Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (8): 1221-1228.doi: 10.3969/j.issn.2095-4344.2017.08.013
Previous Articles Next Articles
Paracrine effect of endothelial colony-forming cells on biological functions of human umbilical vein endothelial cells
Wang Bu-lin1, 2, Guo Xiao-rui3, Li Qin2
- 1Southern Medical University, Guangzhou 510515, Guangdong Province, China; 2Guangzhou General Hospital of Guangzhou Military Command of PLA, Guangzhou 510010, Guangdong Province, China; 3Zhongshan Second People’s Hospital, Zhongshan 528447, Guangdong Province, China
-
Received:2017-01-18Online:2017-03-18Published:2017-04-14 -
Contact:Li Qin, M.D., Chief physician, Guangzhou General Hospital of Guangzhou Military Command of PLA, Guangzhou 510010, Guangdong Province, China -
About author:Wang Bu-lin, Studying for master’s degree, Physician, Southern Medical University, Guangzhou 510515, Guangdong Province, China; Guangzhou General Hospital of Guangzhou Military Command of PLA, Guangzhou 510010, Guangdong Province, China -
Supported by:the National Natural Science Foundation of China, No. 81571910; the Science and Technology Program of Guangdong Province, No. 2014A020212256; the Science and Technology Program of Guangzhou, No. 201607010394
CLC Number:
Cite this article
Wang Bu-lin1, 2, Guo Xiao-rui3, Li Qin2. Paracrine effect of endothelial colony-forming cells on biological functions of human umbilical vein endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2017, 21(8): 1221-1228.
share this article
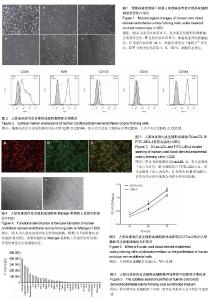
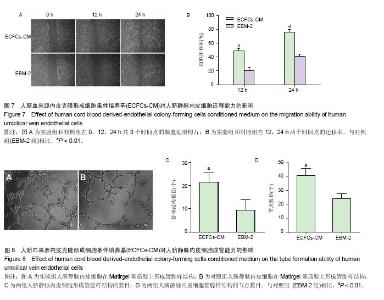
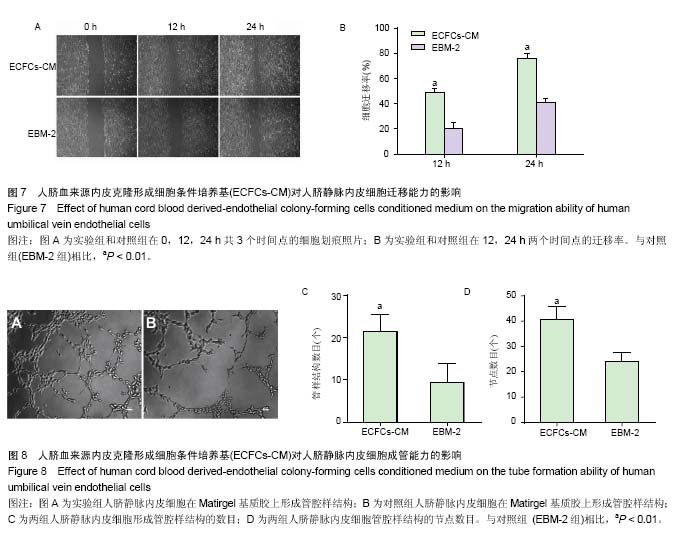
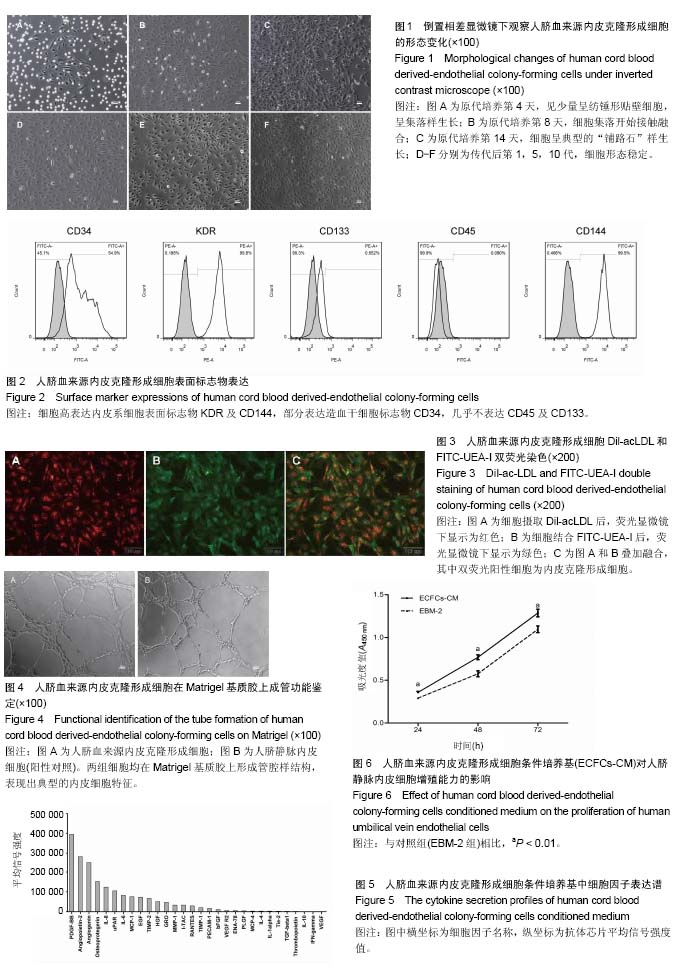
2.1 内皮克隆形成细胞形态 倒置相差显微镜下连续观察显示,刚接种后的单个核细胞呈圆形,细胞体积较小,悬浮分布;原代培养第4天,视野内大量仍为圆形悬浮细胞,仔细观察可见少量呈纺锤形或椭圆形贴壁细胞,呈集落样生长,其增殖速度较快;继续培养至第8天时各细胞集落开始接触融合;至第14天时镜下观察细胞呈“铺路石”样生长,并可见细胞相互连接形成管腔样结构;传代后细胞形态稳定(图1),增殖能力旺盛,按1∶3比例进行传代,可每2 d传1代。 2.2 内皮克隆形成细胞的鉴定结果 通过流式细胞分析细胞表面标志物表达率,结果显示,CD34,KDR,CD133,CD45,CD144的阳性率分别为(54.50±0.57)%,(99.78± 0.04)%,(0.60±0.07)%,(0.11±0.02)%和(99.62±0.16)%,为典型的内皮克隆形成细胞表型,见图2。 免疫荧光染色显示培养的细胞同时具有摄取Dil-acLDL和结合FITC-UEA-I的能力,镜下观察双染色阳性细胞率为(98.95±0.49)%,见图3。 原代培养的细胞与人脐静脉内皮细胞均可在Matrigel基质胶上形成管腔样结构,见图4。以上结果显示所培养的细胞符合内皮克隆形成细胞的特征。 2.3 ECFCs-CM中细胞因子表达情况 人抗体芯片试剂盒AAH-CYT-G2000检测结果显示,ECFCs-CM高表达PDGF-BB,Angiopoietin-2,Angiogenin,Osteoprotegerin,白细胞介素8,uPAR,白细胞介素6,MCP-1,EGF等30种促血管新生因子,见图5。 2.4 ECFCs-CM对人脐静脉内皮细胞增殖能力的影响CCK-8法检测结果显示,ECFCs-CM组的人脐静脉内皮细胞在24,48,72 h共3个时间点的增殖活性均高于对照组 (P < 0.01),见图6。 2.5 ECFCs-CM对人脐静脉内皮细胞迁移能力的影响 细胞划痕实验结果显示,与对照组相比,ECFCs-CM组人脐静脉内皮细胞迁移速度较快,其中两组12,24 h迁移率差异有显著性意义(P < 0.01),见图7。 2.6 ECFCs-CM对人脐静脉内皮细胞体外成管能力的影响 与对照组相比,实验组人脐静脉内皮细胞在Matrigel基质胶上形成管腔样结构数目及节点数目均显著增高,差异有显著性意义(P < 0.01),见图8。"
| [1] Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736-1743.[2] King A, Balaji S, Keswani SG, et al. The role of stem cells in wound angiogenesis. Adv Wound Care. 2014;3(10):614-625.[3] Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763-771.[4] Demidova-Rice TN, Durham JT, Herman IM. Wound healing angiogenesis: innovations and challenges in acute and chronic wound healing. Adv Wound Care. 2012;1(1):17-22.[5] Chong MS, Ng WK, Chan JK. Concise review: endothelial progenitor cells in regenerative medicine: applications and challenges. Stem Cells Transl Med. 2016;5(4):530-538.[6] Asai J, Takenaka H, Ii M, et al. Topical application of ex vivo expanded endothelial progenitor cells promotes vascularisation and wound healing in diabetic mice. Int Wound J. 2013;10(5):527-533.[7] Bai YY, Wang L, Chang D, et al. Synergistic Effects of Transplanted Endothelial Progenitor Cells and RWJ 67657 in Diabetic Ischemic Stroke Models. Stroke. 2015;46(7): 1938-1946.[8] Yin Y, Liu H, Wang F, et al. Transplantation of cryopreserved human umbilical cord blood-derived endothelial progenitor cells induces recovery of carotid artery injury in nude rats. Stem Cell Res Ther. 2015;6:37.[9] Urbich C, Heeschen C, Aicher A, et al. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108(20):2511-2516.[10] Rigato M, Bittante C, Albiero M, et al. Circulating Progenitor Cell Count Predicts Microvascular Outcomes in Type 2 Diabetic Patients. J Clin Endocrinol Metab. 2015;100(7): 2666-2672.[11] Heublein H, Bader A, Giri S. Preclinical and clinical evidence for stem cell therapies as treatment for diabetic wounds. Drug Discov Today. 2015;20(6):703-717.[12] Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int. 2014;2014:1-14.[13] Lin RZ, Moreno-Luna R, Li D, et al. Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc Natl Acad Sci U S A. 2014;111(28):10137-10142.[14] Flex A, Biscetti F, Iachininoto MG, et al. Human cord blood endothelial progenitors promote post-ischemic angiogenesis in immunocompetent mouse model. Thromb Res. 2016;141: 106-111.[15] Asahara T. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964-966.[16] Hur J, Yoon C H, Kim H S, et al. Characterization of Two Types of Endothelial Progenitor Cells and Their Different Contributions to Neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24(2):288-293.[17] Rehman J. Peripheral Blood "Endothelial Progenitor Cells" Are Derived From Monocyte/Macrophages and Secrete Angiogenic Growth Factors. Circulation. 2003;107(8): 1164-1169.[18] Urbich C, Aicher A, Heeschen C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39(5):733-742.[19] Yang Z, von Ballmoos MW, Faessler D, et al. Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis. 2010;211(1):103-109.[20] Cheng CC, Chang SJ, Chueh YN, et al. Distinct angiogenesis roles and surface markers of early and late endothelial progenitor cells revealed by functional group analyses. BMC Genomics. 2013;14:182.[21] Guillevic O, Ferratge S, Pascaud J, et al. A novel molecular and functional stemness signature assessing human cord blood-derived endothelial progenitor cell immaturity. PLoS One. 2016;11(4):e0152993.[22] 乔威,冉峰,刘长建.人外周血内皮祖细胞的分离、培养及鉴定[J].中国组织工程研究,2013,17(36):6508-6514.[23] Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5): 1801-1809.[24] Pelosi E, Castelli G, Testa U. Endothelial progenitors. Blood Cells, Molecules, and Diseases.2014;52(4):186-194.[25] 彭艳,程培,徐勇.脐血内皮祖细胞尾静脉与局部注射治疗糖尿病下肢缺血[J].中国组织工程研究与临床康复, 2011,15(19): 3499-3502.[26] Park S, Moon S, Lee H, et al. A comparison of human cord blood- and embryonic stem cell-derived endothelial progenitor cells in the treatment of chronic wounds. Biomaterials. 2013;34(4):995-1003.[27] Schmidt-Lucke C, Rossig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111(22):2981-2987.[28] Cantinieaux D, Quertainmont R, Blacher S, et al. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS One. 2013;8(8): e69515.[29] Li L F, Liu YY, Yang CT, et al. Improvement of ventilator-induced lung injury by IPS cell-derived conditioned medium via inhibition of PI3K/Akt pathway and IP-10-dependent paracrine regulation. Biomaterials. 2013; 34(1):78-91.[30] Chang CP, Chio CC, Cheong CU, et al. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci (Lond). 2013; 124(3):165-176.[31] Hynes B, Kumar AH, O'Sullivan J, et al. Potent endothelial progenitor cell-conditioned media-related anti-apoptotic, cardiotrophic, and pro-angiogenic effects post-myocardial infarction are mediated by insulin-like growth factor-1. Eur Heart J. 2013;34(10):782-789.[32] Bhang S H, Lee S, Shin J Y, et al. Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis. Mol Ther. 2014;22(4): 862-872.[33] Kim HO, Choi S, Kim H. Mesenchymal stem cell-derived secretome and microvesicles as a cell-free therapeutics for neurodegenerative disorders. Tissue Eng Regen Med. 2013;10(3):93-101.[34] Bae YU, Choi JH, Nagy A, et al. Antisenescence effect of mouse embryonic stem cell conditioned medium through a PDGF/FGF pathway. FASEB J. 2016;30(3):1276-1286.[35] He T. Transplantation of Circulating Endothelial Progenitor Cells Restores Endothelial Function of Denuded Rabbit Carotid Arteries. Stroke. 2004;35(10):2378-2384.[36] Dumortier J, Streblow DN, Moses AV, et al. Human Cytomegalovirus Secretome Contains Factors That Induce Angiogenesis and Wound Healing. J Virol. 2008;82(13): 6524-6535.[37] Lacoste E, Martineau I, Gagnon G. Platelet concentrates: effects of calcium and thrombin on endothelial cell proliferation and growth factor release. J Periodontol. 2003; 74(10):1498-507.[38] 折涛,胡大海,张彦刚,等.胰岛素干预后脂肪干细胞旁分泌对人血管内皮细胞的作用[J].中华烧伤杂志,2011,27(1):32-36.[39] Bertrand-Duchesne MP, Grenier D, Gagnon G. Epidermal growth factor released from platelet-rich plasma promotes endothelial cell proliferation in vitro. J Periodontal Res. 2010; 45(1):87-93.[40] 王卿,王大虎,王爱学,等.银屑病患者损伤处皮肤间充质干细胞对T细胞增殖的影响[J].中国组织工程研究, 2016,20(23): 3451-3456.[41] Kwon HM, Hur SM, Park KY, et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vascul Pharmacol. 2014;63(1):19-28.[42] Miya M, Maeshima A, Mishima K, et al. Enhancement of in vitro human tubulogenesis by endothelial cell-derived factors: implications for in vivo tubular regeneration after injury. Am J Physiol Renal Physiol. 2011;301(2):F387-F395.[43] Wang J, Wang Y, Wang S, et al. Bone marrow-derived mesenchymal stem cell-secreted IL-8 promotes the angiogenesis and growth of colorectal cancer. Oncotarget. 2015;6(40):42825-42837 |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [3] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [4] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [5] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [6] | Zhou Ying, Zhang Huan, Liao Song, Hu Fanqi, Yi Jing, Liu Yubin, Jin Jide. Immunomodulatory effects of deferoxamine and interferon gamma on human dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1012-1019. |
| [7] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [8] | Wang Jifang, Bao Zhen, Qiao Yahong. miR-206 regulates EVI1 gene expression and cell biological behavior in stem cells of small cell lung cancer [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1027-1031. |
| [9] | Liu Feng, Peng Yuhuan, Luo Liangping, Wu Benqing. Plant-derived basic fibroblast growth factor maintains the growth and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1032-1037. |
| [10] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [11] | Fan Yiming, Liu Fangyu, Zhang Hongyu, Li Shuai, Wang Yansong. Serial questions about endogenous neural stem cell response in the ependymal zone after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1137-1142. |
| [12] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [13] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [14] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [15] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||