Chinese Journal of Tissue Engineering Research ›› 2026, Vol. 30 ›› Issue (10): 2475-2483.doi: 10.12307/2026.639
Previous Articles Next Articles
Regulation of bone metabolism by myokines under resistance exercise intervention
Li Xinying1, Zhang Wenhua1, Li Xun2, Zhang Shihua2, Wang Xiaoqiang2
- 1School of Postgraduate Education, 2School of Sports and Health, Shandong Sport University, Jinan 250102, Shandong Province, China
-
Received:2025-05-06Accepted:2025-06-14Online:2026-04-08Published:2025-08-28 -
Contact:Wang Xiaoqiang, PhD, Associate professor, School of Sports and Health, Shandong Sport University, Jinan 250102, Shandong Province, China -
About author:Li Xinying, MS candidate, School of Postgraduate Education, Shandong Sport University, Jinan 250102, Shandong Province, China Zhang Wenhua, MS candidate, School of Postgraduate Education, Shandong Sport University, Jinan 250102, Shandong Province, China Li Xinying and Zhang Wenhua contributed equally to this work. -
Supported by:Shandong Province Social Science Planning Program, No. 21DTYJ03 (to WXQ)
CLC Number:
Cite this article
Li Xinying, Zhang Wenhua, Li Xun, Zhang Shihua, Wang Xiaoqiang. Regulation of bone metabolism by myokines under resistance exercise intervention[J]. Chinese Journal of Tissue Engineering Research, 2026, 30(10): 2475-2483.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
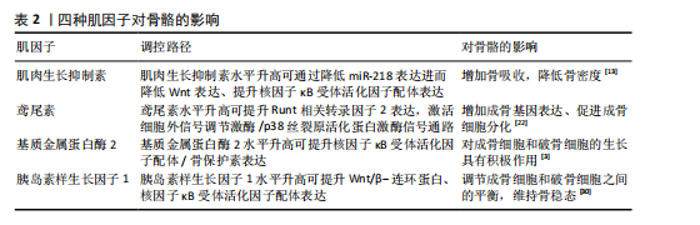
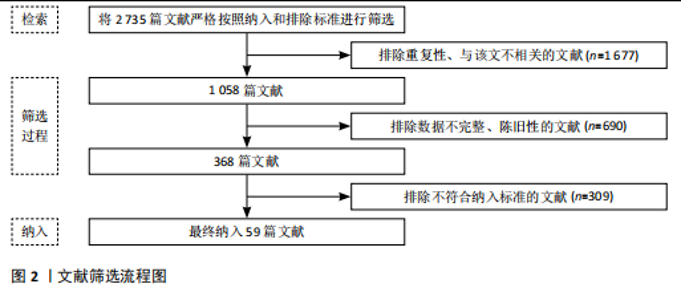
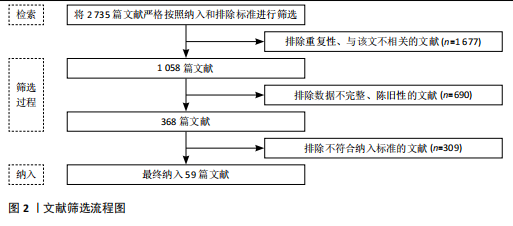
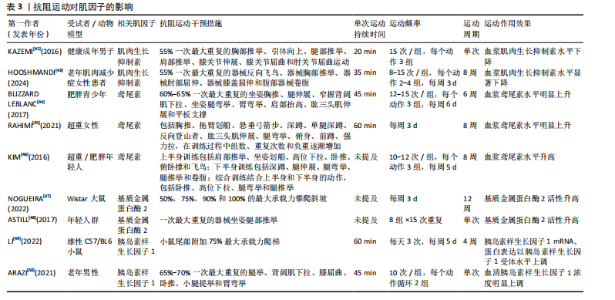
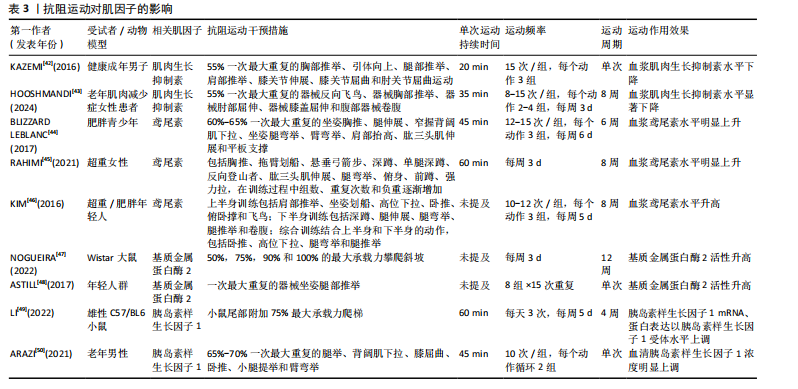
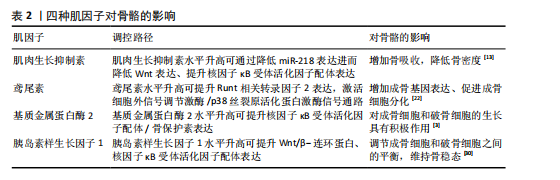
由骨骼肌分泌的可促进骨骼生成的活性因子,为骨骼肌和骨骼间的生化通讯提供了直接证据。肌因子是指一类由骨骼肌细胞特异性表达、制造并释放到细胞外空间的一系列蛋白质及多肽类信使物质。肌因子通过旁分泌、自分泌乃至内分泌途径参与到骨骼肌及其相邻骨骼系统功能的精密调控网络之中,肌因子表达水平的高低与肌肉收缩状态密切相关[20]。运动诱导的肌因子鸢尾素、肌肉生长抑制素、基质金属蛋白酶2和胰岛素样生长因子1等在骨生成与骨吸收过程扮演着无可替代的角色,对骨骼的维护与重塑起到关键性调控作用。 2.2.1 肌肉生长抑制素与骨代谢 肌肉生长抑制素作为转化生长因子β超家族的重要成员,在调控肌肉质量和骨代谢平衡中发挥关键作用。研究表明,肌肉生长抑制素基因缺失或功能抑制可导致肌肉肥大,而它在慢性疾病患者中的过度表达与肌肉萎缩密切相关[21]。临床数据显示,血浆肌肉生长抑制素水平与老年人群骨密度呈显著负相关,作用机制可能涉及肌肉生长抑制素对成骨细胞分化的抑制和对破骨细胞生成的促进作用[22]。动物实验显示,肌肉生长抑制素基因敲除小鼠表现出增强的骨强度和成骨分化能力,而糖尿病模型小鼠中肌肉生长抑制素的过度表达导致骨再生缺陷和肌肉萎缩[23]。这些发现提示肌肉生长抑制素在肌肉-骨骼交互调控中扮演重要角色。 在分子机制层面,肌肉生长抑制素通过多途径影响骨代谢平衡。肌肉生长抑制素可抑制骨细胞外泌体中miR-218的表达,进而减弱Wnt信号通路活性,降低成骨细胞分化能力[12]。肌肉生长抑制素通过增强核因子κB配体活性促进破骨细胞生成,这一过程涉及Smad同源物2依赖的T细胞胞质核因子1核转位机制[12];此外,肌肉生长抑制素缺失小鼠的骨再生能力增强、皮质厚度增加,胫骨、椎骨皮质组织矿物质密度增加,这为相关疾病的治疗提供了潜在靶点[24]。以上研究结果说明,肌肉生长抑制素对骨代谢的调控具有双向性:一方面通过抑制成骨分化减少骨形成,另一方面通过促进破骨细胞生成加剧骨吸收。 2.2.2 鸢尾素与骨代谢 鸢尾素是一种由骨骼肌分泌的运动诱导型肌因子,其前体蛋白Ⅲ型纤连蛋白结构域包含蛋白5的表达受过氧化物酶体增殖物激活受体γ辅激活物1α信号通路的精密调控。运动刺激通过激活过氧化物酶体增殖物激活受体γ辅激活物1α促进Ⅲ型纤连蛋白结构域包含蛋白5基因转录,随后经蛋白水解生成功能性鸢尾素。研究表明,肌肉生长抑制素可抑制Ⅲ型纤连蛋白结构域包含蛋白5的表达,而心肌细胞等其他组织也具有合成鸢尾素的能力,提示其多组织来源特性[25-26]。 在骨代谢调控中,鸢尾素通过激活细胞外信号调节激酶/p38丝裂原活化蛋白激酶信号通路促进活化转录因子4磷酸化,进而增强Runt相关转录因子2的转录活性,上调碱性磷酸酶和骨钙素等成骨标志物的表达。临床研究显示,长期高强度运动可显著提升血清鸢尾素水平,并且鸢尾素水平与骨密度和骨超声传导速度呈正相关[23-24]。动物实验显示,Ⅲ型纤连蛋白结构域包含蛋白5基因敲除小鼠表现出明显的骨量减少和成骨细胞分化抑制,提示鸢尾素在骨形成中的生理必要性[27]。 鸢尾素对破骨细胞活性具有浓度依赖性的双相调控作用。低浓度鸢尾素可促进破骨细胞分化,而高浓度鸢尾素通过抑制核因子κB通路显著减少破骨细胞数量[28]。动物模型显示,鸢尾素缺陷小鼠骨表面破骨细胞覆盖率显著增加,同时伴随炎症因子水平升高,表明鸢尾素对核因子κB配体/骨保护素平衡具有调节作用[29]。这种双向调节机制使鸢尾素既能维持生理性骨改建,又可防止病理性骨吸收过度,在骨稳态调控中发挥关键作用。 2.2.3 基质金属蛋白酶2与骨代谢 基质金属蛋白酶2是锌离子依赖性内肽酶家族成员,通过酶切细胞外基质成分参与组织重塑过程。基质金属蛋白酶2活性可被对氨基苯汞乙酸盐等有机汞化合物激活,并通过与组织金属蛋白酶抑制剂2形成复合物维持动态平衡[30]。运动生理学研究证实,骨骼肌中基质金属蛋白酶2的分泌具有负荷依赖性特征:8周自主跑轮运动可使大鼠腓肠肌基质金属蛋白酶2 mRNA表达量升高2.3倍,血清基质金属蛋白酶2浓度增加,而制动或损伤状态则显著抑制基质金属蛋白酶2的分泌;运动诱导的机械应力通过激活磷脂酰肌醇3激酶/蛋白激酶B信号通路促进基质金属蛋白酶2/基质金属蛋白酶抑制物2比值向蛋白水解活性增强方向偏移[31],这为运动改善肌肉-骨骼系统重塑能力提供了分子依据。 在骨代谢调控中,基质金属蛋白酶2是评估骨基质动态平衡的关键生物标志物。临床研究证实,基质金属蛋白酶2对维持骨结构完整性和矿盐稳态具有不可替代的作用[32]。基质金属蛋白酶2通过降解Ⅰ型胶原C端肽促进成骨细胞迁移,同时调节骨形态发生蛋白2的活性形式释放,从而加速骨缺损修复进程。 基质金属蛋白酶2对骨稳态具有双向调节特性。在乳腺癌骨转移模型中,肿瘤细胞分泌的基质金属蛋白酶2水平与溶骨性病灶数量呈正相关,基质金属蛋白酶2通过切割骨保护素分子使核因子κB配体/骨保护素比值升高3.5倍,显著促进破骨细胞分化[33]。另外,基质金属蛋白酶2可水解成骨细胞膜表面免疫调节分子B7同源物3、抑制Runt相关转录因子2和成骨细胞特异性转录因子的转录活性,导致骨钙素表达量下降[34],这种对成骨-破骨系统的交叉调控揭示了基质金属蛋白酶2在骨代谢疾病中的复杂作用网络。 2.2.4 胰岛素生长因子1与骨代谢 胰岛素样生长因子1是由70个氨基酸组成的多肽激素,在机体生长发育过程中发挥核心调控作用。胰岛素样生长因子1不仅参与血管生成、神经发育与修复等生理过程,更是调节骨骼肌蛋白质代谢的关键因子。 在骨代谢调控方面,胰岛素样生长因子1通过双重机制维持骨稳态。一方面,胰岛素样生长因子1通过激活Wnt/β-连环蛋白信号通路抑制成骨细胞凋亡并促进其分化[35]。临床研究发现,努南综合征患儿血清胰岛素样生长因子1水平降低与骨形成指标下降、骨吸收指标升高显著相关[36]。动物实验显示,成骨细胞特异性敲除胰岛素样生长因子1基因会导致骨形成减少和骨量下降[37]。在骨骼卸载模型中,干骺端骨胰岛素样生长因子1 mRNA表达呈现双相变化,与骨形成标志物(如胶原蛋白1和骨钙素)表达水平密切相关,提示胰岛素样生长因子1在机械应力介导的骨重建中起关键作用[38]。另一方面,胰岛素样生长因子1通过促进核因子κB配体合成调控破骨细胞分化,维持骨重塑平衡[39]。研究表明,运动诱导的胰岛素样生长因子1水平升高与骨吸收标志物的变化密切相关[40]。动物实验显示,全身性胰岛素样生长因子1基因敲除小鼠表现出骨体积增加,这与破骨细胞数量减少有关,提示胰岛素样生长因子1对维持成骨细胞与破骨细胞的动态平衡至关重要[41]。这些发现为理解胰岛素样生长因子1在运动介导的骨代谢调控中的作用提供了重要依据。 四种肌因子对骨骼的影响见表2。 2.3 抗阻运动对肌因子的调控 由工作肌肉释放的肌因子参与抗炎、代谢和免疫过程,并能够以积极方式影响人类健康。不同类型运动对肌因子的激活与释放程度影响大不相同,见表3。以下将阐述抗阻运动对肌因子的影响。 2.3.1 抗阻运动对肌肉生长抑制素的影响 作为肌肉生长负调节因子的肌肉生长抑制素会因抗阻运动的干预而下降。KAZEMI[42]招募24名健康成年男子进行循环抗阻运动,发现单次抗阻训练就可降低受试者血浆肌肉生长抑制素水平,循环抗阻运动为55%一次最大重复的胸部推举、引体向上、腿部推举、肩部推举、膝关节伸展、膝关节屈曲和肘关节屈曲运动,15次/组,每个动作3组。一项针对老年肌肉减少症女性的抗阻运动研究表明,与对照组相比,抗阻运动组受试者血浆肌肉生长抑制素显著下降[43],抗阻运动具体干预措施为,每周3次的肌肥大和肌耐力训练,训练内容针对全身主要肌群,包括器械反向飞鸟、器械胸部推举、器械肘部屈伸、器械"
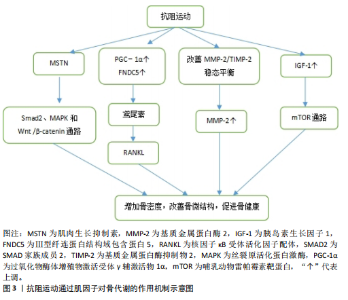
膝盖屈伸和腹部器械卷腹,每个动作2-4组,每组重复8-15次,负荷为55%一次最大重复,持续8周。 2.3.2 抗阻运动对鸢尾素的影响 BLIZZARD LEBLANC等[44]比较了急性有氧运动与抗阻运动6周后受试者的血清鸢尾素水平,急性有氧运动单次时长为45 min,心率储备为60%的蹬自行车训练;急性抗阻运动为60%-65%一次最大重复的坐姿胸推、腿伸展、窄握背阔肌下拉、坐姿腿弯举、臂弯举、肩部抬高、肱三头肌伸展和平板支撑,每个练习进行3组,每组12-15次,强度为60%-65%一次最大重复,每组之间休息60 s,排除热身与拉伸运动,每次训练总时长约为45 min,每周6次,结果表明一次抗阻运动后受试者血清鸢尾素水平没有变化,然而经过6周的抗阻训练后受试者血清鸢尾素水平明显增加。 RAHIMI等[45]招募了30名超重女性进行每周3次、持续8周的普拉提和全身抗阻运动,抗阻运动包括胸推、拖臂划船、悬垂弓箭步、深蹲、单腿深蹲、反向登山者、肱三头肌伸展、腿弯举、俯身、前蹲、强力拉,训练过程中组数、重复次数和负重逐渐增加;普拉提动作为单腿转圈、深蹲、球行、仰卧抬腿、肘部屈伸、上下肢外展、肩桥、侧踢、前后踢等,结果表明与对照组相比,普拉提组和抗阻运动组鸢尾素明显增加,而后两组间鸢尾素水平无显著差异。KIM等[46]的一项随机对照试验比较有氧运动和抗阻运动对受试者血清鸢尾素水平的影响,有氧运动为8周内每天交替进行两种有氧运动模式(50 min跑步机或25 min骑自行车和25 min跑步机登山者相结合),每周5次;抗阻运动训练计划为周一和周三进行上半身抗阻运动(肩推、坐姿划船、引体向上、卧推等),周二和周四为下半身抗阻运动(深蹲、腿伸展、腿弯举等),周五进行全身抗阻运动(卧推、引体向上、腿弯举和腿举),负荷为65%-80%一次最大重复,每个动作进行3组,每组重复10-12次,间歇为1 min,结果表明仅抗阻运动组受试者血清鸢尾素水平升高。 2.3.3 抗阻运动对基质金属蛋白酶2的影响 急性抗阻运动与慢性抗阻运动均会提升基质金属蛋白酶2活性。一项动物实验对Wistar大鼠在垂直梯子上进行为期12周每周3次的负重攀爬运动,负荷为50%,75%,90%和100%最大承载力,之后的下一次攀爬增加30 g,直到大鼠力竭无法完成爬梯,与对照组相比,抗阻运动组大鼠总基质金属蛋白酶2活性升高[47]。一项试验招募18名年轻人群探究急性运动对基质金属蛋白酶2的影响,受试者需完成15次一次最大重复器械坐姿腿部推举动作,共8组,急性运动15 min后显示受试者血清基质金属蛋白酶2水平增加至训练前的两三倍[48]。 2.3.4 抗阻运动对胰岛素样生长因子1的影响 抗阻运动是肌因子胰岛素样生长因子1的“强激活剂”。LI等[49]探究不同运动方式对小鼠胰岛素样生长因子1的影响,将12月龄雄性C57/BL6小鼠随机分为对照组、有氧运动组、阻力运动组、全身振动组和电刺激组,其中抗阻运动方案为在小鼠尾部附加75%最大承载力进行爬梯训练3次,每周5 d,持续4周,结果表明与对照组相比,4种运动训练后小鼠胰岛素样生长因子1 mRNA、蛋白表达及胰岛素样生长因子1受体水平均有明显增加,与其他3种运动相比,抗阻运动组胰岛素样生长因子1 mRNA、蛋白及胰岛素样生长因子1受体水平上调更为显著。ARAZI等[50]招募了30名老年男性探究急性抗阻运动对胰岛素样生长因子1浓度的影响,抗阻运动方案为进行循环式抗阻训练(2个循环),依次为腿举、背阔肌下拉、膝屈曲、卧推、小腿提举和臂弯举,每组重复10次,强度为65%-70%一次最大重复,动作和组间循环之间的休息时间分别为60 s和120 s,结果表明即便是急性运动也会提高受试者血清胰岛素样生长因子1浓度。 2.4 抗阻运动通过肌因子对骨代谢的研究 骨骼与肌肉作为力学敏感组织,通过力学-生物学耦合机制形成功能协同单元。抗阻运动不仅通过机械应力直接刺激骨组织,还可通过诱导肌肉分泌多种肌因子间接调控骨代谢,见图3。然而,目前关于抗阻运动诱导的肌因子分泌谱及其对骨代谢的具体调控机制尚未完全阐明,特别是不同运动强度、频率和持续时间对肌因子表达的影响及其与骨代谢标志物的剂量-效应关系仍需进一步探讨。以下将从抗阻运动的角度来分析抗阻运动通过肌因子对骨代谢的影响。 2.4.1 抗阻运动通过肌肉生长抑制素调控骨代谢 抗阻运动可以改善血清和肌肉中肌肉生长抑制素上调诱发的骨细微结构破坏以及力学性能下降,同时也能改善骨量、增强骨微结构,延缓骨质疏松症的发生。负重训练作为抗阻运动,对骨骼健康颇有益处。TANG等[51]研究对去卵巢大鼠进行跑速20 m/min、负重35%体质量,每周6 d、持续10周的负重训练,结果显示负重训练组大鼠股骨总骨密度和骨小梁密度分别增加8.13%和57.44%,骨骼力学性能增强。有研究对大鼠进行为期8周的负重爬梯训练,结果显示爬梯训练有效降低了肌肉生长抑制素水平,同时改善了骨量、增强了骨微结构[52]。一项研究为探讨负重跑步对1型糖尿病大鼠骨骼的影响及可能机制,将大鼠随机分为正常对照组、糖尿病组和糖尿病运动组,其中糖尿病运动组进行为期6周、每周6 d、每次35 min(含 5 min跑步与2 min间歇)、速度15 m/min、负重35%体质量的跑步训练,结果显示,负重跑步运动通过抑制肌肉生长抑制素表达下调激活素A受体2B型/Smad同源物2/3信号通路活性,同时激活Wnt/糖原合酶激酶3β/β-连"
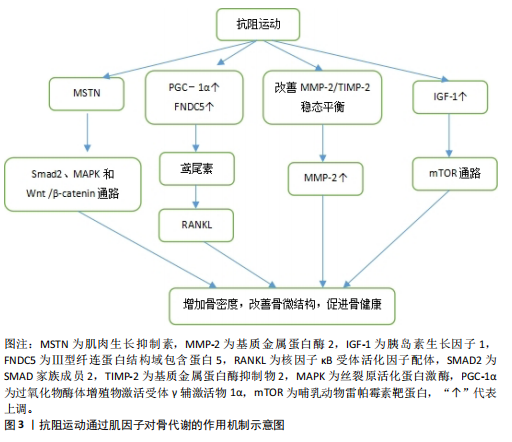
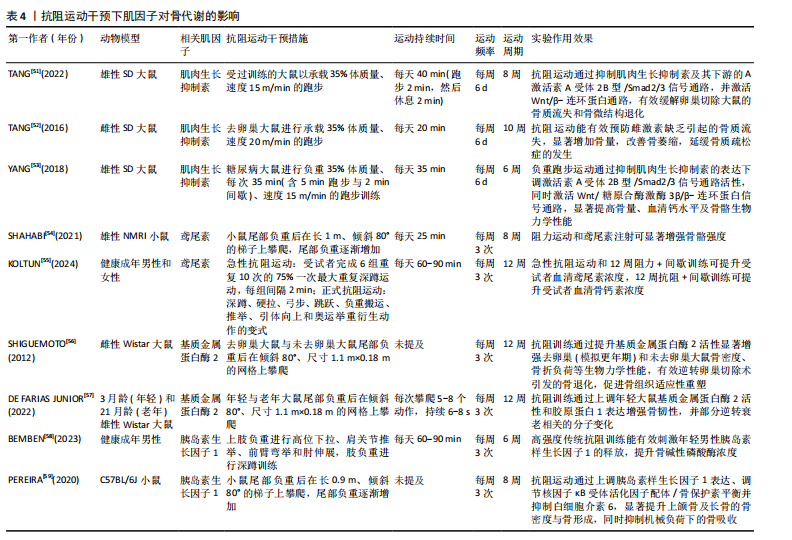
环蛋白信号通路,显著提高骨量、血清钙水平及骨骼生物力学性能,从而改善链脲佐菌素诱导的糖尿病大鼠骨代谢紊乱[53]。 2.4.2 抗阻运动通过鸢尾素调控骨代谢 抗阻运动干预后,鸢尾素浓度升高对骨代谢产生正向调控作用。SHAHABI等[54]将35只雄性小鼠分为对照组、安慰剂组、鸢尾素注射组、抗阻运动组和耐力运动组,其中抗阻运动为负重爬坡,耐力运动为跑台运动,8周干预后实验结果显示,与其他组相比,抗阻运动大幅度提高了小鼠血浆鸢尾素水平与骨强度,说明鸢尾素对骨骼机械性能及预防骨质疏松具有良好作用。近期一项研究招募了39位年轻健康受试者,其中男21人、女18人,所有受试者进行为期12周的抗阻训练和间歇训练,其中抗阻训练包括深蹲、硬拉等多关节参与的运动,间歇运动为负重短跑,结果显示,12周的抗阻与间歇训练可提升受试者胰岛素样生长因子1、鸢尾素以及骨形成标志物浓度[55]。 2.4.3 抗阻运动通过基质金属蛋白酶2调控骨代谢 抗阻运动能提高基质金属蛋白酶2活性,这种作用有助于增加骨细胞外基质的重塑。SHIGUEMOTO等[56]将去卵巢雌性大鼠分为久坐组、急性运动组和慢性运动组,急性、慢性运动组进行为期12周的阻力训练,结果显示,与久坐组相比,急性、慢性运动组大鼠骨密度、骨矿物质含量以及骨断裂载荷显著增加,其中慢性运动组大鼠基质金属蛋白酶2活性显著升高。近期一项研究将3月龄年轻大鼠和21月龄老年大鼠分别分为久坐组和抗阻运动组,抗阻运动组均进行每周3次、为期12周的负重爬梯运动,其中负重为65%,85%,95%和100%的渐进载荷,结果显示,衰老使骨密度、韧性和与细胞外基质重塑相关基因表达发生剧烈负面变化,老年大鼠抗阻训练组Ⅰ型胶原蛋白水平和基质金属蛋白酶2活性增加,对骨骼重塑的影响更为显著[57]。 2.4.4 抗阻运动通过胰岛素样生长因子1调控骨代谢 抗阻运动启动胰岛素样生长因子1,激发哺乳动物雷帕霉素靶蛋白通路,通过mRNA的翻译以及核糖体的生物合成导致肌肉蛋白质的合成。近期一项研究对18-25岁男性青年进行为期6周的高、中、低强度血流限制结合传统抗阻运动,结果显示,低强度血流限制结合传统抗阻运动后受试者血清胰岛素样生长因子1水平升高,急性骨形成标志物(骨特异性碱性磷酸酶)显著增加[58],因此,可以推测血流限制运动结合传统抗阻运动不但能减少肌肉质量的损失,同时对保持骨骼健康、促进骨生长起着至关重要作用。PEREIRA等[59]将42只C57BL/6小鼠分为久坐组、抗阻训练组和有氧训练组,结果显示,与久坐组和有氧运动组相比,抗阻训练组小鼠上颌骨以及股骨胫骨中胰岛素样生长因子1表达明显增加,骨密度、骨小梁体积和骨体积/总体积比均有增加。 抗阻运动干预下肌因子对骨代谢的影响,见表4。"
| [1] 马剑雄,匡明杰,何伟伟,等.肌肉减少症与骨质疏松的相关性:研究与应用[J].中国组织工程研究,2018,22(32):5222-5227. [2] BINKLEY N, BUEHRING B. Beyond FRAX: it’s time to consider “sarco-osteopenia”. J Clin Densitom. 2009;12(4):413-416. [3] KIRK B, FEEHAN J, LOMBARDI G, et al. Muscle, Bone, and Fat Crosstalk: the Biological Role of Myokines, Osteokines, and Adipokines. Curr Osteoporos Rep. 2020;18(4):388-400. [4] GOMARASCA M, BANFI G, LOMBARDI G. Myokines: The endocrine coupling of skeletal muscle and bone. Adv Clin Chem. 2020;94:155-218. [5] ZHANG L, LV J, WANG C, et al. Myokine, a key cytokine for physical exercise to alleviate sarcopenic obesity. Mol Biol Rep. 2023;50(3):2723-2734. [6] 徐帅,徐道明,沈飞.肌骨系统中运动干预肌肉与骨骼交互功能的机制研究进展[J].山东体育学院学报,2022,38(2):91-99. [7] RINDERKNECHT E, HUMBEL RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978;253(8):2769-2776. [8] OKADA Y, NAGASE H, HARRIS ED. Matrix metalloproteinases 1, 2, and 3 from rheumatoid synovial cells are sufficient to destroy joints. J Rheumatol. 1987;14 Spec No:41-42. [9] CANALIS E, CENTRELLA M, BURCH W, et al. Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest. 1989;83(1):60-65. [10] WAGNER KR, COHEN JS. Myostatin-Related Muscle Hypertrophy-RETIRED CHAPTER, FOR HISTORICAL REFERENCE ONLY. 2005 Oct 5 [updated 2013 Jul 3].//Adam MP, Feldman J, Mirzaa GM, et al. editors. GeneReviews®[Internet]. Seattle (WA): University of Washington, Seattle; 1993-2025. [11] BORST E, DE HOYOS DV, GARZARELLA L, et al. Effects of resistance training on insulin-like growth factor-I and IGF binding proteins. Med Sci Sports Exerc. 2001;33(4):648-653. [12] ROTH SM, MARTEL GF, FERRELL RE, et al. Myostatin gene expression is reduced in humans with heavy-resistance strength training: a brief communication. Exp Biol Med (Maywood). 2003;228(6):706-709. [13] MOSIG RA, DOWLING O, DIFEO A, et al. Loss of MMP-2 disrupts skeletal and craniofacial development and results in decreased bone mineralization, joint erosion and defects in osteoblast and osteoclast growth. Hum Mol Genet. 2007;16(9):1113-1123. [14] PEDERSEN BK. Muscles and their myokines. J Exp Biol. 2011;214(Pt 2):337-346. [15] BOSTRÖM P, WU J, JEDRYCHOWSKI MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463-468. [16] SOUZA MV, LEITE RD, SOUZA LINO AD, et al. Resistance training improves body composition and increases matrix metalloproteinase 2 activity in biceps and gastrocnemius muscles of diet-induced obese rats. Clinics (Sao Paulo). 2014;69(4):265-270. [17] QIN Y, PENG Y, ZHAO W, et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J Biol Chem. 2017;292(26):11021-11033. [18] KIM H, WRANN CD, JEDRYCHOWSKI M, et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell. 2018;175(7):1756-1768.e17. [19] RICE JC, WEEKLEY BH, KANHOLM T, et al. MMP-2 is a novel histone H3 N-terminal protease necessary for myogenic gene activation. Epigenetics Chromatin. 2021; 14(1):23. [20] LI M, LIU Q, XIE S, et al. LncRNA TCONS_00323213 Promotes Myogenic Differentiation by Interacting with PKNOX2 to Upregulate MyoG in Porcine Satellite Cells. Int J Mol Sci. 2023;24(7):6773. [21] JU CR, CHEN C. Serum myostatin levels and skeletal muscle wasting in chronic obstructive pulmonary disease. Respir Med. 2012;106(1):102-108. [22] WU L, ZHU D, WANG B, et al. Relative abundance of mature myostatin rather than total myostatin is negatively associated with bone mineral density in Chinese. J Cell Mol Med. 2018;22(2):1329-1336. [23] SHANAZARI Z, FARAMARZI M, BANITALEBI E, et al. Effect of moderate and high-intensity endurance and resistance training on serum concentrations of MSTN and IGF-1 in old male Wistar rats. Horm Mol Biol Clin Investig. 2019;38(2):/j/hmbci.2019.38.issue-2/hmbci-2018-0066/hmbci-2018-0066.xml. doi: 10.1515/hmbci-2018-0066. [24] OMOSULE CL, PHILLIPS CL. Deciphering Myostatin’s Regulatory, Metabolic, and Developmental Influence in Skeletal Diseases. Front Genet. 2021;12:662908. [25] WU Z, PUIGSERVER P, ANDERSSON U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999; 98(1):115-124. [26] STORLINO G, COLAIANNI G, SANESI L, et al. Irisin Prevents Disuse-Induced Osteocyte Apoptosis. J Bone Miner Res. 2020;35(4):766-775. [27] ZHU X, LI X, WANG X, et al. Irisin deficiency disturbs bone metabolism. J Cell Physiol. 2021;236(1):664-676. [28] ESTELL EG, LE PT, VEGTING Y, et al. Irisin directly stimulates osteoclastogenesis and bone resorption in vitro and in vivo. Elife. 2020;9:e58172. [29] LUO Y, QIAO X, MA Y, et al. Disordered metabolism in mice lacking irisin. Sci Rep. 2020;10(1):17368. [30] KOLKENBROCK H, ORGEL D, HECKER-KIA A, et al. The complex between a tissue inhibitor of metalloproteinases (TIMP-2) and 72-kDa progelatinase is a metalloproteinase inhibitor. Eur J Biochem. 1991;198(3): 775-781. [31] 任磊,苗杰,秦永生,等.自主跑轮运动调控MMP-2/TIMP-2稳态平衡改善去卵巢大鼠心脏重塑[J].天津体育学院学报, 2019,34(4):330-336. [32] VANATKA R, ROUZIER C, LAMBERT JC, et al. Winchester syndrome: the progression of radiological findings over a 23-year period. Skeletal Radiol. 2011;40(3):347-351. [33] NI X, XIA T, ZHAO Y, et al. Downregulation of miR-106b induced breast cancer cell invasion and motility in association with overexpression of matrix metalloproteinase 2. Cancer Sci. 2014;105(1):18-25. [34] DERIGGI-PISANI GF, STOTZER US, MARQUETI RC, et al. Role of resistance training in bone macro and micro damages in an estrogen absence animal model. Life Sci. 2023;317:121417. [35] ZHOU M, GRAVES DT. Impact of the host response and osteoblast lineage cells on periodontal disease. Front Immunol. 2022;13:998244. [36] DELAGRANGE M, ROUSSEAU V, CESSANS C, et al. Low bone mass in Noonan syndrome children correlates with decreased muscle mass and low IGF-1 levels. Bone. 2021;153:116170. [37] SHENG MHC, ZHOU XD, BONEWALD LF, et al. Disruption of the insulin-like growth factor-1 gene in osteocytes impairs developmental bone growth in mice. Bone. 2013;52(1):133-144. [38] DRISSI H, LOMRI A, LASMOLES F, et al. Skeletal unloading induces biphasic changes in insulin-like growth factor-I mRNA levels and osteoblast activity. Exp Cell Res. 1999; 251(2):275-284. [39] SHIMONTY A, BONEWALD LF, HUOT JR. Metabolic Health and Disease: A Role of Osteokines? Calcif Tissue Int. 2023;113(1): 21-38. [40] EHRNBORG C, LANGE KHW, DALL R, et al. The growth hormone/insulin-like growth factor-I axis hormones and bone markers in elite athletes in response to a maximum exercise test. J Clin Endocrinol Metab. 2003; 88(1):394-401. [41] KOK HJ, CROWDER CN, KOO MIN CHEE L, et al. Muscle insulin-like growth factor-I modulates murine craniofacial bone growth. Eur Cell Mater. 2021;42:72-89. [42] KAZEMI F. The correlation of resistance exercise-induced myostatin with insulin resistance and plasma cytokines in healthy young men. J Endocrinol Invest. 2016; 39(4):383-388. [43] HOOSHMANDI Z, DARYANOOSH F, AHMADI HEKMATIKAR A H, et al. Highlighting the effect of reduced training volume on maintaining hormonal adaptations obtained from periodized resistance training in sarcopenic older women. Expert Rev Endocrinol Metab. 2024;19(2):187-197. [44] BLIZZARD LEBLANC DR, RIOUX BV, PELECH C, et al. Exercise-induced irisin release as a determinant of the metabolic response to exercise training in obese youth: the EXIT trial. Physiol Rep. 2017;5(23):e13539. [45] RAHIMI M, NAZARALI P, ALIZADEH R. Pilates and TRX training methods can improve insulin resistance in overweight women by increasing an exercise-hormone, Irisin. J Diabetes Metab Disord. 2021;20(2):1455-1460. [46] KIM HJ, LEE HJ, SO B, et al. Effect of aerobic training and resistance training on circulating irisin level and their association with change of body composition in overweight/obese adults: a pilot study. Physiol Res. 2016;65(2):271-279. [47] NOGUEIRA ME, SOUSA NETO IV, MOTTA-SANTOS D, et al. High-protein diet associated with resistance training reduces cardiac TNF-α levels and up-regulates MMP-2 activity in rats. Arch Physiol Biochem. 2022;128(6):1630-1636. [48] ASTILL BD, KATSMA MS, CAUTHON DJ, et al. Sex-based difference in Achilles peritendinous levels of matrix metalloproteinases and growth factors after acute resistance exercise. J Appl Physiol (1985). 2017;122(2):361-367. [49] LI B, FENG L, WU X, et al. Effects of different modes of exercise on skeletal muscle mass and function and IGF-1 signaling during early aging in mice. J Exp Biol. 2022; 225(21):jeb244650. [50] ARAZI H, BABAEI P, MOGHIMI M, et al. Acute effects of strength and endurance exercise on serum BDNF and IGF-1 levels in older men. BMC Geriatr. 2021;21(1):50. [51] TANG L, ZHAO T, KANG Y, et al. MSTN is an important myokine for weight-bearing training to attenuate bone loss in ovariectomized rats. J Physiol Biochem. 2022;78(1):61-72. [52] TANG L, GAO X, YANG X, et al. Ladder-Climbing Training Prevents Bone Loss and Microarchitecture Deterioration in Diet-Induced Obese Rats. Calcif Tissue Int. 2016; 98(1):85-93. [53] YANG J, SUN L, FAN X, et al. Effect of exercise on bone in poorly controlled type 1 diabetes mediated by the ActRIIB/Smad signaling pathway. Exp Ther Med. 2018;16(4):3686-3693. [54] SHAHABI S, ESFARJANI F, REISI J, et al. The Effects of 8-Week Resistance and Endurance Trainings on Bone Strength Compared to Irisin Injection Protocol in Mice. Adv Biomed Res. 2021;10:40. [55] KOLTUN KJ, STERCZALA AJ, SEKEL NM, et al. Effect of acute resistance exercise on bone turnover in young adults before and after concurrent resistance and interval training. Physiol Rep. 2024;12(3):e15906. [56] SHIGUEMOTO GE, PRESTES J, LEITE RD, et al. Effects of resistance training on matrix metalloproteinase-2 activity and biomechanical and physical properties of bone in ovariectomized and intact rats. Scand J Med Sci Sports. 2012;22(5):607-617. [57] DE FARIAS JUNIOR GC, DE SOUSA NETO IV, GUZZONI V, et al. Remodeling process in bone of aged rats in response to resistance training. Life Sci. 2020;256:118008. [58] BEMBEN DA, SHERK VD, BUCHANAN SR, et al. Acute and chronic bone marker and endocrine responses to resistance exercise with and without blood flow restriction in young men. J Strength Cond Res. 2023; 37(4):893-901. [59] PEREIRA LJ, MACARI S, COIMBRA CC, et al. Aerobic and resistance training improve alveolar bone quality and interferes with bone-remodeling during orthodontic tooth movement in mice. Bone. 2020;138:11549. |
| [1] | Chen Huiting, Zeng Weiquan, Zhou Jianhong, Wang Jie, Zhuang Congying, Chen Peiyou, Liang Zeqian, Deng Weiming. Tail anchoring technique of vertebroplasty in treatment of osteoporotic vertebral compression fractures with intravertebral cleft: a finite element analysis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2145-2152. |
| [2] | Zeng Xuan, Weng Rui, Ye Shicheng, Tang Jiadong, Mo Ling, Li Wenchao. Two lumbar rotary manipulation techniques in treating lumbar disc herniation: a finite element analysis of biomechanical differences [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2153-2161. |
| [3] | Cheng Qisheng, Julaiti·Maitirouzi, Xiao Yang, Zhang Chenwei, Paerhati·Rexiti. Finite element analysis of novel variable-diameter screws in modified cortical bone trajectory of lumbar vertebrae [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(9): 2162-2171. |
| [4] | Hu Xiongke, Liu Shaohua, Tan Qian, Liu Kun, Zhu Guanghui. Shikonin intervention with bone marrow mesenchymal stem cells improves microstructure of femur in aged mice [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(7): 1609-1615. |
| [5] | Wen Guangwei, Zhen Yinghao, Zheng Taikeng, Zhou Shuyi, Mo Guoye, Zhou Tengpeng, Li Haishan, Lai Yiyi. Effects and mechanisms of isoginkgetin on osteoclastogenesis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1348-1358. |
| [6] | Wu Zhilin, , He Qin, Wang Pingxi, Shi Xian, Yuan Song, Zhang Jun, Wang Hao . DYRK2: a novel therapeutic target for rheumatoid arthritis combined with osteoporosis based on East Asian and European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1569-1579. |
| [7] | Zhang Haiwen, Zhang Xian, Xu Taichuan, Li Chao. Bibliometric and visual analysis of the research status and trends of senescence in osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1580-1591. |
| [8] | Huang Jie, Zeng Hao, Wang Wenchi, Lyu Zhucheng, Cui Wei. Visualization analysis of literature on the effect of lipid metabolism on osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(6): 1558-1568. |
| [9] | Yang Zhijie, Zhao Rui, Yang Haolin, Li Xiaoyun, Li Yangbo, Huang Jiachun, Lin Yanping, Wan Lei, HuangHongxing. Postmenopausal osteoporosis: predictive values of muscle mass, grip strength, and appendicular skeletal muscle index [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1073-1080. |
| [10] | Zhou Jian, Zhang Tao, Zhou Weili, Zhao Xingcheng, Wang Jun, Shen Jie, Qian Li, Lu Ming. Effects of resistance training on quadriceps mass and knee joint function in patients with osteoporosis and sarcopenia [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(5): 1081-1088. |
| [11] | Cao Wenqi, Feng Xiuzhi, Zhao Yi, Wang Zhimin, Chen Yiran, Yang Xiao, Ren Yanling. Effect of macrophage polarization on osteogenesis-angiogenesis coupling in type 2 diabetic osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 917-925. |
| [12] | Zeng Hao, Sun Pengcheng, Chai Yuan, Huang Yourong, Zhang Chi, Zhang Xiaoyun. Association between thyroid function and osteoporosis: genome-wide data analysis of European populations [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(4): 1019-1027. |
| [13] | Xu Jiamu, Yang Cheng, Li Weimin, Wang Chunqing. Role and pathogenesis of pyroptosis and inflammatory factors in osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 691-700. |
| [14] | Wang Meng, Lu Tan, Li Minjie, Liu Zhicheng, Guo Xiaoyong. Finite element analysis of stress distribution of anchors at different implantation depths under different bone density conditions in rotator cuff tears [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(3): 561-569. |
| [15] | Yang Fengli, Zhou Chao, Xiong Wei, Zhou Yuxiang, Li Dengshun, Wang Xin, Li Zhanzhen. 3D printed poly-L-lactic acid bone scaffolds in repair of bone defects [J]. Chinese Journal of Tissue Engineering Research, 2026, 30(2): 507-515. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||