Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (22): 3602-3608.doi: 10.3969/j.issn.2095-4344.2017.22.026
Effect of combination of acellular nerve grafts and stem cells for sciatic nerve regeneration: a Meta-analysis
- 1Department of Bone and Joint Surgery, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China; 2Southwest Medical University, Luzhou 646000, Sichuan Province, China
-
Received:2017-03-09Online:2017-08-08Published:2017-09-01 -
Contact:Yang Yun-kang, Associate professor, Department of Bone and Joint Surgery, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China -
About author:Xiang Fei-fan, Department of Bone and Joint Surgery, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China -
Supported by:the Project of Sichuan Provincial Science and Technology Department, No. 2014JY0248
CLC Number:
Cite this article
Xiang Fei-fan, Yang Yun-kang, Tan Xiao-qi, Wei Dai-qing, Yang Kun, Sun Yuan-lin, Zhou Ju.
share this article
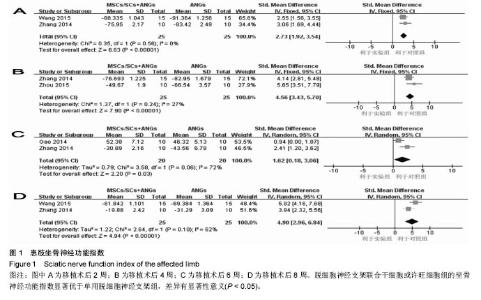
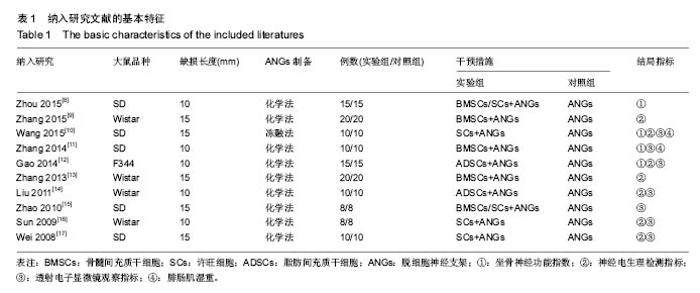
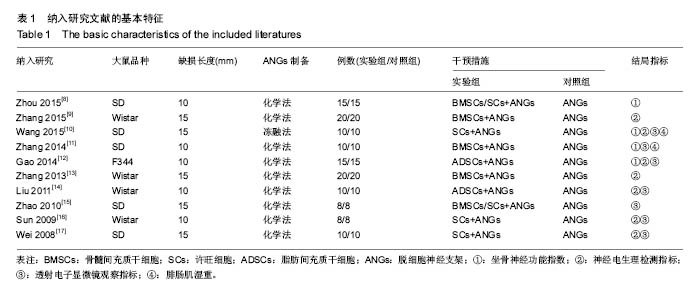
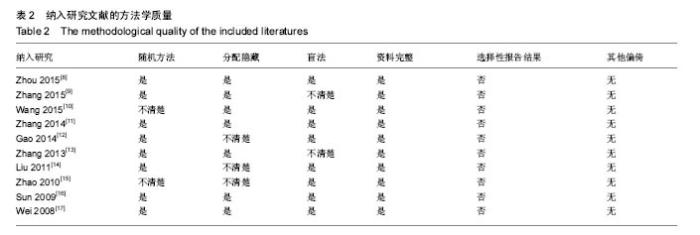
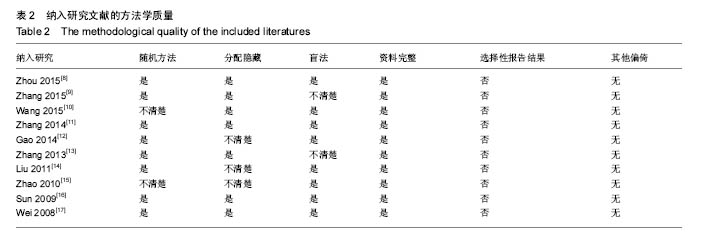
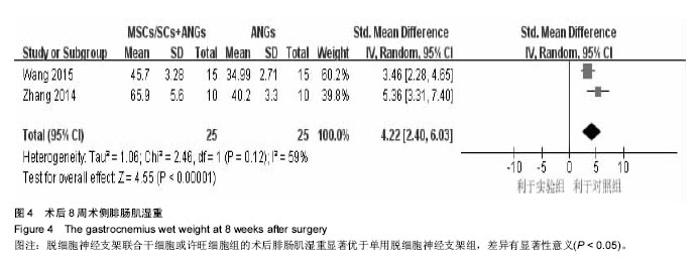
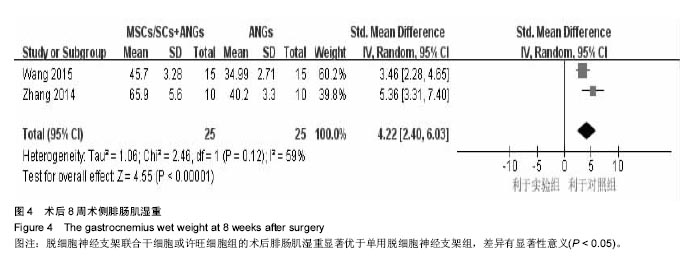
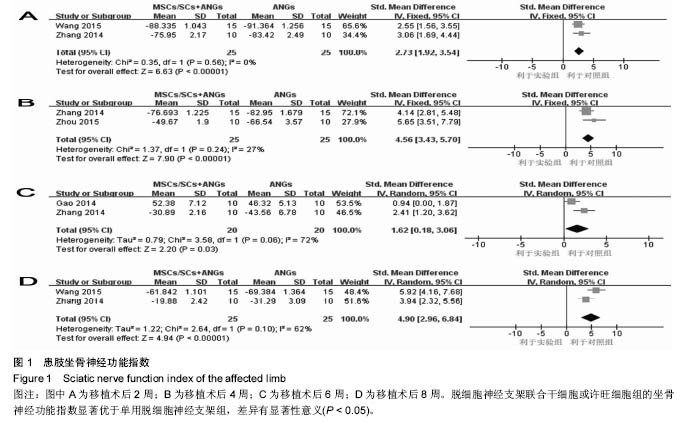
2.3 Meta分析结果 2.3.1 坐骨神经功能指数 2篇文献报道了脱细胞神经支架联合干细胞或许旺细胞与单用脱细胞神经支架移植术后2周患肢的坐骨神经功能指数[10-11],I2 < 50%,使用固定效应模型meta分析结果显示,脱细胞神经支架联合干细胞或许旺细胞组的坐骨神经功能指数显著优于单用脱细胞神经支架组,两者间的差异有显著性意义[SMD=2.73,95%CI(1.92,3.54),P < 0.000 01],见图1A。2篇文献报道了术后4周患肢的坐骨神经功能指数[8, 11],I2=27%,使用固定效应模型meta分析结果显示,脱细胞神经支架联合干细胞或许旺细胞组的坐骨神经功能指数显著优于单用脱细胞神经支架组,两者间的差异有显著性意义[SMD=4.57,95%CI(3.43,5.70),P < 0.000 01],见图1B。2篇文献报道了术后6周患肢的坐骨神经功能指数[11-12],I2=72%,使用随机效应模型meta分析结果显示,脱细胞神经支架联合干细胞或许旺细胞组坐骨神经功能指数显著优于单用脱细胞神经支架组,两者间的差异有显著性意义[SMD=1.62,95%CI(0.18,3.06),P=0.03],见图1C。2篇文献报道了术后8周患肢的坐骨神经功能指数[10-11],I2=62%,使用随机效应模型meta分析结果显示,脱细胞神经支架联合干细胞或许旺细胞组的坐骨神经功能指数显著优于单用脱细胞神经支架组,两者间的差异有显著性意义[SMD=4.90,95%CI(2.96,6.84),P < 0.000 01],见图1D。"
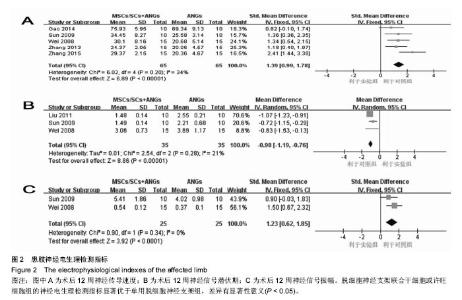
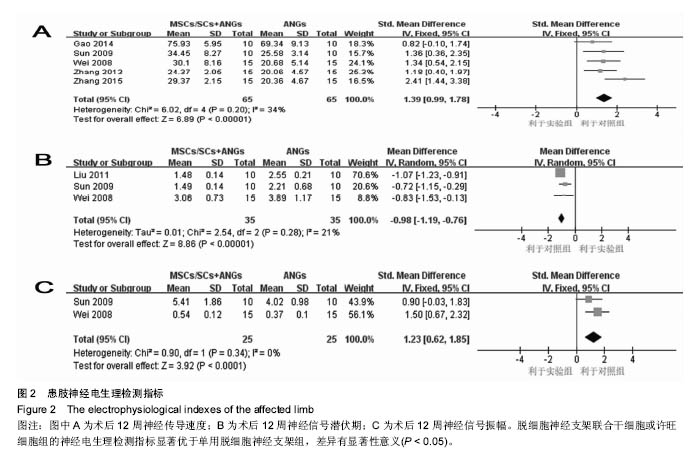
2.3.2 神经电生理检测指标 5篇文献报道脱细胞神经支架联合干细胞或许旺细胞与单用脱细胞神经支架移植术后12周患肢神经的传导速度[9, 12-13, 16-17],I2=34%,使用固定效应模型meta分析结果显示,脱细胞神经支架联合干细胞或许旺细胞组的神经传导速度显著优于单用脱细胞神经支架组,两者间的差异有显著性意义[SMD=1.39,95%CI(0.99,1.78),P < 0.000 01],见图2A。3篇文献报道了术后12周患肢神经信号潜伏期[14, 16-17],I2=21%,使用固定效应模型meta分析结果显示,脱细胞神经支架联合干细胞或许旺细胞组的潜伏期明显短于单用脱细胞神经支架组,两者间的差异有显著性意义[MD=-0.98,95%CI(-1.19,-0.76),P < 0.000 01],见图2B。2篇文献报道了术后12周患肢神经信号振幅[16- 17],I2 < 50%,使用固定效应模型meta分析结果显示,脱细胞神经支架联合干细胞或许旺细胞组的神经信号振幅显著优于单用脱细胞神经支架组,两者间的差异有显著性意义[SMD=1.23,95%CI(0.62,1.85),P < 0.000 1],见图2C。"
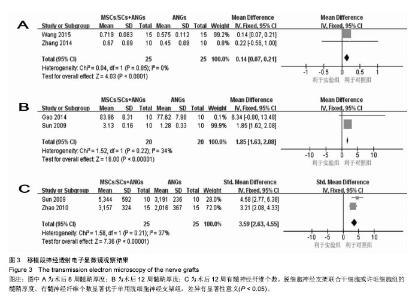
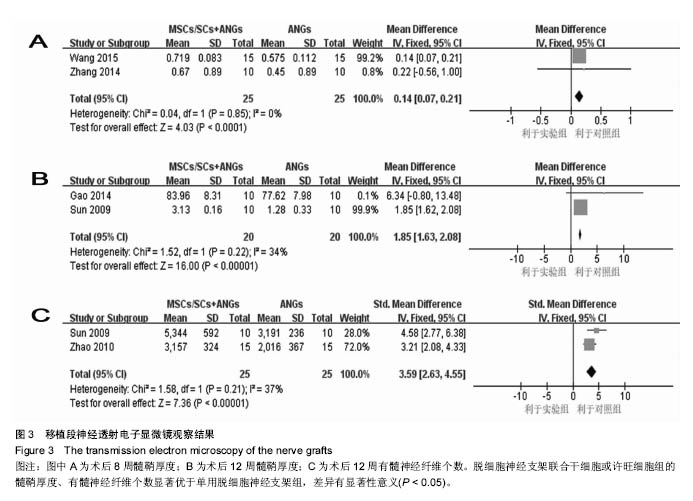
2.3.3 透射电子显微镜观察指标 2篇文献报道了脱细胞神经支架联合干细胞或许旺细胞与单用脱细胞神经支架移植术后8周移植神经髓鞘厚度[10-11],I2 < 50%,采用固定效应模型meta分析结果显示,脱细胞神经支架联合干细胞或许旺细胞组的髓鞘厚度显著优于单用脱细胞神经支架组,两者间的差异有显著性意义[MD=0.14,95%CI(0.07,0.21),P < 0.000 1],见图3A。2篇文献报道了术后12周髓鞘厚度[12, 16],I2=34%,采用固定效应模型meta分析结果显示,脱细胞神经支架联合干细胞或许旺细胞组的髓鞘厚度显著优于单用脱细胞神经支架组,两者间的差异有显著性意义[SMD=1.85,95%CI(1.63,2.08),P < 0.000 01],见图3B。2篇文献报道了术后12周有髓神经纤维数[15, 16],I2=37%,采用固定效应模型meta分析结果显示,脱细胞神经支架联合干细胞或许旺细胞组的有髓神经纤维数显著优于单用脱细胞神经支架组,两者间的差异有显著性意义[SMD=3.59,95%CI(2.63,4.55),P < 0.000 01],见图3C。"
| [1]Isaacs J. Major peripheral nerve injuries. Hand Clin. 2013; 29(3):371-382.[2]Sachanandani NF, Pothula A, Tung TH. Nerve gaps. Plast Reconstr Surg. 2014;133(2):313-319.[3]Isaacs J, Browne T. Overcoming short gaps in peripheral nerve repair: conduits and human acellular nerve allograft. Hand (N Y). 2014;9(2):131-137.[4]He B, Zhu Q, Chai Y, et al. Safety and efficacy evaluation of a human acellular nerve graft as a digital nerve scaffold: a prospective, multicentre controlled clinical trial. J Tissue Eng Regen Med. 2015;9(3):286-295.[5]Zhu S, Liu J, Zheng C, et al. Analysis of human acellular nerve allograft reconstruction of 64 injured nerves in the hand and upper extremity: a 3 year follow-up study. J Tissue Eng Regen Med. 2016 Apr 21. [Epub ahead of print][6]Fan L, Yu Z, Li J, et al. Schwann-like cells seeded in acellular nerve grafts improve nerve regeneration. BMC Musculoskelet Disord. 2014;15:165.[7]Santosa KB, Jesuraj NJ, Viader A, et al. Nerve allografts supplemented with schwann cells overexpressing glial-cell-line-derived neurotrophic factor. Muscle Nerve. 2013;47(2):213-223.[8]Zhou LN, Zhang JW, Liu XL, et al. Co-Graft of Bone Marrow Stromal Cells and Schwann Cells Into Acellular Nerve Scaffold for Sciatic Nerve Regeneration in Rats. J Oral Maxillofac Surg. 2015;73(8):1651-1660.[9]Zhang YR, Ka K, Zhang GC, et al. Repair of peripheral nerve defects with chemically extracted acellular nerve allografts loaded with neurotrophic factors-transfected bone marrow mesenchymal stem cells. Neural Regen Res. 2015;10(9):1498-1506.[10]Wang X, Wang S, Xiao Y. An experimental study on repair of sciatic nerve injury by schwann-like cells derived from umbilical cord blood mesenchymal stem cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2015;29(2):213-220.[11]Zhang Y, Zhang H, Zhang G, et al. Combining acellular nerve allografts with brain-derived neurotrophic factor transfected bone marrow mesenchymal stem cells restores sciatic nerve injury better than either intervention alone. Neural Regen Res. 2014;9(20):1814-1819.[12]Gao S, Zheng Y, Cai Q, et al. Combination of acellular nerve graft and schwann cells-like cells for rat sciatic nerve regeneration.Neural Plast. 2014;2014:139085.[13]Zhang C, Lv G. Repair of sciatic nerve defects using tissue engineered nerves. Neural Regen Res. 2013;8(21):1985-1994.[14]Liu G, Cheng Y, Guo S, et al. Transplantation of adipose-derived stem cells for peripheral nerve repair. Int J Mol Med. 2011;28(4):565-572.[15]Zhao Z, Zhao B, Wang Y, et al. Functional evaluation of chemically extracted acellular nerve allograft supplement with different tissues of Schwann cells for peripheral nerve regeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24(11):1281-1287.[16]Sun XH, Che YQ, Tong XJ, et al. Improving nerve regeneration of acellular nerve allografts seeded with SCs bridging the sciatic nerve defects of rat. Cell Mol Neurobiol. 2009;29(3):347-353.[17]Wei AL, Liu SQ, Tao HY, et al. Repairing peripheral nerve defects with tissue engineered artificial nerves in rats. Chin J Traumatol. 2008;11(1):28-33.[18]Lin MY, Manzano G, Gupta R. Nerve allografts and conduits in peripheral nerve repair. Hand Clin. 2013;29(3):331-348.[19]Sridharan R, Reilly RB, Buckley CT. Decellularized grafts with axially aligned channels for peripheral nerve regeneration. J Mech Behav Biomed Mater. 2015;41:124-135.[20]Sullivan R, Dailey T, Duncan K, et al. Peripheral Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer. Int J Mol Sci. 2016;17(12): E2101.[21]Goulart CO, Jürgensen S, Souto A, et al. A combination of Schwann-cell grafts and aerobic exercise enhances sciatic nerve regeneration. PLoS One. 2014;9(10):e110090.[22]Xiang F, Wei D, Yang Y, et al. Tissue-engineered nerve graft with tetramethylpyrazine for repair of sciatic nerve defects in rats. Neurosci Lett. 2017;638:114-120.[23]Jiang CQ, Hu J, Xiang JP, et al. Tissue-engineered rhesus monkey nerve grafts for the repair of long ulnar nerve defects: similar outcomes to autologous nerve grafts. Neural Regen Res. 2016;11(11):1845-1850.[24]García-Pérez MM, Martínez-Rodríguez HG, López-Guerra GG, et al. A modified chemical protocol of decellularization of rat sciatic nerve and its recellularization with mesenchimal differentiated schwann-like cells: morphological and functional assessments. Histol Histopathol. 2016 Nov 18. [Epub ahead of print] |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [3] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [4] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [5] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [6] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [7] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [8] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [9] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [10] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [11] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [12] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [13] | Huang Chuanjun, Zou Yu, Zhou Xiaoting, Zhu Yangqing, Qian Wei, Zhang Wei, Liu Xing. Transplantation of umbilical cord mesenchymal stem cells encapsulated in RADA16-BDNF hydrogel promotes neurological recovery in an intracerebral hemorrhage rat model [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 510-515. |
| [14] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [15] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||