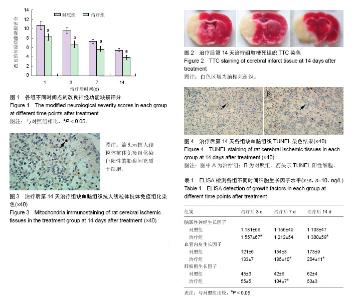
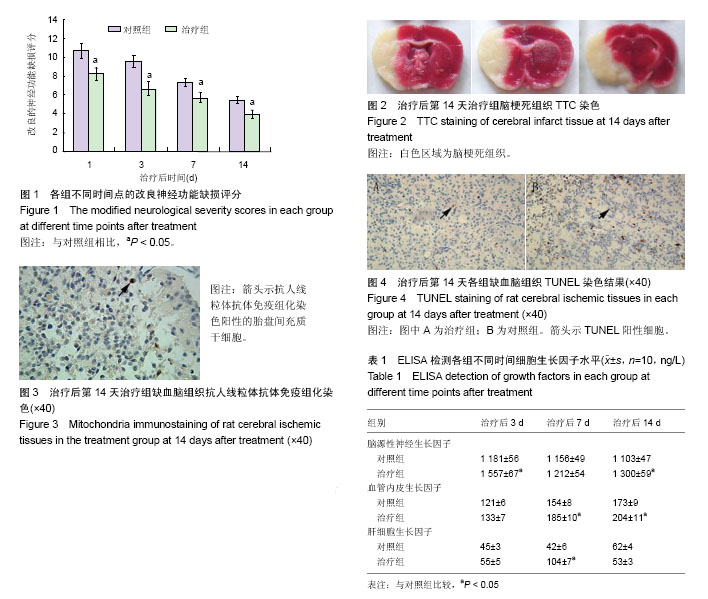
| [1] Schäfer R, Bieback K. Characterization of mesenchymal stem or stromal cells: tissue sources, heterogeneity, and function. Transfusion. 2016;56(4):2S-5S.[2] Jeon YJ, Kim J, Cho JH, et al. Comparative Analysis of Human Mesenchymal Stem Cells Derived From Bone Marrow, Placenta, and Adipose Tissue as Sources of Cell Therapy. J Cell Biochem. 2016; 117(5):1112-1125.[3] Shichinohe H, Ishihara T, Takahashi K, et al. Bone marrow stromal cells rescue ischemic brain by trophic effects and phenotypic change toward neural cells. Neurorehabil Neural Repair. 2015;29(1):80-89.[4] Chen J, Shehadah A, Pal A, et al. Neuroprotective effect of human placenta-derived cell treatment of stroke in rats. Cell Transplant. 2013;22(5):871-879.[5] Trueman RC, Diaz C, Farr TD, et al. Systematic and detailed analysis of behavioural tests in the rat middle cerebral artery occlusion model of stroke: Tests for long-term assessment. J Cereb Blood Flow Metab. 2017;37(4):1349-1361.[6] Ye X, Hu J, Cui G. Therapy Effects of Bone Marrow Stromal Cells on Ischemic Stroke. Oxid Med Cell Longev. 2016;2016:7682960.[7] Kanawa M, Igarashi A, Ronald VS, et al. Age-dependent decrease in the chondrogenic potential of human bone marrow mesenchymal stromal cells expanded with fibroblast growth factor-2. Cytotherapy. 2013;15(9):1062-1072.[8] Luan X, Li G, Wang G, et al. Human placenta-derived mesenchymal stem cells suppress T cell proliferation and support the culture expansion of cord blood CD34? cells: a comparison with human bone marrow-derived mesenchymal stem cells. Tissue Cell. 2013; 45(1):32-38.[9] Prather WR, Toren A, Meiron M, et al. The role of placental-derived adherent stromal cell (PLX-PAD) in the treatment of critical limb ischemia. Cytotherapy. 2009;11(4):427-434.[10] Cargnoni A, Gibelli L, Tosini A, et al. Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis. Cell Transplant. 2009;18(4):405-422.[11] Yuan W, Zong C, Huang Y, et al. Biological, immunological and regenerative characteristics of placenta-derived mesenchymal stem cell isolated using a time-gradient attachment method. Stem Cell Res. 2012;9(2):110-123.[12] Kranz A, Wagner DC, Kamprad M, et al. Transplantation of placenta-derived mesenchymal stromal cells upon experimental stroke in rats. Brain Res. 2010;1315:128-136. [13] Chang CJ, Yen ML, Chen YC, et al. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24(11):2466-2477.[14] Radak D, Katsiki N, Resanovic I, et al. Apoptosis and Acute Brain Ischemia in Ischemic Stroke. Curr Vasc Pharmacol. 2017;15(2): 115-122.[15] Catanese L, Tarsia J, Fisher M. Acute Ischemic Stroke Therapy Overview. Circ Res. 2017;120(3):541-558.[16] Yarygin KN, Kholodenko IV, Konieva AA, et al. Mechanisms of positive effects of transplantation of human placental mesenchymal stem cells on recovery of rats after experimental ischemic stroke. Bull Exp Biol Med. 2009;148(6):862-868.[17] Dulamea AO. The potential use of mesenchymal stem cells in stroke therapy--From bench to bedside. J Neurol Sci. 2015;352(1-2):1-11.[18] Lindvall O, Kokaia Z. Stem cell research in stroke: how far from the clinic. Stroke. 2011;42(8):2369-2375. [19] Borlongan CV, Hadman M, Sanberg CD, et al. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35(10): 2385-2389.[20] De Feo D, Merlini A, Laterza C, et al. Neural stem cell transplantation in central nervous system disorders: from cell replacement to neuroprotection. Curr Opin Neurol. 2012;25(3):322-333.[21] Huang B, Tabata Y, Gao JQ. Mesenchymal stem cells as therapeutic agents and potential targeted gene delivery vehicle for brain diseases. J Control Release. 2012;162(2):464-473.[22] Yang T, Tsang KS, Poon WS, et al. Neurotrophism of bone marrow stromal cells to embryonic stem cells: noncontact induction and transplantation to a mouse ischemic stroke model. Cell Transplant. 2009;18(4):391-404.[23] Yasuhara T, Hara K, Maki M, et al. Mannitol facilitates neurotrophic factor up-regulation and behavioural recovery in neonatal hypoxic-ischaemic rats with human umbilical cord blood grafts. J Cell Mol Med. 2010;14(4):914-921.[24] Bronckaers A, Hilkens P, Martens W, et al. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143(2):181-196.[25] Chen X, Li Y, Wang L, et al. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22(4):275-279.[26] Nishishita T, Ouchi K, Zhang X, et al. A potential pro-angiogenic cell therapy with human placenta-derived mesenchymal cells. Biochem Biophys Res Commun. 2004;325(1):24-31.[27] Guan W, Somanath PR, Kozak A, et al. Vascular protection by angiotensin receptor antagonism involves differential VEGF expression in both hemispheres after experimental stroke. PLoS One. 2011;6(9):e24551.[28] Rajpathak SN, Wang T, Wassertheil-Smoller S, et al. Hepatocyte growth factor and the risk of ischemic stroke developing among postmenopausal women: results from the Women's Health Initiative. Stroke. 2010;41(5):857-862.[29] Doeppner TR, Kaltwasser B, ElAli A, et al. Acute hepatocyte growth factor treatment induces long-term neuroprotection and stroke recovery via mechanisms involving neural precursor cell proliferation and differentiation. J Cereb Blood Flow Metab. 2011;31(5):1251-1262.[30] Han Q, Li B, Feng H, et al. The promotion of cerebral ischemia recovery in rats by laminin-binding BDNF. Biomaterials. 2011; 32(22):5077-5085. |