Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (30): 4885-4892.doi: 10.3969/j.issn.2095-4344.2017.30.022
Previous Articles Next Articles
Application of chitosan-based hydrogels carrying antimicrobial agents in wound healing
- Department of Orthopedics, Second Hospital of Jilin University, Changchun 130041, Jilin Province, China
-
Received:2017-05-07Online:2017-10-28Published:2017-11-07 -
Contact:Wang Jin-cheng, Chief physician, Professor, Ph.D. supervisor, Department of Orthopedics, Second Hospital of Jilin University, Changchun 130041, Jilin Province, China -
About author:Li Zu-hao, Studying for master’s degree, Department of Orthopedics, Second Hospital of Jilin University, Changchun 130041, Jilin Province, China -
Supported by:the National Natural Science Foundation of China, No. 81671804; the Natural Science Foundation of Jilin Province, No. 20160101109JC, 20150414006GH, 20150312028ZG, 20130206060GX; the Excellent Talent Culture Plan for Doctoral Candidates in the Norman Bethune Health Science Center of Jilin University, No. YB201501; the Graduate Innovation Research Plan of Jilin University, No. 2017032
CLC Number:
Cite this article
Li Zu-hao, Wang Chen-yu, Wang Zhong-han, Zhong Lei, Li Chen, Qin Yan-guo, Liu He, Wang Jin-cheng.
share this article
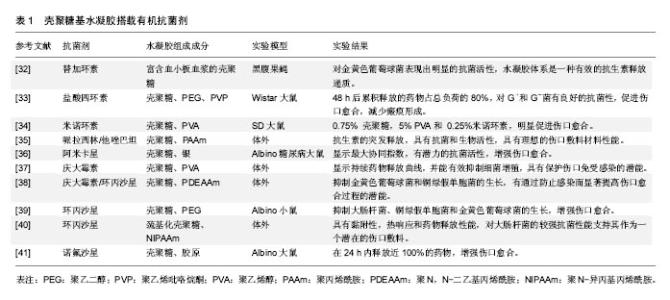
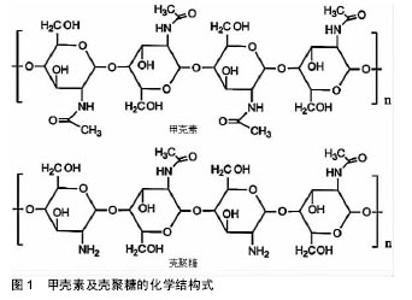
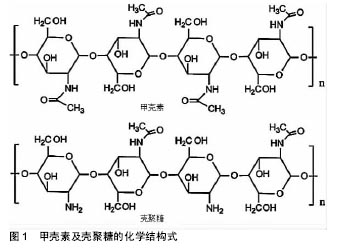
壳聚糖的一些基团,如氨基和羟基容易发生反应而变性,使其具有较高的化学通用性。使用氨基的化学修饰会影响脱乙酰度,所以得到的材料带有阳离子性质。壳聚糖易溶解在相对低pH值溶液中[18]。在酸性条件下,壳聚糖带有正电荷,使其更容易与存在于皮肤伤口的带负电荷分子(如蛋白质、阴离子多糖和核酸)相互作用[19]。此外,壳聚糖还具有成膜能力、温和的凝胶特性以及强大的粘接伤口组织的性能[20],一方面壳聚糖能与黏液和上皮细胞相互作用,并最终导致细胞的紧密连接开放,从而增加上皮通透性,另一方面,这种聚合物的其他结构元素可能有助于他们的渗透活性增强[21]。此外,壳聚糖及其衍生物还能够促进血液凝固和提高伤口愈合速度。特别是低脱乙酰度的壳聚糖膜修复皮肤表面伤口已被证明是有显著效果的[22]。其他的研究也表明该材料可以增强巨噬细胞和中性粒细胞的炎症作用,为肉芽组织提供一个适当的炎症反应环境[23]。 壳聚糖通过与其他聚合物或交联剂进行络合或者交联可以改变及提高其理化性质和机械性质。通过这种方法,设计具有增强愈合效果的壳聚糖基水凝胶敷料,这种水凝胶具有增强黏附和抗菌性能,增加渗出物的吸收能力,刺激血管生成、皮肤组织再上皮化和胶原沉积,抗菌剂缓释等。壳聚糖基水凝胶保护伤口防止继发感染,清除创面渗出,促进组织再生,并具有良好的生物相容性[6,24-25]。对于慢性难愈性感染伤口,壳聚糖基水凝胶搭载抗菌剂在提高伤口局部药物有效浓度,减少全身性药物副反应,抑制微生物生长,促进伤口愈合方面发挥着特有的优势。 2.2 壳聚糖基水凝胶搭载抗菌剂的应用 金黄色葡萄球菌、表皮葡萄球菌、大肠杆菌、铜绿假单胞菌和白色念珠菌是伤口感染的常见病原体[26]。具有抗菌作用的壳聚糖基水凝胶作为伤口敷料使用是非常有吸引力的。由于其高的水含量,为伤口区域提供了一个潮湿的、大量水化的愈合环境,以及促进伤口愈合过程中必不可少的细胞免疫活性。然而,这种相同的水化环境也可以促进微生物感染[27]。这是一对矛盾,尤其是在一些感染较为严重的慢性伤口中。因此,对于慢性感染性伤口,壳聚糖基水凝胶除了发挥其主要功能(如伤口愈合等),加强抗菌作用也是必要的。壳聚糖基水凝胶本身具有抗菌性能,对于无感染的急性伤口可单独使用。壳聚糖水凝胶的早期治疗已广泛应用于皮肤创面和妇科阴道炎。然而,随着耐药菌的增加,壳聚糖水凝胶作为一种携带其他抗菌剂的输送系统引起了人们的极大关注。材料中可以加入随时间释放的抗菌剂,或将广谱抗菌药物如抗菌肽(AMPs)、金属银等共价修饰固定在材料表面[28-29]。 通常抗菌剂可分为两类,一类是有机抗菌剂,如抗生素及人工合成抗菌药、有机矿物盐等;另一类是无机抗菌剂,如银、锌、铜等及其金属氧化物。壳聚糖基水凝胶的搭载抗菌剂已被广泛研究,在这里,将综述使用壳聚糖基水凝胶搭载抗菌剂在抑制伤口感染和促进伤口愈合方面的研究进展。 2.2.1 有机抗菌剂 伤口愈合过程中感染的情况越来越常见,如糖尿病溃疡、烧伤等愈合过程。El-Naggar等[30]从糖尿病患者分离获得65种细菌,其中金黄色葡萄球菌(37%)和铜绿假单胞菌(18.5%)是溃疡的优势菌群。Heunis等[31]在文献中也提到金黄色葡萄球菌是一种致命的病原体,也是浅表性和浸润性皮肤和软组织感染的主要病因。 无论是口服还是注射给药,抗生素(如青霉素类、大环内酯类、四环素类、多烯类等)及人工合成抗菌药(磺胺类、喹诺酮类、呋喃类药物等)对于感染都有着极为广泛的应用。近年来,在伤口局部应用抗菌剂引起了研究者兴趣,因为这样可以加强伤口局部的药物浓度,而不对身体其他部位产生明显的药物作用。针对不同病原体,选择特定抗菌药物搭载于壳聚糖基水凝胶,可以很好地起到抑制感染、促进伤口愈合的作用。 Nimal等[32]将纳米替加环素搭载于壳聚糖基水凝胶中,在体内稳定地释放,很大程度上抑制细菌生长,这个水凝胶体系是抗生素的有效递质,可应用于感染部位,有效防止金黄色葡萄球菌引起的各种皮肤感染。Anjum等[33]通过棉织物涂层混合壳聚糖、聚乙二醇和聚乙烯吡咯烷酮制备抗菌及瘢痕预防伤口敷料。在水凝胶基质内加入盐酸四环素为模型药物。48 h后累积释放的药物占总负荷的80%,载药的敷料表现出对革兰阳性和革兰阴性细菌良好的抗菌性。与对照组相比,搭载盐酸四环素的壳聚糖基水凝胶组观察到伤口快速愈合与最小的瘢痕。这些结果表明,搭载盐酸四环素的壳聚糖基水凝胶敷料可以抑制细菌生长,促进伤口愈合,减少瘢痕形成。 此外,四环素、万古霉素、庆大霉素、哌拉西林/他唑巴坦、卡那霉素、环丙沙星等搭载于壳聚糖基水凝胶,见表1。最近的研究已经证明了含有机抗菌剂的壳聚糖基水凝胶敷料能有效减少细菌感染,有利于肉芽组织的形成和刺激更快的伤口愈合[32-41]。 "
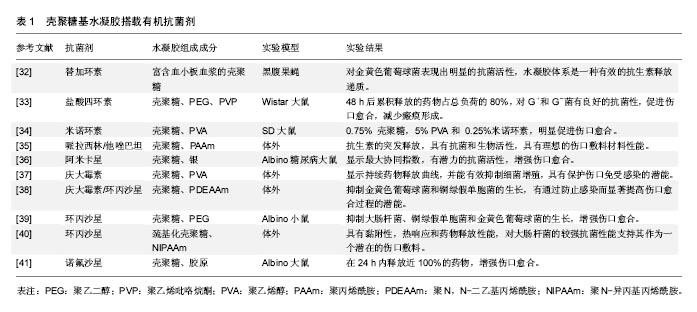
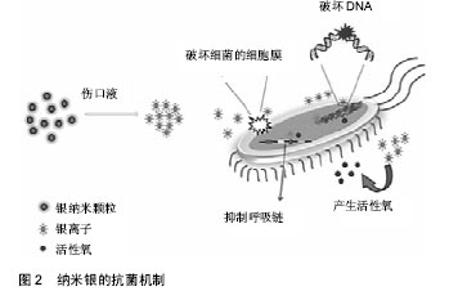
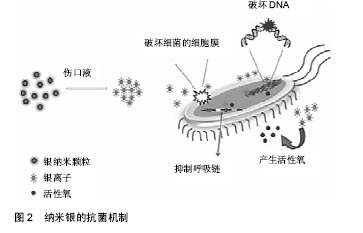
2.2.2 无机抗菌剂 抗生素只在一个方面攻击细菌,细菌适应后可产生耐药性。感染创口耐药细菌的发展是医生面临的一个挑战。细菌耐药的出现通常是由于滥用和过度使用抗生素,急需抗生素的替代品来应对耐药性问题[42]。 银基抗菌剂:银是一种广谱抗菌剂,通过多种机制对微生物作用,显著降低耐药的机会。20世纪60年代莫耶记录了烧伤后银的抗菌效果[43]。纳米银由于其更好的渗透和保留效果,与离子银相比是一种更有效的抗菌剂[44]。一些含银伤口敷料已经通过FDA批准上市,如 ActicoatTM,Bactigrass®,SilvaSorb®,Fucidin®,PolyMem® Silver等。 随着纳米银敷料的广泛研究,其毒性是一个无法忽视的问题。以前认为纳米银的毒性是银离子和银纳米粒子的联合作用[45],但最近的一项研究证明纳米银的毒性完全是由银离子的释放引起的[46]。银纳米粒子与水分或伤口渗出液接触后在伤口表面被氧化,并释放银离子。这些离子附着在细菌细胞膜上,通过与细菌膜中的硫蛋白相互作用引起膜的损伤,并进入细菌内,破坏DNA[47-48]。它攻击细菌的呼吸链,抑制细胞分裂,并最终导致细胞死亡。银离子可以产生自由基,并诱导氧化应激进一步促进杀菌活动。图2显示以纳米银为代表的金属敷料的抗菌机制。但纳米银可能对人类真核细胞有相同的效果,研究表明纳米银水凝胶敷料对人皮肤成纤维细胞的细胞毒作用呈浓度依赖性[49]。因此,建立一个治疗窗,银被控制在一个范围内,既可以抑制细菌,又不会对人体细胞产生毒性,这对于壳聚糖基纳米银水凝胶的应用是一个极为关键的问题。"
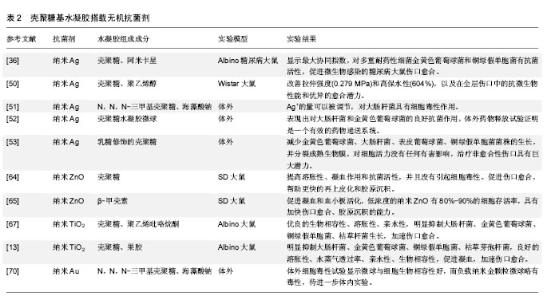
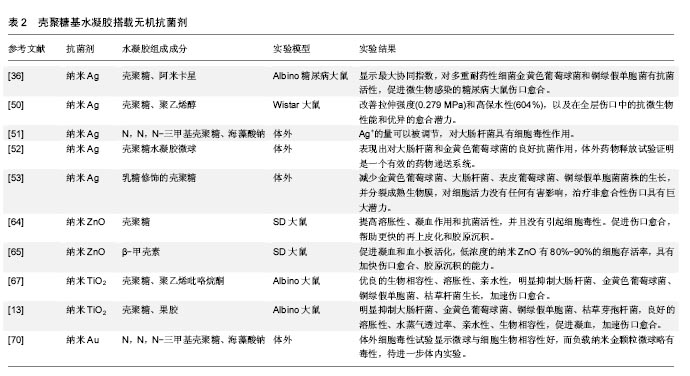
从糖尿病患者中分离出56株细菌株,用10种抗生素对分离菌株进行体外试验,最耐药的金黄色葡萄球菌和铜绿假单胞菌被挑选出来进一步研究。3种来源的壳聚糖分别螯合银纳米粒子进行测试,结果显示虾来源的壳聚糖基纳米银水凝胶结合阿米卡星对耐药细菌具有最高的活性,混合使用表现出最大的协同指数,明显加速伤口愈合[36]。此外,纳米银搭载于壳聚糖基水凝胶已被广泛研究[50-53],其结果均表明搭载纳米银的壳聚糖基水凝胶明显抑制微生物,促进伤口愈合,在伤口敷料方面具有极大的应用前景。 锌基抗菌剂:锌作为人体所需的元素,也是一种重要的抗菌剂,其优势在于较好的抗菌性,对于某些耐抗生素的菌株同样有效。锌的抗菌机制与银相似,与银相比,虽抗菌作用相对较弱,但毒性也较低[54-55],Iyigundogdu等[56]发现锌离子对细菌(大肠杆菌、铜绿假单胞菌和金黄色葡萄球菌)、酵母菌(白色念珠菌和光滑念珠菌)和真菌都有一定抑制作用。相关文献提到单纯的锌离子抗菌作用弱,因此氧化锌才是发挥抗菌作用的主要形式。目前的研究以氧化锌的纳米形式为主,氧化锌的抗菌机制较为复杂,这也使得细菌不容易对其产生耐药[57]。 氧化锌纳米粒子在体外对人体角质形成细胞、神经细胞、表皮细胞的遗传毒性已被报道[58-60]。但是,Nair等[61]报道称纳米氧化锌具有有效的抗菌活性,但适当浓度在体内对正常细胞没有不利影响。这些在体外能产生毒性的浓度可能与体内结果矛盾[62-63]。因此,进行动物体内实验,研制适当浓度的含锌水凝胶敷料,充分发挥抗菌作用和促进伤口愈合的同时又不对细胞产生毒性,这是十分重要的。 Kumar等[64]对纳米氧化锌进行了一系列的研究。他们将纳米氧化锌搭载于壳聚糖基水凝胶中制备了一种柔性多微孔的伤口敷料,结果表明这一材料能提高溶胀性、凝血作用和抗菌活性,并且没有引起细胞毒性。此外,在大鼠体内实验显示,该纳米复合材料能促进伤口愈合,帮助更快的再上皮化和胶原沉积。所得到的结果表明该复合材料可用于烧伤创面、慢性伤口、糖尿病足溃疡。此外,还评估了在β-壳聚糖中加载纳米氧化锌促进伤口愈合的潜力,结果表明促进凝血功能和血小板活化,伤口愈合加快,胶原沉积能力提高[65]。 钛基抗菌剂:二氧化钛的纳米颗粒已被用于化妆品和过滤器,表现出强大的杀菌性能和消除异味的能力,并结合银作为抗菌剂。Jayakumar等[66]研究了甲壳素-壳聚糖/纳米TiO2复合支架,利用成骨细胞、成纤维细胞和人间充质干细胞进行细胞相容性和细胞附着研究。结果表明没有明显的毒性,细胞附着在支架内的孔壁上。它被认为是无毒的,并已由美国食品和药物管理局(FDA)批准用于人类食品、药物、化妆品和食品接触材料[67]。从纳米颗粒中缓慢释放的钛离子可以抑制微生物的增殖,因此它能够加速伤口愈合[68]。据报道壳聚糖-二氧化钛复合膜具有良好的表面性能和杀菌活性[69]。 Archana等[67]制备了壳聚糖-PVP-二氧化钛复合物作为伤口敷料,结果显示该材料具有较好的生物相容性,明显抑制大肠杆菌、金黄色葡萄球菌、铜绿假单胞菌、枯草杆菌生长,促进伤口愈合。此外,Archana等[13]还将二氧化钛搭载于壳聚糖-果胶,明显抑制大肠杆菌、金黄色葡萄球菌、铜绿假单胞菌、枯草芽孢杆菌,具有良好的溶胀性、水蒸气透过率、亲水性、生物相容性,促进凝血,加速伤口愈合。 其他金属基抗菌剂:除上述几种金属外,其他金属的抗菌性能也逐渐被研究者发现。Martins等[70]成功制备了N,N,N-三甲基壳聚糖/海藻酸钠复合物负载纳米金(AuNPs)的水凝胶微球,该复合物具有较好的生物相容性,表征显示有作为伤口敷料的潜质,其应用有待进一步体内实验研究证明。有研究表明Au对金黄色葡萄球菌有着显著的抗菌性能[71]。此外,Al2O3对枯草芽孢杆菌、大肠杆菌[72],CuO对枯草芽孢杆菌和恶臭假单胞菌KT2440[73-74],Cu掺杂TiO2对耻垢分枝杆菌[75],氧化镍对大肠杆菌显著的抗菌活性一一被报道[76]。这些金属及氧化物搭载于壳聚糖基水凝胶作为伤口敷料的研究有待进一步开展。 大量涉及使用无机抗菌剂(如银、锌、铜和其他金属及其氧化物)搭载于壳聚糖基水凝胶中作为伤口敷料促进伤口愈合的研究已被报道,尤其是纳米银的应用最为广泛,见表2。"
| [1]Boucard N, Viton C, Agay D, et al. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials. 2007;28(24):3478-3488.[2]Gupta B, Agarwal R, Alam MS. Textile-based smart wound dressings. Indian J Fibre Text. 2010;35(2):174-187.[3]Choudhury AJ, Gogoi D, Chutia J, et al. Controlled antibiotic-releasing Antheraea assama silk fibroin suture for infection prevention and fast wound healing. Surgery. 2016; 159(2):539-547.[4]Obermeier A, Schneider J, Wehner S, et al. Novel high efficient coatings for anti-microbial surgical sutures using chlorhexidine in fatty acid slow-release carrier systems. PLoS One. 2014;9(7):e101426.[5]Schreml S, Szeimies RM, Prantl L,et al. Wound healing in the 21st century. J Am Acad Dermatol. 2010;63(5):866-881.[6]Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. 2010; 62(1):83-99.[7]符旭东,郭家平.壳聚糖类水凝胶在组织工程领域的研究进展[J].中国医院药学杂志,2010,30(15):1308-1310.[8]Amsden B. Novel biodegradable polymers for local growth factor delivery. Eur J Pharm Biopharm. 2015;97(Pt B): 318-328.[9]Tessmar JK, Göpferich AM. Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Deliv Rev. 2007; 59(4-5):274-291.[10]Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869-1879.[11]Jayakumar R, Prabaharan M, Sudheesh Kumar PT, et al. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29(3):322-337.[12]Elviri L, Bianchera A, Bergonzi C, et al. Controlled local drug delivery strategies from chitosan hydrogels for wound healing. Expert Opin Drug Deliv. 2016. [Epub ahead of print][13]Archana D, Dutta J, Dutta PK. Evaluation of chitosan nano dressing for wound healing: characterization, in vitro and in vivo studies. Int J Biol Macromol. 2013;57:193-203.[14]Dai T, Tanaka M, Huang YY, et al.Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects (vol 9, pg 857, 2011). Expert Rev Anti-Infe. 2013; 11(8):866.[15]Pérez RA, Won JE, Knowles JC, et al. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv Drug Deliv Rev. 2013;65(4):471-496.[16]Ferreira MO, Leite LL, de Lima IS, et al. Chitosan Hydrogel in combination with Nerolidol for healing wounds. Carbohydr Polym. 2016;152:409-418.[17]Li M, Ke QF, Tao SC, et al. Fabrication of hydroxyapatite/ chitosan composite hydrogels loaded with exosomes derived from miR-126-3p overexpressed synovium mesenchymal stem cells for diabetic chronic wound healing. J Mater Chem B. 2016;4(42):6830-6841.[18]Zubair M, Malik A, Ahmad J. Clinico-microbiological study and antimicrobial drug resistance profile of diabetic foot infections in North India. Foot (Edinb). 2011;21(1):6-14.[19]Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. 2010; 62(1):83-99.[20]Jayakumar R, Prabaharan M, Sudheesh Kumar PT, et al. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29(3):322-337.[21]Amidi M, Mastrobattista E, Jiskoot W, et al. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010;62(1):59-82.[22]Borderud SP, Li Y, Burkhalter JE, et al. Electronic cigarette use among patients with cancer: Characteristics of electronic cigarette users and their smoking cessation outcomes (vol 120, pg 3527, 2014). Cancer-Am Cancer Soc. 2015;121(5): 800-801.[23]王冰洋,牛广明,杜华,等.不同敷料在糖尿病足溃疡伤口治疗中的研究与应用[J].中国组织工程研究,2016,20(34): 5155-5162.[24]Moura LI, Dias AM, Carvalho E, et al. Recent advances on the development of wound dressings for diabetic foot ulcer treatment--a review. Acta Biomater. 2013;9(7):7093-7114.[25]Hennink WE, van Nostrum CF. Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev. 2002;54(1):13-36.[26]Doyle JS, Buising KL, Thursky KA, et al. Epidemiology of infections acquired in intensive care units. Semin Respir Crit Care Med. 2011;32(2):115-138.[27]Yousafzai MT, Siddiqui AR, Rozi S, et al. Determinants of Compliance with Universal Precautions at First Level Care Facilities in North West Frontier Province of Pakistan. Am J Epidemiol. 2010;171:120-137.[28]Gao G, Lange D, Hilpert K, et al. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials. 2011;32(16):3899-3909.[29]Flores CY, Diaz C, Rubert A, et al. Spontaneous adsorption of silver nanoparticles on Ti/TiO2 surfaces. Antibacterial effect on Pseudomonas aeruginosa. J Colloid Interface Sci. 2010; 350(2):402-408.[30]El-Naggar MY, Gohar YM, Sorour MA, et al. Hydrogel Dressing with a Nano-Formula against Methicillin-Resistant Staphylococcus aureus and Pseudomonas aeruginosa Diabetic Foot Bacteria. J Microbiol Biotechnol. 2016; 26(2): 408-420.[31]Heunis TD, Smith C, Dicks LM. Evaluation of a nisin-eluting nanofiber scaffold to treat Staphylococcus aureus-induced skin infections in mice. Antimicrob Agents Chemother. 2013; 57(8):3928-3935.[32]Nimal TR, Baranwal G, Bavya MC, et al. Anti-staphylococcal Activity of Injectable Nano Tigecycline/Chitosan-PRP Composite Hydrogel Using Drosophila melanogaster Model for Infectious Wounds. ACS Appl Mater Interfaces. 2016; 8(34):22074-22083.[33]Anjum S, Arora A, Alam MS, et al. Development of antimicrobial and scar preventive chitosan hydrogel wound dressings. Int J Pharm. 2016;508(1-2):92-101.[34]Sung JH, Hwang MR, Kim JO, et al. Gel characterisation and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int J Pharm. 2010;392(1-2):232-240.[35]Pulat M, Kahraman AS, Tan N, et al. Sequential antibiotic and growth factor releasing chitosan-PAAm semi-IPN hydrogel as a novel wound dressing. J Biomater Sci Polym Ed. 2013;24(7): 807-819.[36]El-Naggar MY, Gohar YM, Sorour MA, et al. Hydrogel Dressing with a Nano-Formula against Methicillin-Resistant Staphylococcus aureus and Pseudomonas aeruginosa Diabetic Foot Bacteria. J Microbiol Biotechnol. 2016;26(2): 408-420.[37]Zhang D, Zhou W, Wei B, et al. Carboxyl-modified poly(vinyl alcohol)-crosslinked chitosan hydrogel films for potential wound dressing. Carbohydr Polym. 2015;125:189-199.[38]Ngadaonye JI, Geever LM, McEvoy KE, et al. Evaluation of Novel Antibiotic-Eluting Thermoresponsive Chitosan-PDEAAm Based Wound Dressings. Int J Polym Mater. 2014;63(17):873-883.[39]Sinha M, Banik RM, Haldar C, et al. Development of ciprofloxacin hydrochloride loaded poly(ethylene glycol)/chitosan scaffold as wound dressing. J Porous Mat. 2012;20(4):799-807.[40]Radhakumary C, Antonty M, Sreenivasan K. Drug loaded thermoresponsive and cytocompatible chitosan based hydrogel as a potential wound dressing. Carbohyd polym. 2011;83(2):705-713.[41]Mahmoud AA, Salama AH. Norfloxacin-loaded collagen/chitosan scaffolds for skin reconstruction: Preparation, evaluation and in-vivo wound healing assessment. Eur J Pharm Sci. 2016;83:155-165.[42]Ong SY, Wu J, Moochhala SM, et al. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials. 2008;29(32): 4323-4332.[43]Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1): 76-83.[44]Hajipour MJ, Fromm KM, Ashkarran AA, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10): 499-511.[45]Beer C, Foldbjerg R, Hayashi Y, et al. Toxicity of silver nanoparticles - nanoparticle or silver ion. Toxicol Lett. 2012; 208(3):286-292.[46]Xiu ZM, Zhang QB, Puppala HL, et al. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012;12(8):4271-4275.[47]Kim JS, Kuk E, Yu KN, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3(1):95-101. [48]Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1): 76-83.[49]Anisha BS, Biswas R, Chennazhi KP, et al. Chitosan-hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int J Biol Macromol. 2013;62:310-320.[50]Jaiswal M, Koul V, Dinda AK. In vitro and in vivo investigational studies of a nanocomposite-hydrogel-based dressing with a silver-coated chitosan wafer for full-thickness skin wounds. J Appl Polym Sci. 2016;133(21):43472.[51]Martins AF, Monteiro JP, Bonafe EG, et al. Bactericidal activity of hydrogel beads based on N,N,N-trimethyl chitosan/alginate complexes loaded with silver nanoparticles. Chinese Chem Lett. 2015;26(9):1129-1132.[52]Yadollahi M, Farhoudian S, Namazi H. One-pot synthesis of antibacterial chitosan/silver bio-nanocomposite hydrogel beads as drug delivery systems. Int J Biol Macromol. 2015; 79:37-43.[53]Sacco P, Travan A, Borgogna M, et al. Silver-containing antimicrobial membrane based on chitosan-TPP hydrogel for the treatment of wounds. J Mater Sci Mater Med. 2015;26(3): 128.[54]Hajipour MJ, Fromm KM, Ashkarran AA, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10): 499-511.[55]Coleman NJ. Aspects of the in vitro bioactivity and antimicrobial properties of Ag(+)- and Zn (2+)-exchanged 11 A tobermorites. J Mater Sci Mater Med. 2009;20(6): 1347-1355.[56]Iyigundogdu ZU, Demirci S, Bac N, et al. Development of durable antimicrobial surfaces containing silver- and zinc-ion-exchanged zeolites. Turk J Biol. 2014;38(3): 420-427.[57]Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev. 2013;65(13-14):1803-1815.[58]Kocbek P, Teskac K, Kreft ME, et al. Toxicological aspects of long-term treatment of keratinocytes with ZnO and TiO2 nanoparticles. Small. 2010;6(17):1908-1917.[59]Ng KW, Khoo SP, Heng BC, et al. The role of the tumor suppressor p53 pathway in the cellular DNA damage response to zinc oxide nanoparticles. Biomaterials. 2011;32(32):8218-8225.[60]Sharma V, Singh SK, Anderson D, et al. Zinc oxide nanoparticle induced genotoxicity in primary human epidermal keratinocytes. J Nanosci Nanotechnol. 2011;11(5): 3782-3788.[61]Nair S, Sasidharan A, Divya Rani VV, et al. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J Mater Sci Mater Med. 2009;20 Suppl 1:S235-241.[62]Monteiro-Riviere NA, Wiench K, Landsiedel R, et al. Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanoparticles in UVB sunburned skin: an in vitro and in vivo study. Toxicol Sci. 2011;123(1):264-280.[63]Hackenberg S, Kleinsasser N. Dermal toxicity of ZnO nanoparticles: a worrying feature of sunscreen. Nanomedicine (Lond). 2012;7(4):461-463.[64]Kumar PT, Lakshmanan VK, Anilkumar TV, et al. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: in vitro and in vivo evaluation. ACS Appl Mater Interfaces. 2012;4(5):2618-2629.[65]P T SK, Lakshmanan VK, Raj M, et al. Evaluation of wound healing potential of β-chitin hydrogel/nano zinc oxide composite bandage. Pharm Res. 2013;30(2):523-537.[66]Jayakumar R, Ramachandran R, Divyarani VV, et al. Fabrication of chitin-chitosan/nano TiO2-composite scaffolds for tissue engineering applications. Int J Biol Macromol. 2011;48(2):336-344.[67]Archana D, Singh BK, Dutta J, et al. In vivo evaluation of chitosan-PVP-titanium dioxide nanocomposite as wound dressing material. Carbohydr Polym. 2013;95(1):530-539.[68]Grassian VH, O'shaughnessy PT, Adamcakova-Dodd A, et al. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ Health Perspect. 2007;115(3):397-402.[69]Lieder R, Darai M, Thor MB, et al. In vitro bioactivity of different degree of deacetylation chitosan, a potential coating material for titanium implants. J Biomed Mater Res A. 2012; 100(12):3392-3399.[70]Martins AF, Facchi SP, Monteiro JP, et al. Preparation and cytotoxicity of N,N,N-trimethyl chitosan/alginate beads containing gold nanoparticles. Int J Biol Macromol. 2015;72: 466-471.[71]Khan AU. Medicine at nanoscale: a new horizon. Int J Nanomedicine. 2012;7:2997-2998.[72]Jiang W, Mashayekhi H, Xing B. Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ Pollut. 2009;157(5):1619-1625.[73]Ruparelia JP, Chatterjee AK, Duttagupta SP, et al. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008;4(3):707-716.[74]Gajjar P, Pettee B, Britt DW, et al. Antimicrobial Activity of Commercial Nanoparticles. Adv Mater Nanotechno Proceed. 2009;1151:130-132.[75]Wu B, Huang R, Sahu M, et al. Bacterial responses to Cu-doped TiO(2) nanoparticles. Sci Total Environ. 2010; 408(7):1755-1758.[76]Wang Z, Lee YH, Wu B, et al. Anti-microbial activities of aerosolized transition metal oxide nanoparticles. Chemosphere. 2010;80(5):525-529. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | Li Weiming, Xu Qingwen, Li Yijun, Sun Yanbo, Cui Jin, Xu Pengyuan . Deep seawater promotes wound healing in diabetic mice by activating PI3K/Akt pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 724-729. |
| [5] | Huang Chuanjun, Zou Yu, Zhou Xiaoting, Zhu Yangqing, Qian Wei, Zhang Wei, Liu Xing. Transplantation of umbilical cord mesenchymal stem cells encapsulated in RADA16-BDNF hydrogel promotes neurological recovery in an intracerebral hemorrhage rat model [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 510-515. |
| [6] | Le Guoping, Zhang Ming, Xi Licheng, Luo Hanwen. Preparation and in vitro evaluation of vancomycin hydrochloride@polylactic acid-glycolic acid copolymer-chitosan-hyaluronic acid composite sustained-release microspheres [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 528-534. |
| [7] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [8] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [9] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [10] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [11] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [12] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [13] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [14] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [15] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||