Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (16): 2472-2477.doi: 10.3969/j.issn.2095-4344.2017.16.00
Previous Articles Next Articles
Osteogenic ability of fascia- versus muscle-derived cells in rats
Su Hai-bin, Li Guang-heng
- Department of Orthopedics, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, Henan Province, China
-
Revised:2017-03-27Online:2017-06-08Published:2017-07-06 -
Contact:Li Guang-heng, M.D., Professor, Doctoral supervisor, Department of Orthopedics, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, Henan Province, China -
About author:Su Hai-bin, Studying for master’s degree, Department of Orthopedics, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, Henan Province, China -
Supported by:the National Natural Science Foundation of China, No. 81472136
CLC Number:
Cite this article
Su Hai-bin, Li Guang-heng. Osteogenic ability of fascia- versus muscle-derived cells in rats[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(16): 2472-2477.
share this article
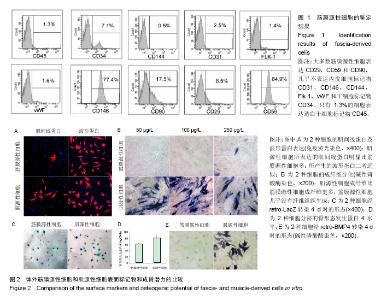
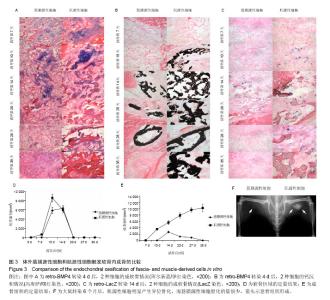
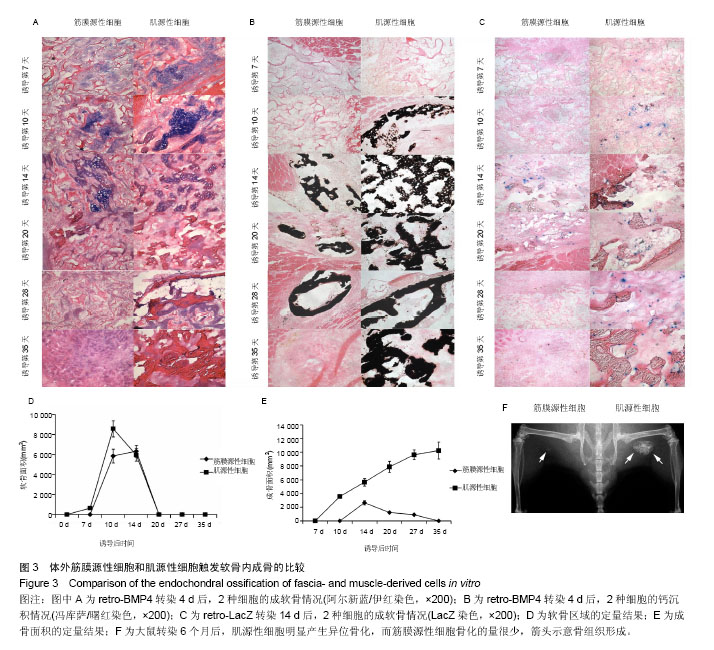
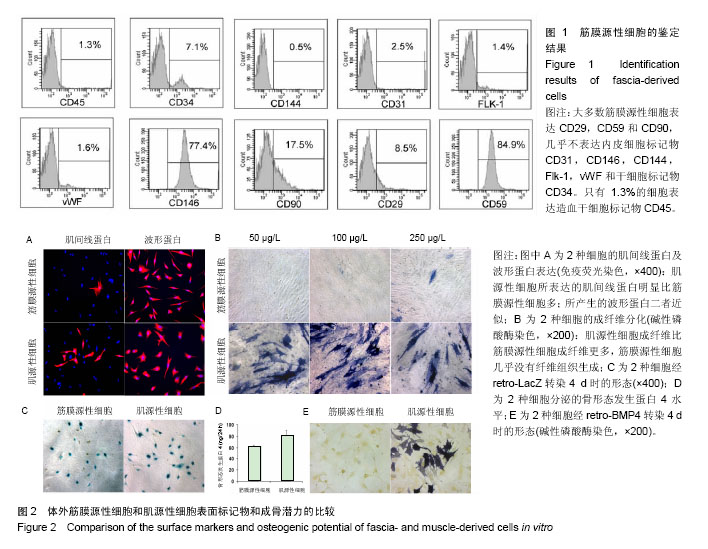
2.1 筋膜源性细胞和肌源性细胞的鉴定结果 流式细胞仪检测结果显示,培养的细胞中大多的筋膜源性细胞表达CD29,CD59和CD90(图1),而筋膜源性细胞很少表达CD31,CD146,CD144,Flk-1,vWF和CD34[24]。 2.2 筋膜源性细胞和肌源性细胞的成骨潜力 肌源性细胞所产生的肌间线蛋白明显比筋膜源性细胞多,二者所产生的波形蛋白近似。骨形态发生蛋白4培养2 d后,碱性磷酸酶染色和LacZ染色显示肌源性细胞向成纤维细胞分化的比筋膜源性细胞多,筋膜源性细胞几乎没有纤维组织生成。同时碱性磷酸酶染色结果显示,肌源性细胞分泌的骨形态发生蛋白4更多,同时产生了更多的肌细胞;筋膜源性细胞分泌骨形态发生蛋白4较少,几乎未产生肌细胞(图2)。 2.3 筋膜源性细胞和肌源性细胞触发软骨内成骨的效果 阿尔新蓝/伊红染色结果显示,随着移植时间的延长,肌源性细胞成软骨多于筋膜源性细胞。冯库萨/曙红染色结果显示,随着移植时间的增长肌源性细胞钙沉积更多。LacZ染色结果显示,肌源性干细胞的软骨细胞多于筋膜源性细胞组。小鼠转染6个月后,肌源性细胞明显产生异位骨化,而筋膜源性细胞骨化的量很少(图3)。"
| [1] Hamdan TA. Missile injuries of the limbs: an Iraqi perspective. J Am Acad Orthop Surg. 2006;14(10 Spec No.):S32-36.[2] Eke PI, Genco RJ. CDC Periodontal Disease Surveillance Project: background, objectives, and progress report. J Periodontol. 2007;78(7 Suppl):1366-1371.[3] Olender E, Brubaker S, Uhrynowska-Tyszkiewicz I, et al. Autologous osteoblast transplantation, an innovative method of bone defect treatment: role of a tissue and cell bank in the process. Transplant Proc. 2014;46(8):2867-2872.[4] Faldini C, Traina F, Perna F, et al. Surgical treatment of aseptic forearm nonunion with plate and opposite bone graft strut. Autograft or allograft? Int Orthop. 2015;39(7):1343-1349. [5] Wang F, Zhang YC, Zhou H, et al. Evaluation of in vitro and in vivo osteogenic differentiation of nano-hydroxyapatite/ chitosan/poly(lactide-co-glycolide) scaffolds with human umbilical cord mesenchymal stem cells. J Biomed Mater Res A. 2014;102(3):760-768.[6] Matsumoto T, Cooper GM, Gharaibeh B, et al. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60(5):1390-1405.[7] Wang Y, Wang Y, Rui Y, et al. In vitro study on multiple differentiation potential of swine synovium-derived MSCs. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009 ;23(6): 737-741. [8] Neumann K, Dehne T, Endres M, et al. Chondrogenic differentiation capacity of human mesenchymal progenitor cells derived from subchondral cortico-spongious bone. J Orthop Res. 2008;26(11):1449-1456.[9] Tuli R, Tuli S, Nandi S, et al. Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem Cells. 2003;21(6):681-693.[10] Wong HL, Siu WS, Fung CH, et al. Characteristics of stem cells derived from rat fascia: in vitro proliferative and multilineage potential assessment. Mol Med Rep. 2015;11(3):1982-1990.[11] Li G, Peng H, Corsi K, et al. Differential effect of BMP4 on NIH/3T3 and C2C12 cells: implications for endochondral bone formation. J Bone Miner Res. 2005;20(9):1611-1623.[12] Foster LJ, Zeemann PA, Li C, et al. Differential expression profiling of membrane proteins by quantitative proteomics in a human mesenchymal stem cell line undergoing osteoblast differentiation. Stem Cells. 2005;23(9):1367-1377.[13] Torrente Y, Tremblay JP, Pisati F, et al. Intraarterial injection of muscle-derived CD34(+)Sca-1(+) stem cells restores dystrophin in mdx mice. J Cell Biol. 2001;152(2):335-348.[14] Williams JT, Southerland SS, Souza J, et al. Cells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypes. Am Surg. 1999;65(1): 22-26.[15] Mariani E, Facchini A. Clinical applications and biosafety of human adult mesenchymal stem cells. Curr Pharm Des. 2012;18(13):1821-1845.[16] Sherwood RI, Christensen JL, Conboy IM, et al. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004; 119(4):543-554.[17] Lee JY, Qu-Petersen Z, Cao B, et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150(5): 1085-1100.[18] Markaryan A, Nelson EG, Kohut RI, et al. Dual immunofluorescence staining of proteoglycans in human temporal bones. Laryngoscope. 2011;121(7):1525-1531.[19] Smith AA. Repeated immunostaining of the same tissue section using alkaline phosphatase as a reporter. Biotech Histochem. 2016;91(6):396-400. [20] Tu Z, Zhang J, Yang G, et al. Characterization of alkaline phosphatase labeled UidA(Gus) probe and its application in testing of transgenic tritordeum. Mol Biol Rep. 2011;38(6): 3629-3634.[21] Verma M, Murkonda BS, Asakura Y, et al. Skeletal Muscle Tissue Clearing for LacZ and Fluorescent Reporters, and Immunofluorescence Staining. Methods Mol Biol. 2016; 1460:129-140. [22] Pati S, Jain S, Behera M, et al. X-gal staining of canine skin tissues: A technique with multiple possible applications. J Nat Sci Biol Med. 2014;5(2):245-249.[23] Burn SF. Detection of β-galactosidase activity: X-gal staining. Methods Mol Biol. 2012;886:241-250.[24] Li G, Zheng B, Meszaros LB, et al. Identification and characterization of chondrogenic progenitor cells in the fascia of postnatal skeletal muscle. J Mol Cell Biol. 2011;3(6): 369-377.[25] Qu-Petersen Z, Deasy B, Jankowski R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157(5):851-864.[26] Couffinhal T, Kearney M, Sullivan A, et al. Histochemical staining following LacZ gene transfer underestimates transfection efficiency. Hum Gene Ther. 1997;8(8):929-934.[27] Lee JY, Peng H, Usas A, et al. Enhancement of bone healing based on ex vivo gene therapy using human muscle-derived cells expressing bone morphogenetic protein 2. Hum Gene Ther. 2002;13(10):1201-1211.[28] Musgrave DS, Pruchnic R, Bosch P, et al. Human skeletal muscle cells in ex vivo gene therapy to deliver bone morphogenetic protein-2. J Bone Joint Surg Br. 2002;84(1): 120-127.[29] Bosch P, Musgrave DS, Lee JY, et al. Osteoprogenitor cells within skeletal muscle. J Orthop Res. 2000;18(6):933-944.[30] Kim SJ, Chung YG, Lee YK, et al. Comparison of the osteogenic potentials of autologous cultured osteoblasts and mesenchymal stem cells loaded onto allogeneic cancellous bone granules. Cell Tissue Res. 2012;347(2):303-310.[31] Kang SH, Chung YG, Oh IH, et al. Bone regeneration potential of allogeneic or autogeneic mesenchymal stem cells loaded onto cancellous bone granules in a rabbit radial defect model. Cell Tissue Res. 2014 ;355(1):81-88. [32] Lee SU, Chung YG, Kim SJ, et al. Does size difference in allogeneic cancellous bone granules loaded with differentiated autologous cultured osteoblasts affect osteogenic potential? Cell Tissue Res. 2014;355(2):337-344.[33] Yin Z, Zhang L, Wang J. Repair of articular cartilage defects with "two-phase" tissue engineered cartilage constructed by autologous marrow mesenchymal stem cells and "two-phase" allogeneic bone matrix gelatin. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2005;19(8):652-657.[34] Liu GP, Li YL, Sun J, et al. Repair of calvarial defects with human umbilical cord blood derived mesenchymal stem cells and demineralized bone matrix in athymic rats. Zhonghua Zheng Xing Wai Ke Za Zhi. 2010;26(1):34-38.[35] Wan C, He Q, Li G. Allogenic peripheral blood derived mesenchymal stem cells (MSCs) enhance bone regeneration in rabbit ulna critical-sized bone defect model. J Orthop Res. 2006;24(4):610-618.[36] Liu G, Li Y, Sun J, Zhou H, et al. In vitro and in vivo evaluation of osteogenesis of human umbilical cord blood-derived mesenchymal stem cells on partially demineralized bone matrix. Tissue Eng Part A. 2010;16(3):971-982. [37] Yang X, Shi W, Du Y, et al. Experimental study of repairing bone defect with tissue engineered bone seeded with autologous red bone marrow and wrapped by pedicled fascial flap. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009; 23(10): 1254-1259. [38] Yew TL, Huang TF, Ma HL, et al. Scale-up of MSC under hypoxic conditions for allogeneic transplantation and enhancing bony regeneration in a rabbit calvarial defect model. J Orthop Res. 2012;30(8):1213-1220. [39] Kasten P, Vogel J, Luginbühl R, et al. Influence of platelet-rich plasma on osteogenic differentiation of mesenchymal stem cells and ectopic bone formation in calcium phosphate ceramics. Cells Tissues Organs. 2006;183(2):68-79. [40] Liu X, Cao L, Jiang Y, et al. Repair of radial segmental bone defects by combined angiopoietin 1 gene transfected bone marrow mesenchymal stem cells and platelet-rich plasma tissue engineered bone in rabbits. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011;25(9):1115-1119. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Zhang Lichuang, Xu Hao, Ma Yinghui, Xiong Mengting, Han Haihui, Bao Jiamin, Zhai Weitao, Liang Qianqian. Mechanism and prospects of regulating lymphatic reflux function in the treatment of rheumatoid arthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1459-1466. |
| [3] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [4] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [5] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [6] | Yang Sidi, Wang Qian, Xu Nuo, Wang Ronghan, Jin Chuanqi, Lu Ying, Dong Ming. Biodentine enhances the proliferation and differentiation of osteoblasts through upregulating bone morphogenetic protein-2 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 516-520. |
| [7] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [8] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [9] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [10] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [11] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [12] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [13] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [14] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [15] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||