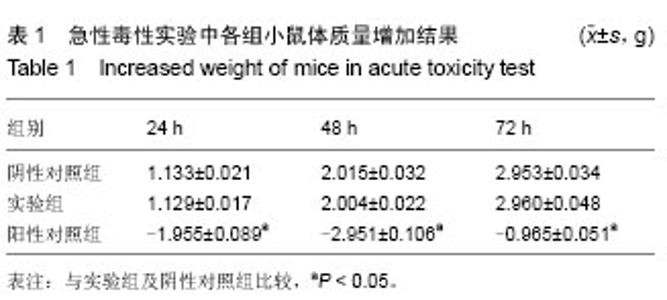
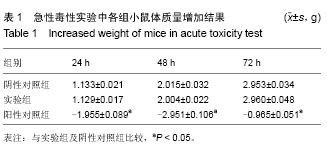
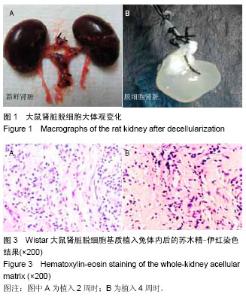
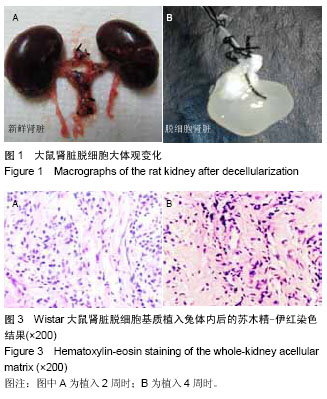
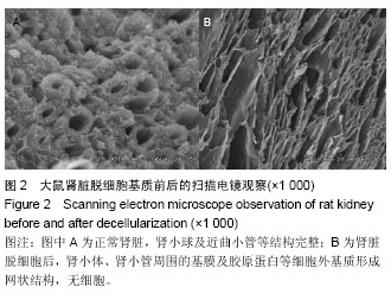
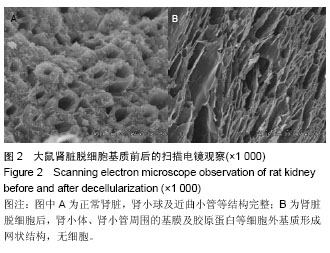
| [1] Badylak SF,Taylor F,Uygun K.Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds.Annu Rev Biomed Eng.2011;13:27-53.
[2] Badylak SF,Weiss DJ,Caplan A,et al.Engineered whole organs and complex tissues.Lancet.2012;379(9819):943-952.
[3] Karlesson JO,Toner M.Long-term storage of tissues by cryopreservation: critical issues. Biomaterials.1996;17(3): 243-256.
[4] Atala A,Schlussel RN,Retrik AB.Renal cell growth in vivo after attachment to biodegradable polymer scaffolds.J Urol.1995; 153:4-8.
[5] Yoo JJ,Ashkar S,Atala A.Creation of functional kidney structures with excretion of kidney-like fluid in vivo.Pediatrics. 1996;988:605-608.
[6] 刘春晓,刘思然,徐啊白,等.灌注法制备大鼠全肾脏脱细胞基质的研究[J].南方医科大学学报,2009,29(5):979-982.
[7] Nakayama KH,Batchelder CA,Lee CI,et al.Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering.Tissue Eng Part A.2010;16(7): 2207-2216.
[8] Wainwright JM,Czajka CA,Patel UB,et al.Preparation of cardiac extracellular matrix from an intact porcine heart.Tissue Eng Part C Methods.2010;16(3):525-532.
[9] Uygun BE,Soto-Gutierrez A,Yagi H,et al.Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16(7): 814-820.
[10] Petersen TH,Calle EA,Zhao L,et al.Tissue-engineered lungs for in vivo implantation. Science.2010;329(5991):538-541.
[11] Cortiella J,Niles J,Cantu A,et al.Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation.Tissue Eng Part A.2010;16(8):2565-2580.
[12] 刘春晓,陈捷,郑少波,等.灌注法制备大鼠全肾脏脱细胞基质支架的细胞相容性[J].中国组织工程研究与临床康复,2009,13(38): 7464-7468.
[13] GB/T 16886-2011/ISO 10993:2006.医疗器械生物学评价.
[14] Scheller EL,Krebsbach PH,Kohn DH.Tissue engineering:state of the art in oral rehabilitation.J Oral Rehabil.2009; 36(5):368-389.
[15] Baker OJ,Schulz DJ,Camden JM,et al.Rat parotid gland cell differertiation in three-dimensional culture.Tissue Eng Part C Methods.2010;16(5):1153-1144.
[16] Jean-Gilles R,Soscia D,Sequeira S,et al.Novel modeling approach to generate a polymeric nanofiber scaffold for salivary gland cells.J Nanotechnol Eng Med. 2010;1(3):31008
[17] Lin CY,Kikuchi N,Hollister SJ.A novel method for biomaterial scaffold internal architecture design to match bone elastic properties with desired porosity.J Biomech.2004;37:623-636.
[18] Takezawa T.A strategy for the development of tissue engineering scaffolds that regulate ceil behavior.Biomaterials. 2003;24:2267-2275.
[19] Soto-Gutierrez A,Yagi H,Uygun BE,et al.Cell delivery: from cell transplantation to organengineering.Cell Transplant.2010; 19(6): 655-665.
[20] Kato N,Higuchi J,Ogata S,et al.Spherule-like acellular stroma in clear cell carcinoma of the ovary:its utility in frozen section diagnosis.Histopathology.2011;59(4):790-794
[21] Ott HC,Matthiesen TS,Goh SK,et al.Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med.2008;14(2):213-221.
[22] Baptista PM,Siddiqui MM,Lozier G,et al.The use of whole organ decellularization for the generation of a vascularized liver organoid.Hepatology.2011;53(2):604-617.
[23] Sellaro TL,Ranade A,Faulk DM,et al.Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels.Tissue Eng Part A.2010;16(3):1075-1082.
[24] Ross EA,Williams MJ,Hamazaki T,et al.Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol.2009;20(11):2338-2347.
[25] Baptista PM,Orlando G,Mirmalek-Sani SH,et al.Whole organ decellularization - a tool for bioscaffold fabrication and organ bioengineering.Conf Proc IEEE Eng Med Biol Soc.2009;2009: 6526-6529.
[26] Gilbert TW,Freund JM,Badylak SF.Quantification of DNA in biologicscaffold materials.J Surg Res.2009;152(1):135-139.
[27] Song JJ,Ott HC.Organ engineering based on decellularized matrix scaffolds.Trends Mol Med.2011;17(8):424-432.
[28] Nagata S,Hanayama R,Kawane K.Autoimmunity and the clearance of dead cells. Cell.2010;140(5):619-630.
[29] Gilbert TW.Strategies for tissue and organ decellularization.J Cell Biochem. 2012;113(7):2217-2222.
[30] Brown BN,Barnes CA,Kasick RT,et al.Surface characterization of extracellular matrix scaffolds.Biomaterials. 2010;31(3): 428-437. |