Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (5): 795-801.doi: 10.3969/j.issn.2095-4344.2017.05.023
Previous Articles Next Articles
The biological features of adipose-derived stem cells and its application in oral tissue regeneration
Luo Yuan1, Huang Yuan-liang2
- 1Department of Oral and Maxillofacial Surgery, the Affiliated Stomatological Hospital of Tongji University, Shanghai Engineering Research Center of Tooth Restoration and Regeneration, Shanghai 200072, China
2Department of Stomatology, Dongfang Hospital of Tongji University, Shanghai 200120, China
-
Online:2017-02-18Published:2017-03-20 -
Contact:Huang Yuan-liang, M.D., Professor, Chief physician, Department of Stomatology, Dongfang Hospital of Tongji University, Shanghai 200120, China -
About author:Luo Yuan, Studying for doctorate, Attending physician, Department of Oral and Maxillofacial Surgery, the Affiliated Stomatological Hospital of Tongji University, Shanghai Engineering Research Center of Tooth Restoration and Regeneration, Shanghai 200072, China -
Supported by:the Funding Project of Shanghai Science and Technology Committee, No. 14411963500; the Young Project of Shanghai Municipal Commission of Health and Family Planning, No. 20154Y0200
CLC Number:
Cite this article
Luo Yuan, Huang Yuan-liang. The biological features of adipose-derived stem cells and its application in oral tissue regeneration[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(5): 795-801.
share this article
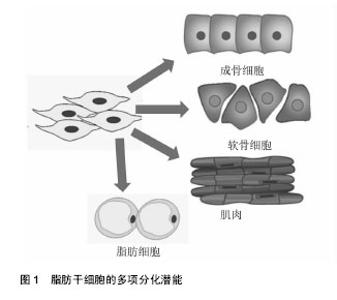
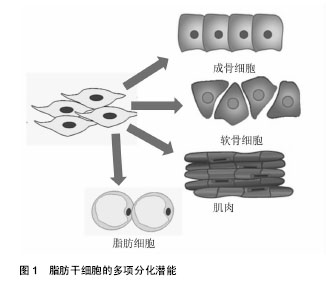
2.1 脂肪干细胞的生物学特性 2.1.1 脂肪干细胞的发现及应用价值 1964年,Rodbel[1]最先从大鼠脂肪组织中分离出成熟的脂肪细胞和脂肪祖细胞,即将分离的大鼠脂肪组织捣碎,Ⅰ型胶原酶 37 ℃消化,离心后除去上清,得到含有脂肪祖细胞的基质血管成分沉淀。2001年Zuk等[2]首次从人体内的脂肪组织中分离得到了脂肪来源的成体干细胞,并进行了体外分离、培养和传代,并证实脂肪干细胞是一类具有多向分化潜能中胚层来源的干细胞,可诱导分化为骨、软骨、脂肪、骨骼肌和神经等细胞。再生医学中理想的种子细胞需满足以下几个条件:①取材方便,供区损伤小;②来源充足;③具有良好的体外扩增能力;④具有一定的免疫调节能力[3]。脂肪干细胞的以下特性使它成为理想的干细胞来源:首先,脂肪干细胞的取材方便,临床中患者的皮下脂肪、腹壁浅层和深层脂肪、上臂脂肪组织、大腿中部和股骨转子部位的脂肪组织,都可提供丰富的脂肪来源;其次,脂肪干细胞获取效率高,一次吸脂手术一般可获得超过200 mL的脂肪组织,每 100 mL脂肪可获得大约2×108个有核细胞,可以产生超过0.5×106的干细胞,相当于20 mL骨髓中骨髓间充质干细胞数量的40倍,而骨髓组织一次可有效抽吸量仅为 50 mL[4]。同时,脂肪干细胞有低的免疫原性。Mclntosh等[5]将人异体脂肪干细胞与淋巴细胞共培养,其不刺激淋巴细胞增殖,证实了脂肪干细胞较弱的异体移植排异,另外脂肪干细胞仅表达中等水平MHCⅠ类分子,而MHCⅡ类分子在多个种属来源的脂肪干细胞中均呈低表达状态,也说明脂肪干细胞移植后不会出现激烈的免疫反应。脂肪干细胞不仅自身表现出低免疫原性,移植后还能够抑制宿主体内的免疫反应。Altman等[6]将脂肪干细胞与脱细胞真皮基质结合后植入到小鼠表皮损伤处,7 d后发现脂肪干细胞可以促进伤口愈合,在植入2周后大部分脂肪干细胞依旧存活,说明脂肪干细胞移植安全性好。 2.1.2 脂肪干细胞的分离培养和体外扩增 目前主要是从抽脂术后废弃的脂肪组织中分离、培养、扩增获得脂肪干细胞,其不仅获取方便,而且对患者创伤小,细胞易于扩增。脂肪干细胞的质量与多种因素有关,包括患者年龄、培养环境、脂肪组织类型、手术类型、取材部位、接种密度等都会影响脂肪干细胞的增殖与分化潜能[7-9],Van Harmelen等[10]通过研究发现来自皮下脂肪的干细胞倍增时间要短于来自腹部脂肪的干细胞,而它们的分化能力没有区别。年轻人皮下脂肪组织中的脂肪干细胞较高龄同性人群有更强的体外扩增能力。脂肪干细胞在脂肪组织中含量较少,必须通过分离和体外扩增才能获得大量的脂肪干细胞。目前分离脂肪干细胞的方法有2种:胶原酶法和组织块法。胶原酶法是用胶原酶消化已剪碎的脂肪组织,离心后沉淀物用含胎牛血清的培养液重悬接种于培养瓶并置入培养箱中静置培养。在培养初期,通过换液去除不贴壁的细胞,在培养过程中,一些前脂肪细胞和始祖细胞逐渐消失。大约培养8周后,得到的是脂肪干细胞[11]。无论是胶原酶法还是组织块法,分离得到的脂肪干细胞均为一种混合细胞。脂肪干细胞体外可以传15代或者可以使用液氮储存。如何能快速有效的分离出脂肪干细胞尚待继续研究,Wu等[12]采用直接将脂肪组织悬浮液通过聚氨酯泡沫膜过滤来得到人脂肪干细胞,此法只需大约30 min,省去了体外细胞培养的过程,并且得到的脂肪干细胞成骨能力极强。 2.1.3 脂肪干细胞的多项分化潜能 2001年,Zuk等[4]首次从人脂肪组织中分离并培养出脂肪干细胞,并证明其在特定的诱导条件下具有多向分化潜能。脂肪干细胞具有强大的向中胚层来源细胞分化的能力,使其在临床中有着广泛的应用前景,如可以分化为骨、软骨、肌腱、脂肪、心肌细胞等(图1);同时最近的一些体外研究显示脂肪干细胞也可向外胚层和内胚层来源的细胞分化,如分化成为神经细胞、许旺细胞及胰腺β细胞、肝细胞和上皮细胞,使其在组织再生和创伤修复过程中发挥着重大作用。对于脂肪干细胞的成脂诱导分化,需在基础培养基中添加地塞米松、胰岛素、吲哚美辛和甲基黄嘌呤[13-14]。成脂分化可以通过油红染色来鉴定。脂肪干细胞在成脂分化过程中,各种转录因子对基因表达的调控影响了脂肪细胞的分化过程,其中最重要的是PPARγ和CEBPα。在两者组成的通路中PPARγ诱导CEBPα的表达,大量转录因子和活性蛋白作用于该通路的各个环节而影响脂肪的分化过程。同时脂肪干细胞在成脂分化过程中也受到多种激素的调节,如胰岛素样生长因子1、胰岛素、糖皮质激素等,其中最重要的是胰岛素样生长因子1,它可以正性调节PPARγ诱导CEBPα的表达。对于脂肪干细胞的成骨诱导分化,目前广泛应用的成骨诱导液配方包括地塞米松或1,25-二羟基维生素D3、抗坏血酸,β-甘油磷酸钠。据报道雌激素、骨形态发生蛋白能促进脂肪干细胞向骨细胞分化。成骨诱导后的鉴定方法主要有碱性磷酸酶检测、茜素红染色及von kossa染色。脂肪干细胞在体外增殖和不同的成骨阶段受到多个信号通路的调节,其中WNT信号通路、BMP信号通路、血小板生长因子信号通路和胰岛素样生长因子1信号通路在脂肪干细胞成骨分化中起到主要作用。在成骨分化过程中不同的信号通路之间还存在交互作用:如转化生长因子β能够诱导脂肪干细胞中β-catenin蛋白的活化,通过经典WNT信号通路促进脂肪干细胞的增殖及成骨分化。TGF-β/BMP信号通路和WNT信号通路的交叉作用,控制着脂肪干细胞的自我更新及成骨分化。当前促进脂肪干细胞向软骨细胞分化的方法有软骨细胞共培养法、外源性生长因子诱导液培养法及基因修饰等,其中诱导液培养法诱导效果更好一些[15-16]。诱导液培养法常用的配方有抗坏血酸、胰岛素、地塞米松、转化生长因子β等,也有学者认为联合应用转化生长因子β和骨形态发生蛋白能提高脂肪干细胞的成软骨潜能。成软骨诱导后的鉴定方法主要有甲苯胺蓝染色和Ⅱ型胶原免疫组化染色[17-18]。此外,脂肪干细胞还可向心肌细胞、上皮细胞、内分泌细胞、肝细胞等分化,显示了较好的多向分化潜能。"
| [1] Rodbell M.Metabolism of isolated fat cells. i. effects of hormones on glucose metabolism and lipolysis.J Biol Chem. 1964;239:375-380.[2] Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211-228.[3] Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33(11):1402-1416.[4] Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12): 4279-4295.[5] McIntosh K, Zvonic S, Garrett S, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24(5):1246-1253.[6] Altman AM, Matthias N, Yan Y, et al. Dermal matrix as a carrier for in vivo delivery of human adipose-derived stem cells. Biomaterials. 2008;29(10):1431-1442.[7] Schipper BM, Marra KG, Zhang W, et al. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg. 2008;60(5):538-544.[8] Bentley DC, Pulbutr P, Chan S, et al. Etiology of the membrane potential of rat white fat adipocytes. Am J Physiol Endocrinol Metab. 2014;307(2):E161-175.[9] Forostyak S, Jendelova P, Kapcalova M, et al. Mesenchymal stromal cells prolong the lifespan in a rat model of amyotrophic lateral sclerosis. Cytotherapy. 2011;13(9):1036-1046.[10] Van Harmelen V, Röhrig K, Hauner H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism. 2004;53(5): 632-637. [11] Zhu M, Heydarkhan-Hagvall S, Hedrick M, et al. Manual isolation of adipose-derived stem cells from human lipoaspirates. J Vis Exp. 2013;(79):e50585. [12] Wu CH, Lee FK, Suresh Kumar S, et al. The isolation and differentiation of human adipose-derived stem cells using membrane filtration. Biomaterials. 2012;33(33):8228-8239.[13] Glaser T, Cappellari AR, Pillat MM, et al. Perspectives of purinergic signaling in stem cell differentiation and tissue regeneration. Purinergic Signal. 2012;8(3):523-537.[14] Jafarzadeh N, Javeri A, Khaleghi M, et al. Oxytocin improves proliferation and neural differentiation of adipose tissue-derived stem cells. Neurosci Lett. 2014;564:105-110.[15] Zhu T, Yu D, Feng J, et al. GDNF and NT-3 induce progenitor bone mesenchymal stem cell differentiation into neurons in fetal gut culture medium.Cell Mol Neurobiol. 2015;35(2): 255-264.[16] Tran TD, Yao S, Hsu WH, et al. Arginine vasopressin inhibits adipogenesis in human adipose-derived stem cells. Mol Cell Endocrinol. 2015;406:1-9.[17] Liu TM, Martina M, Hutmacher DW, et al. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25(3): 750-760.[18] van Dijk A, Niessen HW, Zandieh Doulabi B, et al. Differentiation of human adipose-derived stem cells towards cardiomyocytes is facilitated by laminin. Cell Tissue Res. 2008;334(3):457-467.[19] Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189(1):54-63.[20] Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129(1):118-129.[21] Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012;2012:812693.[22] Garcia-Olmo D, Herreros D, Pascual M, et al. Treatment of enterocutaneous fistula in Crohn's Disease with adipose-derived stem cells: a comparison of protocols with and without cell expansion. Int J Colorectal Dis. 2009;24(1): 27-30.[23] Kim WS, Park BS, Sung JH, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48(1): 15-24.[24] Cui L, Yin S, Liu W, et al. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007;13(6):1185-1195.[25] Taylor BA.Periodontal diseases and systemic health: associations, directions, implications. New South Wales Public Health Bulletin. 1999; 10(3):14-16.[26] Wu L, Zhu F, Wu Y, et al. Dentin sialophosphoprotein- promoted mineralization and expression of odontogenic genes in adipose-derived stromal cells. Cells Tissues Organs. 2008;187(2):103-112.[27] Tobita M, Uysal AC, Ogawa R, et al. Periodontal tissue regeneration with adipose-derived stem cells. Tissue Eng Part A. 2008;14(6):945-953.[28] Tobita M, Uysal CA, Guo X, et al. Periodontal tissue regeneration by combined implantation of adipose tissue-derived stem cells and platelet-rich plasma in a canine model. Cytotherapy. 2013;15(12):1517-1526.[29] Rosenberg M. Bad breath and periodontal disease: how related are they. J Clin Periodontol. 2006;33(1):29-30.[30] Pham TA, Ueno M, Zaitsu T, et al. Clinical trial of oral malodor treatment in patients with periodontal diseases. J Periodontal Res. 2011;46(6):722-729.[31] Salgado AJ, Reis RL, Sousa NJ, et al. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5(2): 103-110.[32] Reichenberger MA, Heimer S, Schaefer A, et al. Adipose derived stem cells protect skin flaps against ischemia-reperfusion injury. Stem Cell Rev. 2012;8(3): 854-862.[33] Cao Y, Sun Z, Liao L, et al. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332(2):370-379.[34] Kaplan FS, Hahn GV, Zasloff MA. Heterotopic Ossification: Two Rare Forms and What They Can Teach Us. J Am Acad Orthop Surg. 1994;2(5):288-296.[35] Hicok KC, Du Laney TV, Zhou YS, et al. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 2004;10(3-4):371-380.[36] Cowan CM, Shi YY, Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22(5):560-567.[37] Yoon E, Dhar S, Chun DE, et al. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007;13(3):619-627.[38] Kulakov AA, Goldshtein DV, Grigoryan AS, et al. Clinical study of the efficiency of combined cell transplant on the basis of multipotent mesenchymal stromal adipose tissue cells in patients with pronounced deficit of the maxillary and mandibulary bone tissue. Bull Exp Biol Med. 2008;146(4): 522-525.[39] Lendeckel S, Jödicke A, Christophis P, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32(6):370-373.[40] Mesimäki K, Lindroos B, Törnwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38(3):201-209.[41] Wolff J, Sándor GK, Miettinen A, et al. GMP-level adipose stem cells combined with computer-aided manufacturing to reconstruct mandibular ameloblastoma resection defects: Experience with three cases. Ann Maxillofac Surg. 2013;3(2): 114-125.[42] Farré-Guasch E, Wolff J, Helder MN, et al. Application of Additive Manufacturing in Oral and Maxillofacial Surgery. J Oral Maxillofac Surg. 2015;73(12):2408-2418.[43] Kakudo N, Shimotsuma A, Kusumoto K. Fibroblast growth factor-2 stimulates adipogenic differentiation of human adipose-derived stem cells. Biochem Biophys Res Commun. 2007;359(2):239-244.[44] Pachón-Peña G, Yu G, Tucker A, et al. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. J Cell Physiol. 2011;226(3):843-851.[45] Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201.[46] Lebedev KA. Homeostasis of adult human stem cells and carcinogenesis. Human Physiology. 2010;36(6): 621-628.[47] Yu JM, Bunnell BA, Kang SK. Neural differentiation of human adipose tissue-derived stem cells. Methods Mol Biol. 2011; 702: 219-231.[48] Mahmoudifar N, Doran PM. Osteogenic differentiation and osteochondral tissue engineering using human adipose-derived stem cells. Biotechnol Prog. 2013;29(1): 176-185.[49] Debnath T, Shalini U, Kona LK, et al. Comparative analysis of chondrogenesis from cartilage tissue and alginate encapsulated human adipose stem cells. Journal of Arthroscopy & Joint Surgery. 2015; 2(2):67-74.[50] Sensebé L. Clinical grade production of mesenchymal stem cells. Biomed Mater Eng. 2008;18(1 Suppl):S3-10.[51] Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series. J Med Case Rep. 2011;5:296.[52] Suga H, Eto H, Aoi N, et al. Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg. 2010;126(6): 1911-1923.[53] Bertolini F, Lohsiriwat V, Petit JY, et al. Adipose tissue cells, lipotransfer and cancer: a challenge for scientists, oncologists and surgeons. Biochim Biophys Acta. 2012;1826(1):209-214.[54] Sensebé L, Bourin P. Producing MSC according GMP: process and controls. Biomed Mater Eng. 2008;18(4-5): 173-177.[55] Busser H, Najar M, Raicevic G, et al. Isolation and Characterization of Human Mesenchymal Stromal Cell Subpopulations: Comparison of Bone Marrow and Adipose Tissue. Stem Cells Dev. 2015;24(18):2142-2157.[56] Bielli A, Scioli MG, Gentile P, et al. Adult adipose-derived stem cells and breast cancer: a controversial relationship. Springerplus. 2014;3:345.[57] Mizuno H. Adipose-derived stem and stromal cells for cell-based therapy: current status of preclinical studies and clinical trials. Curr Opin Mol Ther. 2010;12(4):442-449. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [3] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [4] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [5] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [6] | Zhou Ying, Zhang Huan, Liao Song, Hu Fanqi, Yi Jing, Liu Yubin, Jin Jide. Immunomodulatory effects of deferoxamine and interferon gamma on human dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1012-1019. |
| [7] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [8] | Wang Jifang, Bao Zhen, Qiao Yahong. miR-206 regulates EVI1 gene expression and cell biological behavior in stem cells of small cell lung cancer [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1027-1031. |
| [9] | Liu Feng, Peng Yuhuan, Luo Liangping, Wu Benqing. Plant-derived basic fibroblast growth factor maintains the growth and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1032-1037. |
| [10] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [11] | Fan Yiming, Liu Fangyu, Zhang Hongyu, Li Shuai, Wang Yansong. Serial questions about endogenous neural stem cell response in the ependymal zone after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1137-1142. |
| [12] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [13] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [14] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [15] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||