Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (16): 2606-2611.doi: 10.3969/j.issn.2095-4344.2017.16.025
Previous Articles Next Articles
Construction and vascularization of tissue-engineered sinus node in cardiac tissue engineering
Tong Ling-xi1, Zhang Chuan-sen2, Huang Fei1
- 1Yantai Branch of Binzhou Medical University, Yantai 264003, Shandong Province, China; 2the Second Military Medical University, Shanghai 200000, China
-
Revised:2017-01-21Online:2017-06-08Published:2017-07-06 -
Contact:Zhang Chuan-sen, Professor, the Second Military Medical University, Shanghai 200000, China -
About author:Tong Ling-xi, Studying for master’s degree, Yantai Branch of Binzhou Medical University, Yantai 264003, Shandong Province, China -
Supported by:the National Natural Science Foundation of China, No. 81071603, 30970737; the Natural Science Foundation of Shanghai, No. 09JC1417700
CLC Number:
Cite this article
Tong Ling-xi, Zhang Chuan-sen, Huang Fei. Construction and vascularization of tissue-engineered sinus node in cardiac tissue engineering[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(16): 2606-2611.
share this article
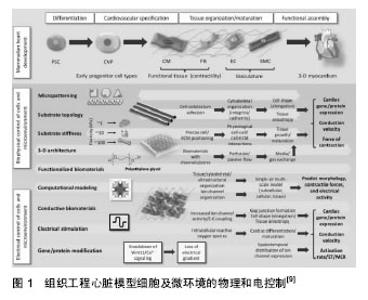
2.1 组织工程窦房结的构建 构建组织工程窦房结要力求接近生理窦房结,同时要能对血管化的形成、移植时长入机体有良好的促进作用,最重要的是让支架材料在体内降解,不对机体产生作副作用。Zimmermann等[4]在2004年提出了心脏组织工程的标准:①具备一定收缩能力;②电生理学稳定;③能够血管化;④低免疫原性。优良的组织工程窦房结也应遵守这一标准。 起搏种子细胞的选择大致为窦房结原代细胞,脂肪、骨髓间充质干细胞,胚胎干细胞等。Marvin等[5]用通过对乳鼠窦房结进行定位,对窦房结细胞进行分离、纯化,培养出的细胞形态、结构均类似于窦房结细胞或心肌细胞,在电镜下观察细胞的超微结构类似于心脏的窦性节段的细胞,用膜片钳记录到该细胞的动作电位,认为其可以广泛的应用于单细胞的研究,也可以作为组织工程窦房结潜在的种子细胞。Fukuda[6]提取鼠类的骨髓间充质干细胞,将其用5-氮杂胞嘧啶核苷处理,同时根据其搏动进行筛选,在1周时细胞结构会类似于成纤维细胞,2周左右会出现自发的搏动,而在3周时搏动统一。这些细胞会同时表达脑钠肽和心钠肽在经5-氮杂胞嘧啶核苷处理前细胞表达Nkx2.5, GATA4,TEF-1, MEF2-C mRNA,而在处理后表达MEF2-A、MEF2-D的mRNA。结构上出现心肌小节和心房颗粒,具有窦房结细胞或心室肌肉细胞的潜能。张喜等[7]以11 d胎鼠为源提取胚胎心脏祖细胞,利用内皮素1诱导其为起搏细胞。免疫组织工程检测表达起搏离子通道蛋白HCN2及HCN4。单细胞膜片钳实验检测出现连续性自发性动作电位,证实此种方法可以构建组织工程窦房结的种子细胞。目前组织工程窦房结研究的种子细胞种类多样,主要的方法不外乎用诱导分化、基因修饰的方法将细胞诱导为起搏细胞,但安全性仍没有统一规范的评价。普遍认为种子细胞应来源广泛,取材方便,可以大规模批量提取,且生存能力强而不具备成瘤的风险。 组织工程支架材料更是种类繁多,活体的窦房结本身就处于胶原支架上[8],因此胶原类材料更为适用于组织工程窦房结(图1)。胶原类材料中含有大量的细胞外基质,细胞外基质可以将心肌细胞排列,使浦肯野纤维排列其中,起到传导的作用[9]。胶原类材料中的细胞外基质就较为丰富,Pen?a等[10]在研究心力衰竭时使用了一种高分子仿生聚合物,反向热导凝胶(reverse thermal gel,RTG),这种凝胶的特殊性在于能够持续性促进心肌细胞的分裂,并且可以作为运载心肌细胞的载体,根据温度将其运送到机体的不同部位。将反向热导凝胶和多聚赖氨酸、层粘蛋白混合进行处理后,能够极大的提高新生及成年大鼠心肌细胞的存活,窦房结的起搏细胞本质上类似于心肌细胞,因此反向热导凝胶不失为一种良好的支架材料。纳米级材料在心脏组织工程中应用也极其广泛。纳米级材料的优势在于能够参与微环境中的信号传导,决定种子细胞的命运,例如促进细胞的诱导分化[11]。Wu等[12]利用碳纳米角作为支架材料能够促进大鼠心肌的存活,同时减少成纤维细胞的形成。而石墨烯作为一种新兴的纳米材料也已经广泛应用于药物输送[13]、细胞成像以及组织支架[14-15]。Shin等[16]将纳米级材料和凝胶混合使用,以氧化还原的石墨烯整合明胶甲基丙烯酰氯水凝胶作为一种新的支架材料,这种支架材料不仅能够促进细胞的增殖、分裂和成熟,还能增强心肌细胞的收缩力,加快其收缩搏动的速率,其降解产物也不会对机体产生不良反应,完全适用与组织工程窦房结。组织工程窦房结不仅需要起搏细胞的自发搏动,还需要移行细胞等进行传导,这种新支架无疑是一种较好的选择。"
| [1] Yang X, Zhou Y, Li H, et al. Mesenchymal stem cells as a gene delivery system to create biological pacemaker cells in vitro. J Int Med Res.2008;36(5):1049-1055. [2] Cho HC, Kashiwakura Y, Marban E. Creation of a biological pacemaker by cell fusion. Circ Res.2007;100(8): 1112-1115. [3] Holnthoner W, Hohenegger K, Husa AM,et al. Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. J Tissue Eng Regen Med.2012;9(2):127-136. [4] Zimmermann WH, Melnychenko I, Eschenhagen T.Engineered heart tissue for regeneration of diseased hearts.Biomaterials. 2004;25(9):1639-1647.[5] Marvin WJ Jr, Chittick VL, Rosenthal JK, et al.The isolated sinoatrial node cell in primary culture from the newborn rat.Circ Res.1984;55(2):253-260.[6] Fukuda K. Development of Regenerative Cardiomyocytes from Mesenchymal Stem Cells for Cardiovascular Tissue Engineering .Artif Organs.2001;25(3):187-193.[7] Zhang X, Zhang CS, Liu YC,et al. Isolation, culture and characterization of cardiac progenitor cells derived from human embryonic heart tubes.Cells Tissues Organs. 2009; 190(4):194-208. [8] 付金利, 廖斌.组织工程窦房结支架材料的选择与展望[J].中国组织工程研究与临床康复, 2007,11(5): 940-943.[9] Thavandiran N, Nunes SS, Xiao Y, et al.Topological and electrical control of cardiac differentiation and assembly. Stem Cell Res Ther. 2013; 4(1):14.[10] Peña B, Martinelli V, Jeong M,et al.Biomimetic Polymers for Cardiac Tissue Engineering.Biomacromolecules. 2016;17(5): 1593-1601. [11] Tian B, Liu J, Dvir T, et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues.Nat Mater. 2012;(11):986-994. [12] Wu Y, Shi X, Li Y, et al. Carbon Nanohorns Promote Maturation of Neonatal Rat Ventricular Myocytes and Inhibit Proliferation of Cardiac Fibroblasts: a Promising Scaffold for Cardiac Tissue Engineering.Nanoscale Res Lett.2016;11(1):284.[13] Geim AK. Graphene: status and prospects.Science. 2009; 324(5934):1530-1534[14] Bitounis D, Ali-Boucetta H, Hong BH, et al.Prospects and challenges of graphene in biomedical applications.Adv Mater. 2013;25(16):2258-68. [15] Schinwald A, Murphy FA, Jones A,,et al. Graphene-based nanoplatelets: a new risk to the respiratory system as a consequence of their unusual aerodynamic properties.ACS Nano. 2012;6(1):736-46. [16] Shin SR, Zihlmann C, Akbari M, et al. Reduced Graphene Oxide-GelMA Hybrid Hydrogels as Scaffolds for Cardiac Tissue Engineering. Small. 2016;12(27):3677-3689.[17] Naderi H, Matin MM, Bahrami AR.Review paper:Critical Issues in Tissue Engineering: Biomaterials, Cell Sources, Angiogenesis, and Drug Delivery Systems.J Biomater Appl. 2011;26(4):383-417 .[18] Baumgartner I, Pieczek A, Manor O,et al.Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97(12):1114-1123.[19] Dew L, MacNeil S. Chong CK.Vascularization strategies for tissue engineers.Regen Med,2015;10(2):211-224 . [20] Sato M, Yamato M, Hamahashi K, et al. Articular cartilage regeneration using cell sheet technology.Anat Rec(Hoboken), 2014;297(1):36-43. [21] Choudhury M, Boyett MR, Morris GM.Biology of the Sinus Node and its Disease.Arrhythm Electrophysiol Rev.2015;4(1): 28-34. [22] Okano T, Yamada N, Sakai H.A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J Biomed Mater Res.1993; 27(10):1243-1251.[23] Sekine H, Shimizu T, Hobo K,et al.Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic Hearts. Circulation. 2008 ;118(14 Suppl):S145-152. [24] Sekine H, Shimizu T, Sakaguchi K.In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4:1399.[25] Sakaguchi K, Shimizu T, Okano T.Construction of three-dimensional vascularized cardiac tissue with cell sheet engineering.J Control Release. 2015;205:83-88.[26] Dvir T, Kedem A, Ruvinov E,et al. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome.Proc Natl Acad Sci U S A. 2009;106(35): 14990-14995. [27] Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 2008 ;26(8):434-441.[28] Laschke MW, Harder Y, Amon M, et al. Angiogenesis in tissue engineering :breathing life into constructed tissue substitutes. Tissue Eng,2006;12(8):2093-2104.[29] Lovett M, Lee K, Edwards A, et al. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15(3): 353-370.[30] Baumen H, Mansouri D, Fromont G, et al. Terminal urothelium differentiation of engineered neoureter after in vivo maturation in the “omental bioreactor”. Eur Urol.2007;52(5):1492-1498.[31] Cheng HL, Wallis C, Shou Z,et al.Quantifying angiogenesis in VEGF-enhanced tissue-engineered bladder constructs by dynamic contrast-enhanced MRI using contrast agents of different molecular weights. Magn Reson Imaging. 2007;25(1): 137-145.[32] Chen W, Shi C, Yi S, et al. Bladder regeneration by collagen scaffolds with collagen binding human basic fibroblast growth factor.J Urol. 2010;183(6):2432-2439. [33] Ohashi K, Yokoyama T, Yamato M,et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets.Nat Med. 2007;13(7):880-885. [34] Wezel F, Southgate J, Thomas DF.Regenerative medicine in urology.BJU Int. 2011;108(7):1046-1065.[35] Maher B. Tissue engineering: How to build a heart.Nature. 2013;499(7456):20-22.[36] Uygun BE,Soto-Gutierrez A,Yagi H,et al.Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16(7): 814-820. [37] Paulsen SJ, Miller JS.Tissue vascularization through 3D printing: Will technology bring us flow? Dev Dyn. 2015; 244(5): 629-640. [38] Marsano A, Maidhof R, Luo J,et al. The effect of controlled expression of VEGF by transduced myoblasts in a cardiac patch on vascularization in a mouse model of myocardial infarction. Biomaterials. 2013;34(2):393-401.[39] Hendrickx B, Vranckx JJ, Luttun A. Cell-based vascularization strategies for skin tissue engineering.issue Eng Part B Rev. 2011;17(1):13-24. [40] Holnthoner W, Hohenegger K, Husa AM.Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix.J Tissue Eng Regen Med. 2015;9(2):127-136. [41] Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis.Science. 1997; 275(5302):964-967.[42] Rouwkema J, Westerweel PE, de Boer J.The use of endothelial progenitor cells for prevascularized bone tissue engineering.Tissue Eng Part A. 2009;15(8):2015-27.[43] He H, Shirota T, Yasui H,et al. Canine endothelial progenitor cell-lined hybrid vascular graft with nonthrombogenic potential.J Thorac Cardiovasc Surg. 2003;126(2):455-464[44] Zhou M, Liu Z, Liu C.Tissue engineering of small-diameter vascular grafts by endothelial progenitor cells seeding heparin-coated decellularized scaffolds.J Biomed Mater Res B Appl Biomater. 2012;100(1):111-20..[45] Chen BS, Xie H, Zhang SL.Tissue engineering of bladder using vascular endothelial growth factor gene-modified endothelial progenitor cells.Int J Artif Organs. 2011;34(12): 1137,1146. [46] 张璐萍.血管化组织工程化窦房结的构建及应用基础研究[D] 上海:第二军医大学.2013. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [13] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [14] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| [15] | Fang Xiaoyang, Tang Tian, Wang Nan, Qian Yuzhang, Xie Lin. Repair and regenerative therapies of the annulus fibrosus [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1582-1587. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||