Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (6): 864-870.doi: 10.3969/j.issn.2095-4344.2017.06.008
Previous Articles Next Articles
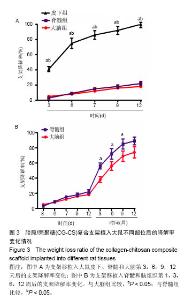
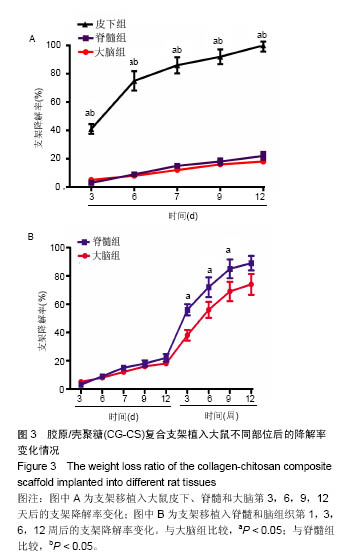
Degradation rate of collagen-chitosan composite scaffold implanted into different rat tissues
- 1Institute of Traumatic Brain Injury and Neurology, Brain Hospital of Affiliated Hospital of Logistics University of Chinese Armed Police Forces, Tianjin Key Laboratory of Neurotrauma Repair, Tianjin 300162, China; 2Center Laboratory of Logistics University of Chinese Armed Police Forces, Tianjin 300162, China
-
Received:2017-01-17Online:2017-02-28Published:2017-03-16 -
Contact:Zhang Sai, M.D., Professor, Chief physician, Doctoral supervisor, Institute of Traumatic Brain Injury and Neurology, Brain Hospital of Affiliated Hospital of Logistics University of Chinese Armed Police Forces, Tianjin Key Laboratory of Neurotrauma Repair, Tianjin 300162, China -
About author:Fu Feng, Studying for master’s degree, Institute of Traumatic Brain Injury and Neurology, Brain Hospital of Affiliated Hospital of Logistics University of Chinese Armed Police Forces, Tianjin Key Laboratory of Neurotrauma Repair, Tianjin 300162, China -
Supported by:the National Natural Science Foundation of China, No. 81301050, 81271392; the Postdoctoral Foundation of China, No. 2013M542583
CLC Number:
Cite this article
Fu Feng, Qin Zhe, Li Xiao-hong, Chen Chong, Wang Li-na, Xu Chao, Tu Yue, Zhang Sai. Degradation rate of collagen-chitosan composite scaffold implanted into different rat tissues[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(6): 864-870.
share this article
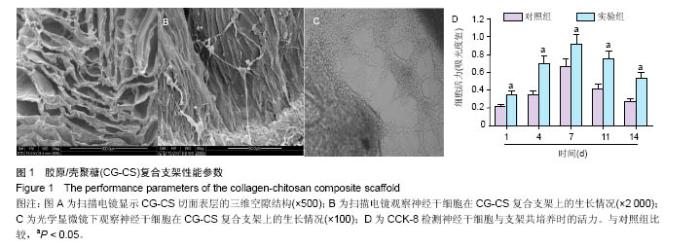
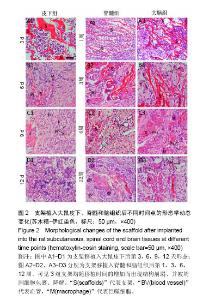
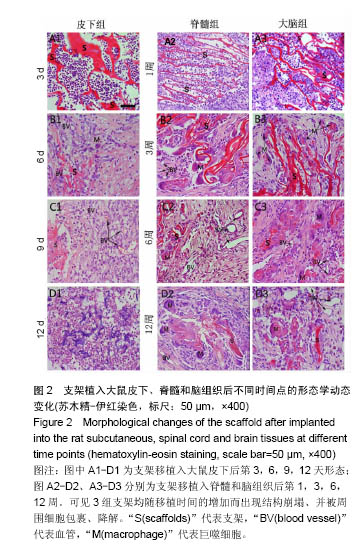
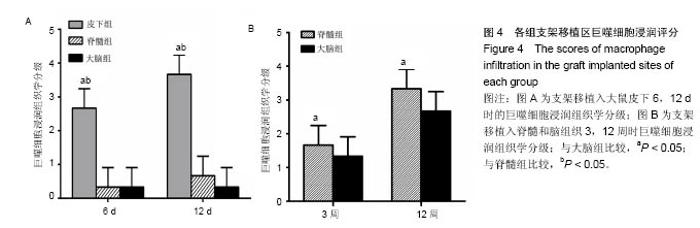
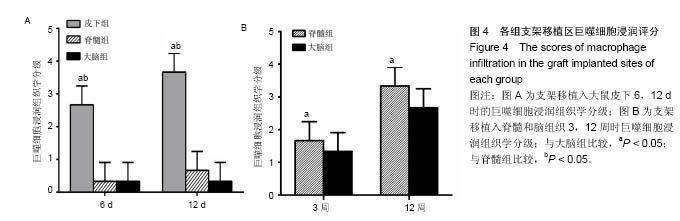
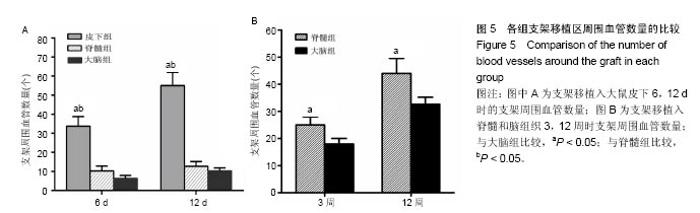
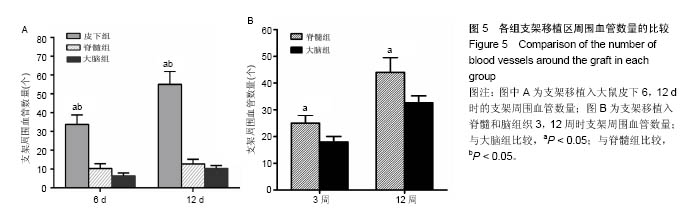
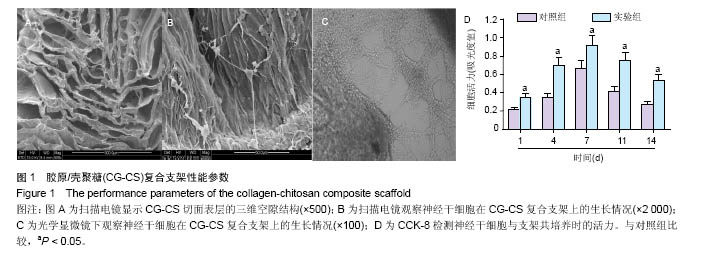
2.1 实验动物数量分析 纳入大鼠72只,均进入结果分析。 2.2 支架性能参数 扫描电镜观察可见,支架呈多孔径立体结构,表面均匀分布孔径为(450±30) μm孔隙(图1A),孔间互通性较好。神经干细胞在支架表面和孔隙内部黏附紧密,生长良好,部分细胞突触相互连接(图1B)。光学显微镜下可见神经干细胞单个细胞、神经球与神经球之间的突起形成相互连接,交织成网(图1C)。CCK-8结果显示,实验组与对照组的吸光度值均随着共培养时间的延长而增高,培养第7天达到高峰,随后呈下降趋势。实验组吸光度值均高于对照组(P < 0.05;图1D)。支架的孔隙率、吸水率和膨胀率分别为(92.5±4.7)%,(1 308.7±29.1)%和(54.2± 8.1)%。"
| [1]Bhattacharya I, Ghayor C, Weber FE. The use of adipose tissue-derived progenitors in bone tissue engineering-a review. Transfus Med Hemother. 2016;43(5):336-343.[2]Hussein KH, Park KM, Kang KS, et al. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater Sci Eng C Mater Biol Appl. 2016;67:766-778.[3]Yamada S, Yamamoto K, Ikeda T, et al. Potency of fish collagen as a scaffold for regenerative medicine. Biomed Res Int. 2014;2014:302932.[4]Yarlagadda PK, Chandrasekharan M, Shyan JY. Recent advances and current developments in tissue scaffolding. Biomed Mater Eng. 2005;15:159-177.[5]Dzul SP, Rocha AG, Rawat S, et al. In vitro characterization of a novel Isu homologue from Drosophila melanogaster for de novo FeS-cluster formation. Metallomics. 2016.[6]Yue S, Lee PD, Poologasundarampillai G, et al. Evaluation of 3-D bioactive glass scaffolds dissolution in a perfusion flow system with X-ray microtomography. Acta Biomater. 2011;7(6):2637-2643.[7]Cheng N, Wang Y, Zhang Y, et al. The osteogenic potential of mesoporous bioglasses/silk and non-mesoporous bioglasses/silk scaffolds in ovariectomized rats: in vitro and in vivo evaluation. PLoS One. 2013;8(11):e81014.[8]Rossi E, Gerges I, Tocchio A, et al. Biologically and mechanically driven design of an RGD-mimetic macroporous foam for adipose tissue engineering applications. Biomaterials. 2016;104:65-77.[9]Yi J, Xiong F, Li B, et al. Degradation characteristics, cell viability and host tissue responses of PDLLA-based scaffold with PRGD and beta-TCP nanoparticles incorporation. Regen Biomater. 2016;3(3):159-166.[10]Muthukumar T, Aravinthan A, Sharmila J, et al. Collagen/chitosan porous bone tissue engineering composite scaffold incorporated with Ginseng compound K. Carbohydr Polym. 2016;152:566-574.[11]Giretova M, Medvecky L, Stulajterova R, et al. Effect of enzymatic degradation of chitosan in polyhydroxybutyrate/ chitosan/calcium phosphate composites on in vitro osteoblast response. J Mater Sci Mater Med. 2016;27(12): 181.[12]Nguyen VT, Ko SC, Oh GW, et al. Anti-inflammatory effects of sodium alginate/gelatine porous scaffolds merged with fucoidan in murine microglial BV2 cells. International journal of biological macromolecules. 2016;93:1620-1632.[13]Neshati Z, Bahrami AR, Eshtiagh-Hosseini H, et al. Evaluating the biodegradability of Gelatin/Siloxane/ Hydroxyapatite (GS-Hyd) complex in vivo and its ability for adhesion and proliferation of rat bone marrow mesenchymal stem cells. Cytotechnology. 2012;64(5): 485-495.[14]杜倩.壳聚糖-胶原角膜修复材料的制备及生物相容性[J].中国组织工程研究,2015,19(52):8433-8437.[15]Zeng S, Liu L, Shi Y, et al. Characterization of Silk Fibroin/Chitosan 3D Porous Scaffold and In Vitro Cytology. PLoS One. 2015;10(6):e0128658.[16]邢冉,陈旭义,朱祥,等.神经干细胞在新型复合支架中的生长和分化[J].中国组织工程研究,2016,20(19):2857-2863.[17]Jinno C, Morimoto N, Ito R, et al. A Comparison of Conventional Collagen Sponge and Collagen-Gelatin Sponge in Wound Healing. Biomed Res Int. 2016;2016: 4567146.[18]Yang Y, Melzer C, Bucan V, et al. Conditioned umbilical cord tissue provides a natural three-dimensional storage compartment as in vitro stem cell niche for human mesenchymal stroma/stem cells. Stem Cell Res Ther. 2016;7:28.[19]陆瑞欣.神经干细胞在胶原凝胶及胶原-壳聚糖支架内的培养[D].大连:大连理工大学,2009.[20]Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue engineering Part B Reviews. 2013;19:485-502.[21]Cheng NC, Wang S, Young TH. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials. 2012;33:1748-1758.[22]Martinez A, Blanco MD, Davidenko N, et al. Tailoring chitosan/collagen scaffolds for tissue engineering: Effect of composition and different crosslinking agents on scaffold properties. Carbohydrate Polymers. 2015;132:606-619.[23]Gorham SD, Light ND, Diamond AM, et al. Effect of chemical modifications on the susceptibility of collagen to proteolysis. II. Dehydrothermal crosslinking. Int J Biol Macromol. 1992;14(3):129-138.[24]Martins AM, Kretlow JD, Costa-Pinto AR, et al. Gradual pore formation in natural origin scaffolds throughout subcutaneous implantation. J Biomed Mater Res A. 2012; 100(3):599-612.[25]Brown BN, Valentin JE, Stewart-Akers AM, et al. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482-1491.[26]Durkut S, Elcin YM, Elcin AE. Biodegradation of chitosan-tripolyphosphate beads: in vitro and in vivo studies. Artif Cells Blood Substit Immobil Biotechnol. 2006;34(2): 263-276.[27]Xia Z, Triffitt JT. A review on macrophage responses to biomaterials. Biomed Mater. 2006;1(1):R1-R9.[28]Stein M, Keshav S, Harris N, et al. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176(1):287-292.[29]Wesley RB 2nd, Meng X, Godin D, et al. Extracellular matrix modulates macrophage functions characteristic to atheroma: collagen type I enhances acquisition of resident macrophage traits by human peripheral blood monocytes in vitro. Arterioscler Thromb Vasc Biol. 1998;18(3):432-440.[30]邓倩莹,李立华,李毅群,等.溶菌酶、过氧化氢对壳聚糖降解性能的影响[J].化学世界,2005,45(6):338-340.[31]Lim SM, Song DK, Oh SH, et al. In vitro and in vivo degradation behavior of acetylated chitosan porous beads. J Biomater Sci Polym Ed. 2008;19(4):453-466.[32]Ishida T, Takeuchi K, Hayashi S, et al. Anatomical structure of the subcutaneous tissue on the anterior surface of human thigh. Okajimas Folia Anat Jpn. 2015;92(1):1-6.[33]Lis A, Szarek D, Laska J. Biomaterials engineering strategies for spinal cord regeneration: state of the art. Polim Med. 2013;43(2):59-80.[34]Breuer Z, Mayevsky A. Brain vasculature and mitochondrial responses to ischemia in gerbils. II. Strain differences and statistical evaluation. Brain Res. 1992;598(1-2):251-256.[35]Ma S, Adayi A, Liu Z, et al. Asymmetric collagen/chitosan membrane containing minocycline-loaded chitosan nanoparticles for guided bone regeneration. Sci Rep. 2016;6:31822.[36]Spiller KL, Anfang RR, Spiller KJ, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477- 4488.[37]Huang KF, Hsu WC, Hsiao JK, et al. Collagen- glycosaminoglycan matrix implantation promotes angiogenesis following surgical brain trauma. Biomed Res Int. 2014;2014:672409. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Yang Shenglin, Pu Xingwei, Luo Chunshan, Yang Jianwen. Neuroprotective effects of tetrandrine preconditioning in rabbits with spinal cord ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1223-1227. |
| [3] | Kan Houming, Fan Lijun, Chen Xuetai, Shen Wen. Application of platelet-rich plasma in neuropathic pain [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1286-1292. |
| [4] | Hu Wei, Xie Xingqi, Tu Guanjun. Exosomes derived from bone marrow mesenchymal stem cells improve the integrity of the blood-spinal cord barrier after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 992-998. |
| [5] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [6] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [7] | Fan Yiming, Liu Fangyu, Zhang Hongyu, Li Shuai, Wang Yansong. Serial questions about endogenous neural stem cell response in the ependymal zone after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1137-1142. |
| [8] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [9] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [10] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [11] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [12] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [13] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [14] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [15] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||