄,中央型椎管狭窄,以及退变性间盘疾病早期[16]。 2.3.2 DIAM系统 DIAM系统在结构上类似于Wallis系统,上下两条所带将中间的主体结构捆绑固定于棘突间隙间。但其主体为硅酮材质,其外部以聚乙烯套包绕,使其更具有吸收震动作用。于2005年获得FDA批准进入IDE临床试验。其主要适应证为:退变性椎间盘疾病,中央型椎管狭窄及侧隐窝狭窄,腰间盘突出症及融合术后预防邻近节段退变[17]。 2.4 经皮置入式棘突间撑开系统 2.4.1 Aperius PercLID系统 Palmer等[18]于2007年发明了Aperius PercLID 独立撑开系统,是全球第一款经皮置入的棘突间撑开器,为经旁正中入路装置,该系统由一组撑开套管针、穿刺置入设备以及撑开器组成,撑开器的核心是由钛合金,而外部的壳是由纯钛构造,局麻下经皮置入棘突间隙,通常在离背部中线5-7 cm 处做1 cm皮肤切口,穿过腰背筋膜及棘突间韧带,在C型臂X射线正侧位透视下,棘突间医用套管置入装置并展开固定翼,该手术还可经皮行独立椎间的减压,Aperius系统还可适用于L5/S1节段[19]。与其他棘突间撑开装置相比,其优点有:经皮置入、独立的减压系统、手术单边进入、保护了腰背筋膜和韧带。 2.4.2 Superion系统 该器械是Moti Altarac团队研发,其材料为钛合金。该装置结构为单一纵轴和单一通道。主体与由上、下臂构成纵轴连接。每个臂有一个具有扩展性“U”型鞍状结构使责任棘突卡入其中并且能够腰部活动而活动。驱动器与通道链接控制superion装置展开或非展开状态[20]。术中在C臂透视下或直视下定位手术节段,在背部正中行12-15 mm切口,superion装置插入切口,暴露棘上韧带并切开,再继续向深部扩张暴露棘突间组织,以确保有足够的操作空间,再用特制医用套管通过superion装置穿过棘上韧带,到达适宜的深度后取出扩张器。再测量棘突之间的距离,确定置入物型号。置入后通过配套驱动器使superion装置撑开[21]。 2.4.3 In-Space系统 In-Space系统由聚醚醚酮材料构成的圆柱形主体和TAN材料构成2个“C”型固定臂构成。In-Space系统经皮从侧方入路置入椎间,术中通过C臂定位置入节段,应用瞄准器联合导丝对棘突间分离,评估置入In-Space型号,通过专用把持器置入,撑开固定臂,置入过程中避免In-Space移位[22]。该装置目前可适用于L1-L5单节段或双节段。推荐适应证为:在屈曲位能缓解的中央、侧方或椎间孔性腰椎管狭窄症、间盘源性腰痛、小关节突综合征、Ⅰ度以内的腰椎活脱、埃莱尔-当洛综合征引起的棘突间痛[23]。 2.5 腰椎棘突间撑开器生物力学特点 2.5.1 棘突间撑开系统对置入节段及邻近节段活动度影响 万宗淼[24]通过体外实验研究发现,正常腰椎置入X-STOP之后,后伸活动度不及正常的50%,旋转、前屈、侧弯时腰椎活动度没有明显变化。于2011年Ilharreborde等[25]通过在尸体标本L3-4间置入Waliis系统通过试验得到其生物力学特点,结果显示置入节段屈伸活动度减少13.8%,轴向旋转及左右侧屈分别0.4%和6.2%。Tsai等[26]研究发现Coflex系统可在屈伸及轴向旋转两个方向提供弹性动态固定,可恢复失稳节段正常的后伸及轴向旋转活动。Park等[27]于2010年研究发现In-Space系统能减少置入节段的椎间压力,在屈伸方向上有稳定脊柱的作用,但在其他方向的活动及临近节段没有该稳定作用。Hartmann等[28]对4种棘突间撑开装置(Aperius PercLID、X-STOP、In-Space、Coflex)的生物力学特点对比研究得出,它们均能明显限制腰椎过伸活动,对该节段的其他活动方向没有限制作用,但增加了整个腰椎侧向和轴向活动度,而这将导致腰椎失稳加剧邻近节段退变。但本组研究人员以为整体腰椎活动度增加可能因为手术置入内置物过程中对棘突上肌肉止点剥离及棘间韧带和周围软组织破坏有关,与棘突间撑开器本身生物力学特性无关。 以上证据得出,腰椎棘突间撑开装置能够有效的限制腰椎过伸活动,可以一定程度恢复置入节段稳定性,对邻近节段活动度无显著影响,能够有效预防邻近节段退变。 2.5.2 棘突间撑开系统对置入节段椎管面积及椎间孔高度的影响 Richards等[5]对比样本发现,在X-Stop置入前后,置入节段后伸位时椎管面积增加18%,椎管矢状径增加10%,椎间孔面积增加约25%,椎间孔宽度增加41%。Wan等[29]也发现X-Stop置入节段在后伸位时,椎间孔横截面积较术前增加32.9%,椎间孔高度增加24.4%,而中立位和前屈位时对椎间孔影响甚微。Richards和Wan研究结果表明X-Stop置入后可预防被置入节段后伸时椎管和椎间孔狭窄。于2013年徐建平 等[30]回顾性分析了40例腰椎退行性疾病患者术后邻近节段解剖变化。结果显示,术后1年时Wallis组(融合术后临近节段置入Wallis系统)与融合组(单纯后路融合术)两者差异无显著性意义;术后3年时Wallis组椎间高度、椎管横截面积显著优于融合组。Zhou等[31]研究得出In-Space系统使置入节段椎间、椎间孔面积增加。Surace等[32]对置入AperiusPercLID术后发现,责任节段椎间孔横截面积与术前相比增加17.6%。Marcia等[33]发现,随访1年由经皮置入腰椎棘突间撑开系统治疗退变性腰椎管狭窄患者,椎管和椎间孔横截面积与术前相比增加15%。Hirsch等[34]通过体外试验研究得出,在L4-5节段对比4种棘突间撑开装置(In-Space、X-STOP、Wallis和DIAM)的生物力学效应,研究结果得出,4种棘突装置均能在过伸位时增加椎间孔横截面积,其中Wallis和DIAM仅在过伸位时有撑开作用,而X-STOP和In-Space在6个体位均有一定程度撑开作用。在对上下相邻节段生物力学研究中发现,4种装置对其上位节段的椎间孔横轮廓与截面积均无影响,而X-STOP和DIAM则减小了下位节段椎间孔的轮廓及横截面积。 总体看来,棘突间撑开装置在伸展位能显著增加置入节段的椎管面积和椎间孔高度,部分撑开器在中立位和屈曲位也有一定撑开作用,且对邻节段影响甚微,同时能有效的缓解因置入节段椎管狭窄对神经产生的机械性刺激症状。 2.5.3 腰椎棘突间撑开系统对置入节段椎间盘及小关节应力影响 姚庆强等[35]通过三维有限元软件进行建立了L4-5棘突间置入X-STOP的虚拟模型并应用三维有限元软件模拟其生物力学并进行分析,结果显示当椎体处于中立位时X-STOP系统所受负荷最小,后伸位时因X-STOP改变了的传导,进而有效降低椎间盘应力负荷,但L5椎弓根应力负荷同时增大,也增加了椎弓根骨折概率。Swanson等[36]在尸体标本中置入X-STOP通过变换尸体体位测量不同体位下椎间盘压力的变化,结果发现在过伸位时后方纤维环和髓核内压力分别减少63%和41%,在中立位时分别减少38%和20%,而邻近节段的椎间盘内压力无明显变化。于2007年Lafage等[37]通过体外生物试验及有限元分析,评估对责任节段置入Wallis系统后该节段的椎间压力及生物力学特性的变化,得出Wallis系统置入节段椎间压力对照组减少,Wallis系统承重负荷明显增加。陈肇辉[38]于2010年通过Coflex三维有限元分析发现,可降低置入节段椎间盘和小关节压力负荷,对上下邻近节段活动度及邻近椎间盘应力负荷影响甚微,但可使上下邻近节段小关节负荷增加;但同时也使Coflex装置本身及相应节段棘突应力负荷升高,手术失败率升高。王乃国[39]通过有限元分析技术模拟DIAM技术及椎弓根螺钉固定融合技术,对比分析得出DIAM可缓解传统手术所导致邻近关节应力集中的影响。Lazaro等[40]在In-Space的体外研究发现,屈曲体位时置入节段小关节压力减少30%,后伸体位时减小69%。Eebulut等[41]研究发现被置入撑开装置的节段椎间盘和小关节压力均减小,而邻近节段椎间盘及小关节压力增加。Nishida等[42]通过在猪体内经皮置入棘突间撑开装置,结果发现在中立位时,硬脊膜外压力无明显变化,而在过伸位时能显著预防硬脊膜压力升高。 以上研究均表明,总体上腰椎棘突间撑开装置可以有效降低置入节段椎间盘和小关节突应力负荷同时保留相应节段的活动度,但内置物本身和置入节段的上下棘突应力升高,警示有增加棘突骨折及内置物疲劳性断裂的风险。 2.6 腰椎棘突间撑开系统的临床应用 Puzzilli等[43]将542例腰椎管狭窄患者随机分为2组,手术组 422例患者行 X-STOP置入术,保守组120例,术后1年随访,手术组83.5% 患者取得满意效果,而保守组为50%;术后3 年,手术组仅24例患者因神经症状恶化再行手术治疗。Kuchta等[44]通过对175例行X-STOP治疗患者长达2年的随访发现,末次随访目测类比评分由术前的61.2分降至39.0分,Oswestry功能障碍指数术前平均为32.6%,术后6周为22.7%,术后2年为20.3%,再手术率为 4%。Pan等[45]对50例应用第2代Wallis系统治疗腰椎退行性疾病患者的术后疗效进行回顾性研究。术后3,12,24个月的日本骨科学会(JOA)评分,分别为25,26,25分,均显著高于术前的12分;术后3,12,24个月的Oswestry功能障碍指数均为5%,显著低于术前的13%,证明二代Wallis系统对腰椎退行性疾病的治疗有显著疗效。Bae等[46]随访了290例腰椎管狭窄患者,发现术后36个月Colfex组有效率高于腰椎融合组有效率的13.3%,证明Coflex系统在不破坏脊柱解剖结构和保留脊柱活动度的情况下可以有效、持久的提高有效率。Errico等[47]随访了127例应用Coflex系统治疗的腰椎椎管狭窄症病例,随访时间平均6.3年,患者满意率为93%。Hrabálek等[17]的研究中,随访了68例应用DIAM系统治疗的腰椎椎间盘突出、椎管狭窄的患者,随访时间3年,较术前比较,Oswestry功能障碍指数改善率63.85% ,轴性、根性疼痛目测类比评分下降了70.75% ,满意度为92.00%。Ramesh等[48]于2016年对应用Aperius系统治疗的腰椎椎管狭窄症的患者进行回顾性研究,平均随访17个月,所研究的23篇文献中报道Aperius系统总并发症发生率为10.62% 。Aperius PercLID现今天临床病例少,随访时间短,没有系统的临床疗效研究结果,但其独特的减压系统,仍具有很好的临床优势,值得今后的学者继续随访研究,验证其临床疗效。 根据以上所有临床证据分析发现,各种腰椎棘突间撑开装置在短、中期随访中均具有良好的临床疗效。但近年来也有一些报道称X-STOP治疗腰间盘突出症复发率据外文献报道10%-15%[49]。Beyer等[50]研究发现单独应用Aperius系统临床疗效不及传统手术。Kantelhardt等[22]研究发现置入In-Space术后1年症状复发率较高,二次手术率为22%。Epstein[51]综合分析了2008年到2011年发表的关于X-STOP临床应用文献,涵盖6篇文献,共计384个病例,平均随访时间为22-42.9个月,结果显示术后并发症发生率11.6%-38%、翻修率4.6%-85%及预后不良病例66.7%-77%,患者平均医疗费用高达41 084美元。但Gazzeri等[52]研究发现手术操作相关并发症(如脑脊液漏、硬膜外血肿)和围手术期并发症(下肢深静脉血栓的发生、坠积性肺炎、泌尿系统感染、压疮、手术切口感染等),腰椎棘突间撑开系统比传统脊柱融合手术并发症率更低。随着关于腰椎间棘突撑开系统的临床实验结果越来越多,Wu等[53]、Hong等[54]、Moojen等[55]通过循证医学的方法对其进行评价。总体看来,腰椎棘突间撑开装置的临床疗效可以与脊柱融合手术相媲美,其局麻下操作、创伤小、手术时间短、恢复快、住院天数短,术后并发症小,保留腰椎活动度和预防邻近节段退变等方面更具优势。但棘突间撑开装置也有不足如医疗费用高、翻修率高、临床疗效不确切等。 "

| [1] Kovacs FM, Urrútia G, Alarcón JD. Surgery versus conservative treatment for symptomatic lumbar spinal stenosis: a systematic review of randomized controlled trials. Spine (Phila Pa 1976). 2011;36(20): E1335-1351.[2] Siddiqui M, Smith FW, Wardlaw D. One-year results of X Stop interspinous implant for the treatment of lumbar spinal stenosis. Spine (Phila Pa 1976). 2007;32(12): 1345-1348.[3] Kaner T, Ozer AF. Dynamic stabilization for challenging lumbar degenerative diseases of the spine: a review of the literature. Adv Orthop. 2013;2013:753470.[4] Sénégas J, Vital JM, Pointillart V, et al. Long-term actuarial survivorship analysis of an interspinous stabilization system. Eur Spine J. 2007;16(8):1279-1287.[5] Richards JC, Majumdar S, Lindsey DP, et al. The treatment mechanism of an interspinous process implant for lumbar neurogenic intermittent claudication. Spine (Phila Pa 1976). 2005;30(7): 744-749.[6] Kabir SM, Gupta SR, Casey AT. Lumbar interspinous spacers: a systematic review of clinical and biomechanical evidence. Spine (Phila Pa 1976). 2010;35(25): E1499-1506.[7] 周大鹏. Wallis与X-stop治疗腰椎间盘突出症疗效及对腰椎功能影响的对比研究[D]. 泰山医学院,2013.[8] Christie SD, Song JK, Fessler RG. Dynamic interspinous process technology. Spine (Phila Pa 1976). 2005;30(16 Suppl): S73-78.[9] Bono CM, Vaccaro AR. Interspinous process devices in the lumbar spine. J Spinal Disord Tech. 2007;20(3):255-261.[10] Talwar V, Lindsey DP, Fredrick A, et al. Insertion loads of the X STOP interspinous process distraction system designed to treat neurogenic intermittent claudication. Eur Spine J. 2006;15(6):908-912.[11] Zucherman JF, Hsu KY, Hartjen CA, et al. A prospective randomized multi-center study for the treatment of lumbar spinal stenosis with the X STOP interspinous implant: 1-year results. Eur Spine J. 2004;13(1): 22-31.[12] Sénégas J. Mechanical supplementation by non-rigid fixation in degenerative intervertebral lumbar segments: the Wallis system. Eur Spine J. 2002;11 Suppl 2: S164-169.[13] 王良意. 腰椎棘突间稳定装置的应用及并发症的进展[J]. 实用骨科杂志, 2014,20(2): 137-141.[14] Davis R, Auerbach JD, Bae H, et al. Can low-grade spondylolisthesis be effectively treated by either coflex interlaminar stabilization or laminectomy and posterior spinal fusion? Two-year clinical and radiographic results from the randomized, prospective, multicenter US investigational device exemption trial: clinical article. J Neurosurg Spine. 2013;19(2): 174-184.[15] 杜敬曾,齐强,陈仲强. 腰椎棘突间撑开系统研究进展[J]. 中国脊柱脊髓杂志,2009,19(11): 870-873.[16] 王洪立,姜建元. 腰椎棘突间分离装置研究进展[J]. 中国脊柱脊髓杂志, 2008,18(8): 631-634.[17] Hrabálek L, Machác J, Vaverka M. The DIAM spinal stabilisation system to treat degenerative disease of the lumbosacral spine. Acta Chir Orthop Traumatol Cech. 2009;76(5):417-423.[18] Palmer S, Mahar A, Oka R. Biomechanical and radiographic analysis of a novel, minimally invasive, extension-limiting device for the lumbar spine. Neurosurg Focus. 2007;22(1): E4.[19] Fabrizi AP, Maina R, Schiabello L. Interspinous spacers in the treatment of degenerative lumbar spinal disease: our experience with DIAM and Aperius devices. Eur Spine J. 2011;20 Suppl 1: S20-26.[20] Miller LE, Block JE. Interspinous spacer implant in patients with lumbar spinal stenosis: preliminary results of a multicenter, randomized, controlled trial. Pain Res Treat. 2012;2012: 823509.[21] Loguidice V, Bini W, Shabat S, et al. Rationale, design and clinical performance of the Superion® Interspinous Spacer: a minimally invasive implant for treatment of lumbar spinal stenosis. Expert Rev Med Devices. 2011;8(4): 419-426.[22] Kantelhardt SR, Török E, Gempt J, et al. Safety and efficacy of a new percutaneously implantable interspinous process device. Acta Neurochir(Wien). 2010;152(11): 1961-1967.[23] 申洪全,汪洋,柯珍勇. 腰椎棘突间分离装置应用进展[J]. 现代医药卫生, 2014,30(8): 1184-1187.[24] 万宗淼. X-Stop棘突间撑开器对老年腰椎管狭窄症病人手术节段和邻近节段脊椎的三维运动生物力学分析及临床疗效评价[D]. 中南大学,2011.[25] Ilharreborde B, Shaw MN, Berglund LJ, et al. Biomechanical evaluation of posterior lumbar dynamic stabilization: an in vitro comparison between Universal Clamp and Wallis systems. Eur Spine J. 2011;20(2): 289-296.[26] Tsai KJ, Murakami H, Lowery GL, et al. A biomechanical evaluation of an interspinous device (Coflex) used to stabilize the lumbar spine.J Surg Orthop Adv.2006;15(3): 167-172.[27] Park SW, Lim TJ, Park J. A biomechanical study of the instrumented and adjacent lumbar levels after In-Space interspinous spacer insertion. J Neurosurg Spine.2010;12(5): 560-569.[28] Hartmann F, Dietz SO, Hely H, et al. Biomechanical effect of different interspinous devices on lumbar spinal range of motion under preload conditions. Arch Orthop Trauma Surg. 2011;131(7): 917-926.[29] Wan Z, Wang S, Kozánek M, et al. Biomechanical evaluation of the X-Stop device for surgical treatment of lumbar spinal stenosis. J Spinal Disord Tech. 2012;25(7): 374-378.[30] 徐建平,易红蕾,李明,等. Wallis棘突间动态稳定系统对缓解腰椎融合术后邻近节段退变的临床疗效分析[J].中国骨伤,2013,26(12):1005-1009.[31] Zhou D, Nong LM, DU R, et al. Effects of interspinous spacers on lumbar degenerative disease. Exp Ther Med. 2013;5(3): 952-956.[32] Surace MF, Fagetti A, Fozzato S, et al. Lumbar spinal stenosis treatment with Aperius perclid interspinous system. Eur Spine J. 2012;21 Suppl 1: S69-74.[33] Marcia S, Hirsch JA, Chandra RV, et al. Midterm clinical and radiologic outcomes after percutaneous interspinous spacer treatment for neurogenic intermittent claudication. J Vasc Interv Radiol. 2015;26(11): 1687-1693.e1-2.[34] Hirsch C, Breque C, Ragot S, et al. Biomechanical study of dynamic changes in L4-L5 foramen surface area in flexion and extension after implantation of four interspinous process devices. Orthop Traumatol Surg Res. 2015;101(2): 215-219.[35] 姚庆强,王黎明,蒋纯志,等. 非融合棘突间撑开器治疗腰椎早期退变性腰痛的三维有限元分析[J]. 中国骨与关节损伤杂志,2008,23(4): 292-295.[36] Swanson KE, Lindsey DP, Hsu KY, et al.The effects of an interspinous implant on intervertebral disc pressures. Spine (Phila Pa 1976). 2003; 28(1): 26-32.[37] Lafage V, Gangnet N, Sénégas J, et al. New interspinous implant evaluation using an in vitro biomechanical study combined with a finite-element analysis. Spine (Phila Pa 1976). 2007;32(16): 1706-1713.[38] 陈肇辉. 腰椎棘突间撑开装置的三维有限元分析及临床应用研究[D]. 第二军医大学,2010.[39] 王乃国. 腰椎棘突间动力固定的三维有限元分析和前瞻性临床研究[D]. 中国协和医科大学,2010.[40] Lazaro BC, Brasiliense LB, Sawa AG, et al. Biomechanics of a novel minimally invasive lumbar interspinous spacer: effects on kinematics, facet loads, and foramen height. Neurosurgery. 2010;66(3 Suppl Operative): 126-132; discussion 132-133.[41] Erbulut DU, Zafarparandeh I, Hassan CR, et al. Determination of the biomechanical effect of an interspinous process device on implanted and adjacent lumbar spinal segments using a hybrid testing protocol: a finite-element study. J Neurosurg Spine. 2015;23(2): 200-208.[42] Nishida K, Doita M, Kakutani K, et al. Development of percutaneously insertable/removable interspinous process spacer for treatment of posture-dependent lumbar spinal-canal stenosis: preclinical feasibility study using porcine model. Eur Spine J. 2012;21(6): 1178-1185.[43] Puzzilli F, Gazzeri R, Galarza M, et al. Interspinous spacer decompression (X-STOP) for lumbar spinal stenosis and degenerative disk disease: a multicenter study with a minimum 3-year follow-up. Clin Neurol Neurosurg. 2014;124: 166-174.[44] Kuchta J, Sobottke R, Eysel P, et al. Two-year results of interspinous spacer (X-Stop) implantation in 175 patients with neurologic intermittent claudication due to lumbar spinal stenosis. Eur Spine J. 2009;18(6): 823-829.[45] Pan B, Zhang ZJ, Lu YS, et al. Experience with the second-generation Wallis interspinous dynamic stabilization device implanted in degenerative lumbar disease: a case series of 50 patients. Turk Neurosurg. 2014;24(5): 713-719.[46] Bae HW, Davis RJ, Lauryssen C, et al. Three-year follow-up of the prospective, randomized, controlled trial of coflex interlaminar stabilization vs instrumented fusion in patients with lumbar stenosis. Neurosurgery. 2016;79(2): 169-181.[47] Errico TJ, Kamerlink JR, Quirno M, et al. Survivorship of coflex Interlaminar-Interspinous Implant. SAS J. 2009;3(2): 59-67.[48] Ramesh A, Lyons F, Kelleher M. Aperius interspinous device for degenerative lumbar spinal stenosis: a review. Neurosurg Rev. 2016; 39(2):197-205; discussion 205.[49] Zaveri GR, Mehta SS. Surgical treatment of lumbar tuberculous spondylodiscitis by transforaminal lumbar interbody fusion (TLIF) and posterior instrumentation. J Spinal Disord Tech. 2009;22(4): 257-262.[50] Beyer F, Yagdiran A, Neu P, et al. Percutaneous interspinous spacer versus open decompression: a 2-year follow-up of clinical outcome and quality of life. Eur Spine J. 2013;22(9): 2015-2021.[51] Epstein NE. A review of interspinous fusion devices: High complication, reoperation rates, and costs with poor outcomes. Surg Neurol Int. 2012; 3:7.[52] Gazzeri R, Galarza M, Alfieri A. Controversies about interspinous process devices in the treatment of degenerative lumbar spine diseases: past, present, and future. Biomed Res Int. 2014;2014: 975052.[53] Wu AM, Zhou Y, Li QL, et al. Interspinous spacer versus traditional decompressive surgery for lumbar spinal stenosis: a systematic review and meta-analysis. PLoS One. 2014;9(5): e97142.[54] Hong P, Liu Y, Li H. Comparison of the efficacy and safety between interspinous process distraction device and open decompression surgery in treating lumbar spinal stenosis: a meta analysis. J Invest Surg. 2015;28(1): 40-49.[55] Moojen WA, Arts MP, Bartels RH, et al. Effectiveness of interspinous implant surgery in patients with intermittent neurogenic claudication: a systematic review and meta-analysis. Eur Spine J. 2011;20(10): 1596-1606. |
| [1] | Xu Feng, Kang Hui, Wei Tanjun, Xi Jintao. Biomechanical analysis of different fixation methods of pedicle screws for thoracolumbar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1313-1317. |
| [2] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [3] | Chen Xinmin, Li Wenbiao, Xiong Kaikai, Xiong Xiaoyan, Zheng Liqin, Li Musheng, Zheng Yongze, Lin Ziling. Type A3.3 femoral intertrochanteric fracture with augmented proximal femoral nail anti-rotation in the elderly: finite element analysis of the optimal amount of bone cement [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1404-1409. |
| [4] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [5] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [6] | Xu Yulin, Shen Shi, Zhuo Naiqiang, Yang Huilin, Yang Chao, Li Yang, Zhao Heng, Zhao Lu. Biomechanical comparison of three different plate fixation methods for acetabular posterior column fractures in standing and sitting positions [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 826-830. |
| [7] | Cai Qunbin, Zou Xia, Hu Jiantao, Chen Xinmin, Zheng Liqin, Huang Peizhen, Lin Ziling, Jiang Ziwei. Relationship between tip-apex distance and stability of intertrochanteric femoral fractures with proximal femoral anti-rotation nail: a finite element analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 831-836. |
| [8] | Song Chengjie, Chang Hengrui, Shi Mingxin, Meng Xianzhong. Research progress in biomechanical stability of lateral lumbar interbody fusion [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 923-928. |
| [9] | Xie Chongxin, Zhang Lei. Comparison of knee degeneration after anterior cruciate ligament reconstruction with or without remnant preservation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 735-740. |
| [10] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [11] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [12] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [13] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [14] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [15] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||