Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (1): 107-112.doi: 10.3969/j.issn.2095-4344.0419
Previous Articles Next Articles
Effect of formyl peptide receptor on the differentiation of neural stem/progenitor cells into neurons
Zhang Liang1, Cheng Hui1, Leng Ming-hao1, Pan Jian-hai1, He Xiang-chun1, Zhang Yi-ming1, Lu Shan1, Chen Hua2
- 1Department of Orthopedics, 2Department of Dermatology, Suizhou Hospital Affiliated to Hubei University of Medicine, Suizhou 441300, Hubei Province, China
-
Revised:2017-08-07Online:2018-01-08Published:2018-01-08 -
Contact:Chen Hua, Nurse in charge, Department of Dermatology, Suizhou Hospital Affiliated to Hubei University of Medicine, Suizhou 441300, Hubei Province, China -
About author:Zhang Liang, M.D., Attending physician, Department of Orthopedics, Suizhou Hospital Affiliated to Hubei University of Medicine, Suizhou 441300, Hubei Province, China -
Supported by:the Scientific Research Fund for Health and Family Planning in Hubei Province, No. WJ2015MB22
CLC Number:
Cite this article
Zhang Liang, Cheng Hui, Leng Ming-hao, Pan Jian-hai, He Xiang-chun, Zhang Yi-ming, Lu Shan, Chen Hua. Effect of formyl peptide receptor on the differentiation of neural stem/progenitor cells into neurons[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(1): 107-112.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
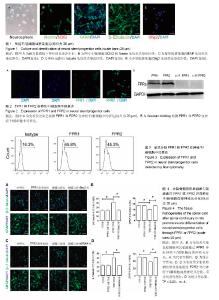
2.1 神经干/祖细胞的培养及鉴定 来源于胎龄12.5 d的胎鼠大脑皮质的细胞,在含有2% B27、20 μg/L表皮生长因子和20 μg/L碱性成纤维细胞生长因子的DMEM/F12培养基中培养7 d后,在倒置显微镜下观察呈典型的神经球(图1A)。 为了进一步鉴定神经球细胞具有干细胞特征,选用两个能够标记神经干/祖细胞特性的分子进行免疫荧光染色:SOX2和Nestin。共聚焦显微镜观察结果显示:几乎所有的细胞胞核SOX2表达阳性,同时大部分细胞胞质Nestin染色阳性(图1B)。 神经干/祖细胞另外一个特征就是能够分化成为类神经元、星形胶质细胞和少突胶质细胞。因此对神经球细胞的分化能力也进行了鉴定。分别用β-Ⅲ tubulin、GFAP和Olig2来标记神经元、星形胶质细胞和少突胶质细胞。共聚焦显微镜观察结果显示:神经球细胞分化后大部分细胞GFAP表达阳性,少部分细胞β-Ⅲ tubulin和Olig2表达阳性,说明培养的神经球细胞具有向类神经元、星形胶质细胞和少突胶质细胞分化的能力,同时说明神经干/祖细胞大部分分化成星形胶质细胞,少部分分化为类神经元和少突胶质细胞(图1C-E)。上述实验结果充分说明在体外成功建立了神经干/祖细胞的培养体系,为进一步实验打下基础。 2.2 FPR1和FPR2在神经干/祖细胞中的表达 免疫荧光染色共聚焦检测结果显示:部分细胞表达FPR1和FPR2,同时二者主要表达在细胞膜和细胞质中;FPR2的免疫荧光强度明显高于FPR1(图2A)。为了进一步证实这种结果,又进行了Western blotting实验,结果提示:神经干/祖细胞表达FPR1和FPR2,同时FPR2的表达量要比FPR1多(图2B)。 2.3 流式分析FPR1和FPR2在神经干/祖细胞中的表达 为了进一步观察神经干/祖细胞中FPR1和FPR2的表达水平,又进行了流式细胞仪检测,结果提示:神经球细胞中,大约有45.8%的细胞表达FPR1,大约有45.3%的细胞表达FPR2(图3)。 2.4 大鼠脊髓损伤后组织匀浆液通过FPR1或FPR2导向神经干/祖细胞向类神经元分化 为了观察脊髓损伤后产生的FPR1和FPR2配体是否能够通过神经干/祖细胞表达的FPR1和FPR2受体对神经干/祖细胞分化产生影响,实验首先提取了大鼠脊髓损伤后2 d的匀浆液,又用流式细胞仪分选出FPR1或FPR2阳性的神经干/祖细胞。共聚焦显微镜 观察结果显示:与对照组相比,匀浆液能够使FPR1或FPR2阳性神经干/祖细胞分化后产生的β-Ⅲ tubulin阳性神经元比例升高,这种作用能够被FPR1或FPR2阻断剂Boc2或WRW4阻断。同时,与对照组相比,匀浆液能够使FPR1或FPR2阳性神经干/祖细胞分化后产生的GFAP阳性星形胶质细胞比例降低,这种作用能够被FPR1或FPR2阻断剂Boc2或WRW4阻断(图4A-D)。这些实验结果说明匀浆液能够导向FPR1或FPR2阳性神经干/祖细胞向类神经元分化,抑制其向星形胶质细胞分化,同时这种作用具有特异性。"

| [1] Yousefifard M, Rahimi-Movaghar V, Nasirinezhad F, et al. Neural stem/progenitor cell transplantation for spinal cord injury treatment; A systematic review and meta-analysis. Neuroscience. 2016;322:377-397.[2] Piltti KM, Avakian SN, Funes GM, et al. Transplantation dose alters the dynamics of human neural stem cell engraftment, proliferation and migration after spinal cord injury. Stem Cell Res. 2015;15(2):341-353.[3] Takeuchi H, Natsume A, Wakabayashi T, et al. Intravenously transplanted human neural stem cells migrate to the injured spinal cord in adult mice in an SDF-1- and HGF-dependent manner. Neurosci Lett. 2007;426(2):69-74.[4] Mothe AJ, Tator CH. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int J Dev Neurosci. 2013;31(7):701-713.[5] Mothe AJ, Tam RY, Zahir T, et al. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials. 2013;34(15): 3775-3783. [6] McCreedy DA, Sakiyama-Elbert SE. Combination therapies in the CNS: engineering the environment. Neurosci Lett. 2012; 519(2):115-121.[7] Lundberg C, Martínez-Serrano A, Cattaneo E, et al. Survival, integration, and differentiation of neural stem cell lines after transplantation to the adult rat striatum. Exp Neurol. 1997; 145(2 Pt 1):342-360.[8] Gao JL, Chen H, Filie JD, et al. Differential expansion of the N-formylpeptide receptor gene cluster in human and mouse. Genomics. 1998;51(2):270-276.[9] Bao L, Gerard NP, Eddy RL Jr, et al. Mapping of genes for the human C5a receptor (C5AR), human FMLP receptor (FPR), and two FMLP receptor homologue orphan receptors (FPRH1, FPRH2) to chromosome 19. Genomics. 1992;13(2):437-440.[10] Murphy PM, Ozçelik T, Kenney RT, et al. A structural homologue of the N-formyl peptide receptor. Characterization and chromosome mapping of a peptide chemoattractant receptor family. J Biol Chem. 1992;267(11):7637-7643.[11] Ye RD, Cavanagh SL, Quehenberger O, et al. Isolation of a cDNA that encodes a novel granulocyte N-formyl peptide receptor. Biochem Biophys Res Commun. 1992;184(2): 582-589.[12] Zhang L, Wang G, Chen X, et al. Formyl peptide receptors promotes neural differentiation in mouse neural stem cells by ROS generation and regulation of PI3K-AKT signaling. Sci Rep. 2017;7(1):206.[13] Wang G, Zhang L, Chen X, et al. Formylpeptide Receptors Promote the Migration and Differentiation of Rat Neural Stem Cells. Sci Rep. 2016;6:25946.[14] Liu M, Chen K, Yoshimura T, et al. Formylpeptide receptors mediate rapid neutrophil mobilization to accelerate wound healing. PLoS One. 2014;9(6):e90613.[15] Chiang N, Serhan CN, Dahlén SE, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58(3):463-487.[16] Ying G, Iribarren P, Zhou Y, et al. Humanin, a newly identified neuroprotective factor, uses the G protein-coupled formylpeptide receptor-like-1 as a functional receptor. J Immunol. 2004;172(11):7078-7085.[17] He R, Sang H, Ye RD. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood. 2003;101(4):1572-1581.[18] He N, Jin WL, Lok KH, et al. Amyloid-β(1-42) oligomer accelerates senescence in adult hippocampal neural stem/progenitor cells via formylpeptide receptor 2. Cell Death Dis. 2013;4:e924. [19] Resnati M, Pallavicini I, Wang JM, et al. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci U S A. 2002;99(3):1359-1364.[20] Liu Y, Liu H, Sauvey C, et al. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nat Protoc. 2013;8(9):1670-1679.[21] Fehlings MG, Tator CH, Linden RD, et al. Motor and somatosensory evoked potentials recorded from the rat. Electroencephalogr Clin Neurophysiol. 1988;69(1):65-78.[22] Cheng TY, Wu MS, Lin JT, et al. Annexin A1 is associated with gastric cancer survival and promotes gastric cancer cell invasiveness through the formyl peptide receptor/extracellular signal-regulated kinase/integrin beta-1-binding protein 1 pathway. Cancer. 2012;118(23):5757-5767.[23] Bizzarro V, Belvedere R, Milone MR, et al. Annexin A1 is involved in the acquisition and maintenance of a stem cell-like/aggressive phenotype in prostate cancer cells with acquired resistance to zoledronic acid. Oncotarget. 2015;6(28):25076-25092.[24] Khau T, Langenbach SY, Schuliga M, et al. Annexin-1 signals mitogen-stimulated breast tumor cell proliferation by activation of the formyl peptide receptors (FPRs) 1 and 2. FASEB J. 2011;25(2):483-496.[25] Liu M, Chen K, Yoshimura T, et al. Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci Rep. 2012;2:786.[26] Rabiet MJ, Huet E, Boulay F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur J Immunol. 2005;35(8):2486-2495.[27] Marasco WA, Phan SH, Krutzsch H, et al. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J Biol Chem. 1984;259(9):5430-5439.[28] Betten A, Bylund J, Christophe T, et al. A proinflammatory peptide from Helicobacter pylori activates monocytes to induce lymphocyte dysfunction and apoptosis. J Clin Invest. 2001;108(8):1221-1228.[29] Deng X, Ueda H, Su SB, et al. A synthetic peptide derived from human immunodeficiency virus type 1 gp120 downregulates the expression and function of chemokine receptors CCR5 and CXCR4 in monocytes by activating the 7-transmembrane G-protein-coupled receptor FPRL1/LXA4R. Blood. 1999;94(4):1165-1173.[30] Gensel JC, Nakamura S, Guan Z, et al. Macrophages promote axon regeneration with concurrent neurotoxicity. J Neurosci. 2009;29(12):3956-3968.[31] Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20(3):269-287.[32] Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23(11):541-548.[33] Shin MK, Jang YH, Yoo HJ, et al. N-formyl-methionyl-leucyl- phenylalanine (fMLP) promotes osteoblast differentiation via the N-formyl peptide receptor 1-mediated signaling pathway in human mesenchymal stem cells from bone marrow. J Biol Chem. 2011 ;286(19):17133-17143.[34] Viswanathan A, Painter RG, Lanson NA Jr, et al. Functional expression of N-formyl peptide receptors in human bone marrow-derived mesenchymal stem cells. Stem Cells. 2007; 25(5):1263-1269.[35] Kim MK, Min do S, Park YJ, et al. Expression and functional role of formyl peptide receptor in human bone marrow-derived mesenchymal stem cells. FEBS Lett. 2007;581(9):1917-1922.[36] Le Y, Hu J, Gong W, et al. Expression of functional formyl peptide receptors by human astrocytoma cell lines. J Neuroimmunol. 2000;111(1-2):102-108.[37] Sozzani S, Sallusto F, Luini W, et al. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J Immunol. 1995;155(7):3292-3295.[38] Becker EL, Forouhar FA, Grunnet ML, et al. Broad immunocytochemical localization of the formylpeptide receptor in human organs, tissues, and cells. Cell Tissue Res. 1998;292(1):129-135.[39] Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J Exp Med. 1982;155(1): 264-275. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [3] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| [4] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [5] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [6] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [7] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [8] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [9] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [10] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [11] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [12] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [13] | Zhou Wu, Wang Binping, Wang Yawen, Cheng Yanan, Huang Xieshan. Transforming growth factor beta combined with bone morphogenetic protein-2 induces the proliferation and differentiation of mouse MC3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(23): 3630-3635. |
| [14] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [15] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||