Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (7): 1669-1678.doi: 10.12307/2026.057
Previous Articles Next Articles
Resveratrol promotes osteogenic differentiation of bone marrow mesenchymal stem cells in an inflammatory microenvironment
Zou Yulian1, 2, 3, Chen Chaopei1, 2, 3, Huang Haixia1, 2, 3, Lan Yuyan1, 2, 3, Liu Min1, 2, 3, Huang Ting1, 2, 3
- 1Department of Prosthodontics, The Affiliated Stomatological Hospital, Southwest Medical University, Luzhou 646000, Sichuan Province, China; 2Luzhou Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Luzhou 646000, Sichuan Province, China; 3Institute of Stomatology, Southwest Medical University, Luzhou 646000, Sichuan Province, China
-
Received:2024-12-05Revised:2025-05-08Accepted:2025-05-29Online:2026-03-08Published:2025-08-18 -
Contact:Huang Haixia, MS, Associate chief physician, Department of Prosthodontics, The Affiliated Stomatological Hospital, Southwest Medical University, Luzhou 646000, Sichuan Province, China; Luzhou Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Luzhou 646000, Sichuan Province, China; Institute of Stomatology, Southwest Medical University, Luzhou 646000, Sichuan Province, China -
About author:Zou Yulian, Master candidate, Department of Prosthodontics, The Affiliated Stomatological Hospital, Southwest Medical University, Luzhou 646000, Sichuan Province, China; Luzhou Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Luzhou 646000, Sichuan Province, China; Institute of Stomatology, Southwest Medical University, Luzhou 646000, Sichuan Province, China -
Supported by:Key Research & Development Science and Technology Plan Project of Luzhou City, No. 2024SYF143 (to HHX)
CLC Number:
Cite this article
Zou Yulian, Chen Chaopei, Huang Haixia, Lan Yuyan, Liu Min, Huang Ting. Resveratrol promotes osteogenic differentiation of bone marrow mesenchymal stem cells in an inflammatory microenvironment[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1669-1678.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
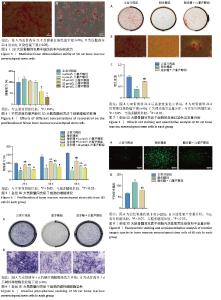
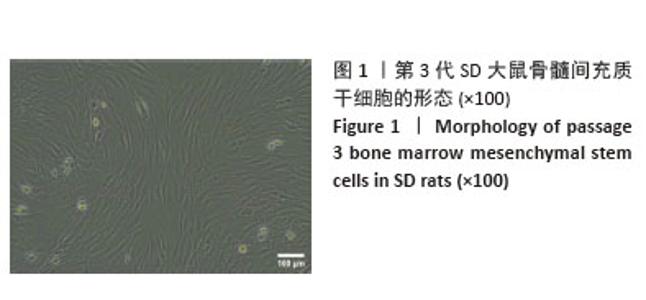
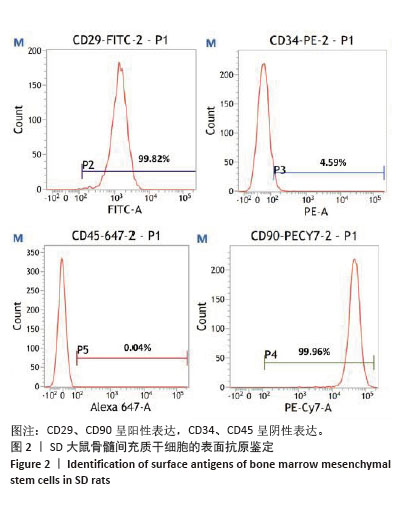
2.3 大鼠BMSCs的多向分化能力 大鼠BMSCs经成骨诱导培养21 d后,茜素红染色可见红染斑块即为钙结节(图3A),证明BMSCs可被诱导成骨分化。成脂诱导培养21 d后,油红O染色可见圆形橙红染色即为脂滴(图3B),证明BMSCs可被诱导成脂分化。 2.4 不同浓度白藜芦醇对大鼠BMSCs增殖的影响 采用CCK-8法检测不同浓度(1,5,10,20,40 μmol/L) 白藜芦醇培养24 h后对BMSCs增殖活性的影响,结果见图4。如图所示,与对照组相比,当白藜芦醇浓度> 10 μmol/L时细胞增殖明显受到抑制,差异有显著性意义(P < 0.05),说明白藜芦醇浓度超过10 μmol/L时,可对BMSCs产生毒性作用。 2.5 白藜芦醇减轻炎症微环境对大鼠BMSCs增殖的抑制 采用CCK-8检测BMSCs在正常环境下、脂多糖刺激环境下以及在脂多糖刺激环境下添加不同浓度(1,5,10 μmol/L)白藜芦醇的增殖情况,见图5。实验结果显示,孵育24,36 h后,与正常对照组相比,脂多糖组BMSCs增殖明显受到抑制(P < 0.05)。与脂多糖组相比,1,5 μmol/L白藜芦醇组BMSCs增殖抑制得到缓解(P < 0.05)。孵育48 h后,脂多糖组增殖率仍低于正常对照组。与脂多糖组相比,白藜芦醇组BMSCs增殖率均升高(P < 0.05),其中加入1 μmol/L白藜芦醇组增殖率最高。综上,1 μmol/L白藜芦醇在炎症微环境中对BMSCs的保护作用最为显著,因此选择1 μmol/L白藜芦醇用于后续实验。 2.6 白藜芦醇减轻炎症微环境中大鼠BMSCs的成骨分化障碍 成骨诱导7 d后各组大鼠BMSCs碱性磷酸酶染色结果见图6。正常对照组碱性磷酸酶染色最深,脂多糖组碱性磷酸酶染色较正常对照组明显变浅,而脂多糖+白藜芦醇组碱性磷酸酶染色较脂多糖组有所加深。成骨诱导21 d后各组大鼠BMSCs茜素红染色结果见图7。正常对照组可见大面积红染钙结节,脂多糖组较正常对照组红染面积明显减少且颜色变浅,而脂多糖+白藜芦醇组红染面积较脂多糖组增加。钙化结节定量检测结果见图7C,正常对照组的吸光度值最高,说明钙化结节数量最多,与其他两组比较差异均有显著性意义(P < 0.05)。脂多糖组的吸光度值最低,说明钙化结节数量最少,与其他两组比较差异有显著性意义(P < 0.05)。脂多糖+白藜芦醇组的吸光度值高于脂多糖组,说明钙化结节数量上升,差异有显著性意义(P < 0.05)。这些结果表明,白藜芦醇可减轻炎症微环境中BMSCs的成骨分化障碍。 2.7 白藜芦醇下调炎症微环境中大鼠BMSCs内活性氧水平 各组大鼠BMSCs内活性氧水平见图8,绿色荧光代表活性氧的产生,如图8A所示,正常对照组的绿色荧光最为微弱,荧光覆盖面积也最小,这表明在正常生理状态下,BMSCs内活性氧含量相对较低。而脂多糖组绿色荧光最亮,荧光面积最大,表明细胞内活性氧产生量显著增加。相比之下,脂多糖+白藜芦醇组绿色荧光强度明显减弱,荧光面积也相对减小,这提示白藜芦醇可能对脂多糖诱导的活性氧过量产生具有一定的抑制作用。为进一步量化这些观察结果,采用Image J软件对荧光显微镜图像进行半定量分析,分析结果如图8B所示,正常对照组的平均荧光强度最低,即在正常条件下,BMSCs内的活性氧水平维持在一个较低的水平,而脂多糖组的平均荧光强度最高,这进一步证实了脂多糖能够有效诱导BMSCs内活性氧的过量产生。更为重要的是,与脂多糖组相比,脂多糖+白藜芦醇组的平均荧光强度显著下降(P < 0.05),这一结果明确表明白藜芦醇能够显著抑制由脂多糖刺激引起的BMSCs内活性氧的过量产生,从而发挥抗氧化作用。"
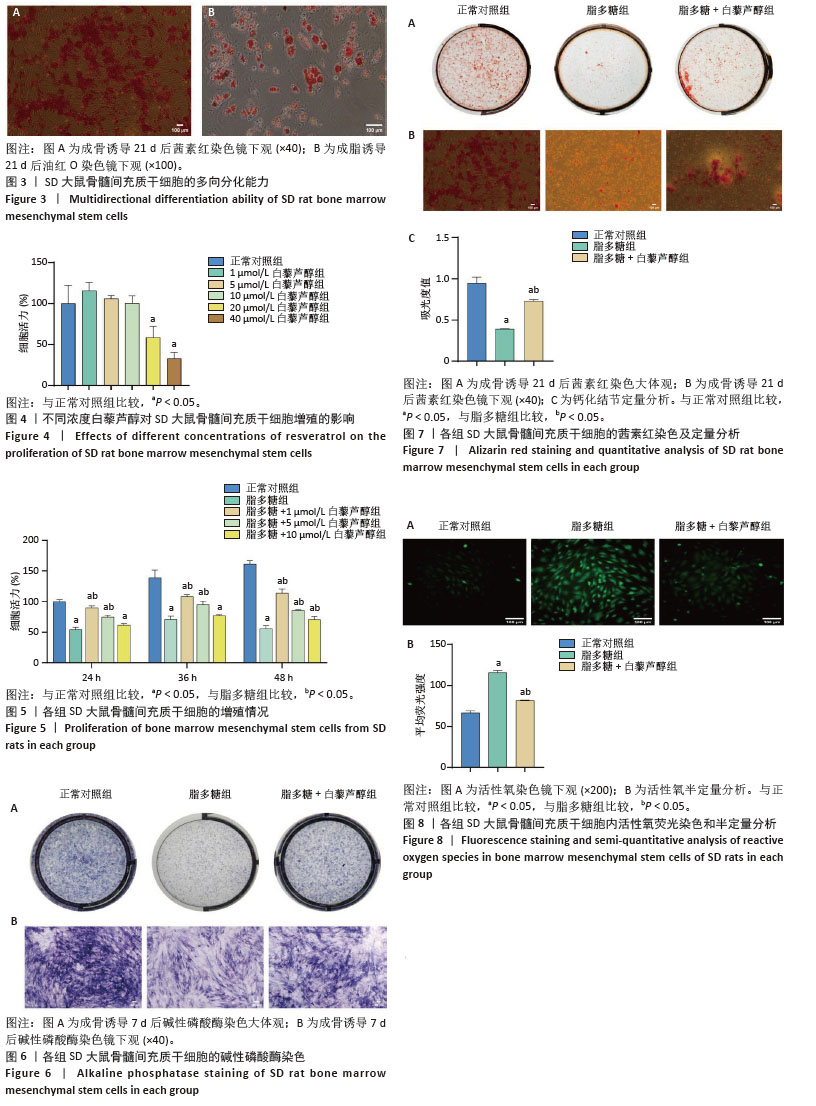

2.8 白藜芦醇缓解炎症微环境中大鼠BMSCs内炎症反应 各组大鼠BMSCs内炎症基因的表达,见图9,与正常对照组比较,脂多糖组白细胞介素1β、白细胞介素18、肿瘤坏死因子α mRNA表达水平均显著上升(P < 0.05),说明脂多糖可诱导BMSCs内炎症产生;与脂多糖组相比,脂多糖+白藜芦醇组上述炎症因子mRNA表达水平均下降(P < 0.05),说明白藜芦醇可抑制脂多糖诱导的大鼠BMSCs内炎症反应。 2.9 白藜芦醇上调炎症微环境中大鼠BMSCs内成骨分化特异基因Runx2、Osx的表达水平 成骨诱导7 d 后,定量分析各组大鼠BMSCs中成骨分化特异基因Runx2、Osx mRNA表达水平,见图10。与正常对照组相比,脂多糖组Runx2、Osx mRNA表达水平显著降低(P < 0.05);与脂多糖组相比,脂多糖+白藜芦醇组 Runx2、Osx mRNA表达水平上升(P < 0.05),表明白藜芦醇可上调脂多糖刺激下大鼠BMSCs内成骨分化特异基因Runx2、Osx表达水平。 2.10 炎症微环境中白藜芦醇对大鼠BMSCs内FoxO1和NLRP3蛋白表达的影响 成骨诱导7 d后,定量分析各组大鼠BMSCs中FoxO1和NLRP3蛋白表达水平。如图11所示,脂多糖组FoxO1蛋白表达量较正常对照组下降(P < 0.05),而NLRP3蛋白表达量较正常对照组显著上升(P < 0.05)。与之相反,脂多糖+白藜芦醇组 FoxO1蛋白表达量较脂多糖组上升(P < 0.05),而NLRP3蛋白表达量较脂多糖组下降(P < 0.05)。"
| [1] ZHAO R, ZHAO W, HUANG J, et al. Prevalence and Risk Factors of Peri-Implant Disease: A Retrospective Case-Control Study in Western China. Int J Environ Res Public Health. 2022;19(19):12667. [2] IVANOVSKI S, BARTOLD PM, HUANG YS. The role of foreign body response in peri‐implantitis: What is the evidence? Periodontol 2000. 2022;90(1):176-185. [3] SHUTO T, WACHI T, SHINOHARA Y, et al. Increase in receptor activator of nuclear factor κB ligand/osteoprotegerin ratio in peri-implant gingiva exposed to Porphyromonas gingivalis lipopolysaccharide. J Dent Sci. 2016;11(1):8-16. [4] INSUA A, MONJE A, WANG HL, et al. Basis of bone metabolism around dental implants during osseointegration and peri‐implant bone loss. J Biomed Mater Res A. 2017;105(7):2075-2089. [5] FU X, LIU G, HALIM A, et al. Mesenchymal stem cell migration and tissue repair. Cells. 2019; 8(8):784. [6] SONG W, BO X, MA X, et al. Craniomaxillofacial derived bone marrow mesenchymal stem/stromal cells (BMSCs) for craniomaxillofacial bone tissue engineering: A literature review. J Stomatol Oral Maxillofac Surg. 2022;123(6):e650-e659. [7] TONIOLO L, CONCATO M, GIACOMELLO E. Resveratrol, a multitasking molecule that improves skeletal muscle health. Nutrients. 2023; 15(15):3413. [8] HECKER A, SCHELLNEGGER M, HOFMANN E, et al. The impact of resveratrol on skin wound healing, scarring, and aging. Int Wound J. 2022;19(1):9-28. [9] MEYER C, BROCKMUELLER A, RUIZ DE PORRAS V, et al. Microbiota and Resveratrol: How Are They Linked to Osteoporosis? Cells. 2024; 13(13):1145. [10] YANG G, CHANG CC, YANG Y, et al. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J Agric Food Chem. 2018;66(49):12953-12960. [11] ZHOU T, YAN Y, ZHAO C, et al. Resveratrol improves osteogenic differentiation of senescent bone mesenchymal stem cells through inhibiting endogenous reactive oxygen species production via AMPK activation. Redox Rep. 2019;24(1):62-69. [12] MOLEZ AM, DO NASCIMENTO EHL, HAITER NETO F, et al. Effect of resveratrol on the progression of experimental periodontitis in an ovariectomized rat model of osteoporosis: Morphometric, immune-enzymatic, and gene expression analysis. J Periodontal Res. 2020;55(6):840-849. [13] HERRERA D, BERGLUNDH T, SCHWARZ F, et al. Prevention and treatment of peri-implant diseases-The EFP S3 level clinical practice guideline. J Clin Periodontol. 2023;50 Suppl 26:4-76. [14] MEYLE J, FISCHER-WASELS L. Non-surgical treatment of peri-implantitis. Br Dent J. 2024;237(10):780-785. [15] SCHWARZ F, JEPSEN S, OBREJA K, et al. Surgical therapy of peri-implantitis. Periodontol 2000. 2022;88(1):145-181. [16] FU JH, WANG HL. Breaking the wave of peri-implantitis. Periodontol 2000. 2020;84(1):145-160. [17] BERGLUNDH T, ARMITAGE G, ARAUJO MG, et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89 Suppl 1:S313-S318. [18] DAVIES JE. Understanding peri‐implant endosseous healing. J Dent Educ. 2003;67(8):932-949. [19] REIS FN, PELÁ VT, CÂMARA JVF, et al. A new role for resveratrol: Protection of enamel against erosion. J Dent. 2024;141:104810. [20] GUO R, PENG W, YANG H, et al. Evaluation of resveratrol-doped adhesive with advanced dentin bond durability. J Dent. 2021;114: 103817. [21] FARHAD SZ, KARBALAEIHASANESFAHANI A, DADGAR E, et al. Promising potential effects of resveratrol on oral and dental health maintenance: a comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2025;398(2):1367-1389. [22] MENG T, XIAO D, MUHAMMED A, et al. Anti-inflammatory action and mechanisms of resveratrol. Molecules. 2021;26(1):229. [23] LI Z, XU Y, LU S, et al. Bone mesenchymal stem cell extracellular vesicles delivered miR let-7-5p alleviate endothelial glycocalyx degradation and leakage via targeting ABL2. Cell Commun Signal. 2023;21(1):205. [24] 岳海云,孙银雪,毕迎春.白藜芦醇促进人乳牙牙髓干细胞的增殖和成骨分化[J].中国组织工程研究,2023,27(19):3011-3016. [25] YUAN J, WANG X, MA D, et al. Resveratrol rescues TNF‑α‑induced inhibition of osteogenesis in human periodontal ligament stem cells via the ERK1/2 pathway. Mol Med Rep. 2020;21(5):2085-2094. [26] LI YY, CAI Q, LI BS, et al. The Effect of Porphyromonas gingivalis Lipopolysaccharide on the Pyroptosis of Gingival Fibroblasts. Inflammation. 2021;44(3):846-858. [27] LIU J, HAN X, ZHANG T, et al. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: from mechanism to therapy. J Hematol Oncol. 2023;16(1):116. [28] IANTOMASI T, ROMAGNOLI C, PALMINI G, et al. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with microRNAs. Int J Mol Sci. 2023;24(4):3772. [29] ZHAO Y, SONG S, WANG D, et al. Nanozyme-reinforced hydrogel as a H2O2-driven oxygenerator for enhancing prosthetic interface osseointegration in rheumatoid arthritis therapy. Nat Commun. 2022;13(1):6758. [30] ZENG L, HE H, SUN M, et al. Runx2 and Nell-1 in dental follicle progenitor cells regulate bone remodeling and tooth eruption. Stem Cell Res Ther. 2022;13(1):486. [31] KOMORI T, YAGI H, NOMURA S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755-764. [32] LIU X, YANG B, ZHANG Y, et al. miR-30a-5p inhibits osteogenesis and promotes periodontitis by targeting Runx2. BMC Oral Health. 2021;21(1):513. [33] NAKASHIMA K, ZHOU X, KUNKEL G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17-29. [34] CHEN S, GLUHAK-HEINRICH J, WANG Y, et al. Runx2, osx, and dspp in tooth development. J Dent Res. 2009;88(10):904-909. [35] ZHOU F, YI Z, WU Y, et al. The role of forkhead box class O1 during implant osseointegration. Eur J Oral Sci. 2021;129(6):e12822. [36] EBRAHIMNEZHAD M, NATAMI M, BAKHTIARI GH, et al. FOXO1, a tiny protein with intricate interactions: Promising therapeutic candidate in lung cancer. Biomed Pharmacother. 2023;169:115900. [37] RACHED MT, KODE A, XU L, et al. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 2010;11(2):147-160. [38] LIANG X, SU Y, HUO Y. Forkhead box protein O1 (FoxO1) /SERPINB1 ameliorates ROS production in diabetic nephropathy. Food Sci Nutr. 2020;9(1):44-51. [39] XIONG Y, ZHANG Y, ZHOU F, et al. FOXO1 differentially regulates bone formation in young and aged mice. Cell Signal. 2022;99:110438. [40] ZHAO H, QIN L, WANG R, et al. FOXO1 regulates NLRP3 inflammasome proteins in LPS-induced cardiotoxicity. Am J Transl Res. 2023;15(8):5446. [41] HUANG X, SU X, MA Q, et al. FoxO1 agonists promote bone regeneration in periodontitis by protecting the osteogenesis of periodontal ligament stem cells. Stem Cells Dev. 2023;32(15-16): 491-503. |
| [1] | Wang Songpeng, Liu Yusan, Yu Huanying, Gao Xiaoli, Xu Yingjiang, Zhang Xiaoming, Liu Min. Bidirectional regulation of reactive oxygen species based on zeolitic imidazolate framework-8 nanomaterials: from tumor therapy and antibacterial activity to cytoprotection [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2033-2013. |
| [2] | Wu Yanting, Li Yu, Liao Jinfeng. Magnesium oxide nanoparticles regulate osteogenesis- and angiogenesis-related gene expressions to promote bone defect healing [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1885-1895. |
| [3] | Jiang Xinghai, Song Yulin, Li Dejin, Shao Jianmin, Xu Junzhi, Liu Huakai, Wu Yingguo, Shen Yuehui, Feng Sicheng. Vascular endothelial growth factor 165 genes transfected into bone marrow mesenchymal stem cells to construct a vascularized amphiphilic peptide gel module [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 1903-1911. |
| [4] | Chen Haojie, Wang Dai, Shen Shan. Immune inflammatory microenvironment mechanisms in peri-implantitis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2054-2062. |
| [5] | Liu Dawei, Cui Yingying, Wang Fanghui, Wang Zixuan, Chen Yuhan, Li Yourui, Zhang Ronghe. Epigallocatechin gallate-mediated bidirectional regulation of reactive oxygen species and its application in nanomaterials [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(8): 2101-2112. |
| [6] | Hu Xiongke, Liu Shaohua, Tan Qian, Liu Kun, Zhu Guanghui. Shikonin intervention with bone marrow mesenchymal stem cells improves microstructure of femur in aged mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1609-1615. |
| [7] | Cai Ziming, Yu Qinghe, Ma Pengfei, Zhang Xin, Zhou Longqian, Zhang Chongyang, Lin Wenping. Heme oxygenase-1 alleviates lipopolysaccharide-induced inflammatory response in nucleus pulposus mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1624-1631. |
| [8] | Yuan Xiaoshuang, Yang Xu, Yang Bo, Chen Xiaoxu, Tian Ting, Wang Feiqing, Li Yanju, Liu Yang, Yang Wenxiu. Effect of conditioned medium of diffuse large B-cell lymphoma cells on proliferation and apoptosis of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1632-1640. |
| [9] | Li Zhenyu, Zhang Siming, Bai Jiaxiang, Zhu Chen. Osthole improves osteogenic differentiation function of bone marrow mesenchymal stem cells under high-glucose conditions [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1641-1648. |
| [10] | Han Nianrong, Huang Yifei, Akram · Osman, Liu Yanlu, Hu Wei . Programmed cell death receptor-1 suppresses osteogenic differentiation of rat bone marrow mesenchymal stem cells in a high-glucose microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1649-1657. |
| [11] | Jin Dongsheng, Zhao Zhanghong, Zhu Ziyin, Zhang Sen, Sun Zuyan, Deng Jiang. Effects of icariin-loaded microsphere-three-dimensional scaffold on osteogenic differentiation of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1658-1668. |
| [12] | Xia Linfeng, Wang Lu, Long Qianfa, Tang Rongwu, Luo Haodong, Tang Yi, Zhong Jun, Liu Yang. Human umbilical cord mesenchymal stem cell-derived exosomes alleviate blood-brain barrier damage in mice with septic encephalopathy [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1711-1719. |
| [13] | Wen Guangwei, Zhen Yinghao, Zheng Taikeng, Zhou Shuyi, Mo Guoye, Zhou Tengpeng, Li Haishan, Lai Yiyi. Effects and mechanisms of isoginkgetin on osteoclastogenesis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1348-1358. |
| [14] | Yin Yongcheng, Zhao Xiangrui, Yang Zhijie, Li Zheng, Li Fang, Ning Bin. Effect and mechanism of peroxiredoxin 1 in microglial inflammation after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1106-1113. |
| [15] | Hu Jing, Zhu Ling, Xie Juan, Kong Deying, Liu Doudou. Autophagy regulates early embryonic development in mice via affecting H3K4me3 modification [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1147-1155. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||