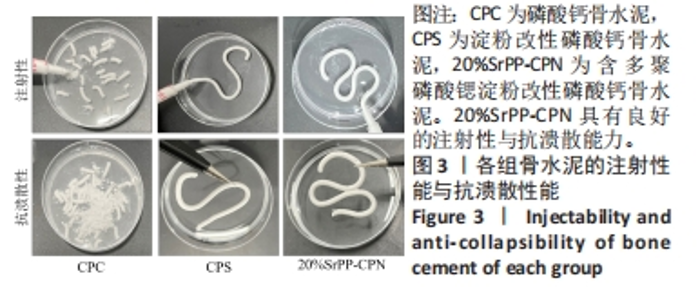
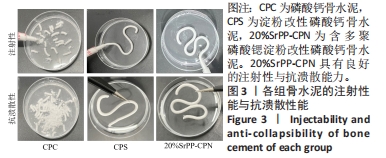
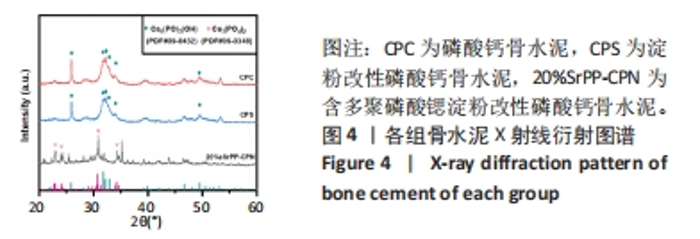
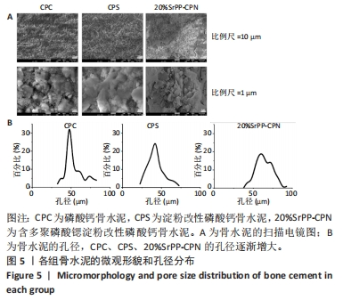
Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (22): 3539-3547.doi: 10.12307/2024.522
Previous Articles Next Articles
Application of strontium polyphosphate with both radiopaque and osteogenic functions in calcium phosphate cement
Tang Ziniu1, 2, Chu Fengcheng1, 2, Wu Kang1, 2, Zhang Lin1, 2, Bai Yanjie3, Lin Xiao1, 2, Yang Huilin1, 2, Zhou Huan4, Liu Huiling1, 2, Yang Lei1, 2, 4
- 1First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China; 2Institute of Orthopedics, Soochow University, Suzhou 215006, Jiangsu Province, China, 3Department of Chemical Engineering; 4School of Health Sciences and Biomedical Engineering, Hebei University of Technology, Tianjin 300131, China
-
Received:2023-08-21Accepted:2023-10-12Online:2024-08-08Published:2024-01-20 -
Contact:Liu Huiling, Master, Senior experimentalist, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China; Institute of Orthopedics, Soochow University, Suzhou 215006, Jiangsu Province, China Yang Lei, PhD, Professor, Doctoral supervisor, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China; Institute of Orthopedics, Soochow University, Suzhou 215006, Jiangsu Province, China; School of Health Sciences and Biomedical Engineering, Hebei University of Technology, Tianjin 300131, China -
About author:Tang Ziniu, Master, First Affiliated Hospital of Soochow University, Suzhou 215006, Jiangsu Province, China; Institute of Orthopedics, Soochow University, Suzhou 215006, Jiangsu Province, China -
Supported by:National Key Research and Development Project, No. 2020YFC1107401 (to YL); National Natural Science Foundation of China, No. 82025025 (to YL); National Natural Science Foundation of China, No. 81802155 (to LHL); National Natural Science Foundation of China, No. 32171321 (to LX); Yunnan Academician Workstations, No. 202205AF150025 (to YL); National Clinical Research Center for Orthopedics, Sports Medicine & Rehabilitation, No. 2021-NCRC-CXJJ-ZH-17 (to YL); Suzhou Key Laboratory of Orthopedics, No. SZS2022017 (to YHL)
CLC Number:
Cite this article
Tang Ziniu, Chu Fengcheng, Wu Kang, Zhang Lin, Bai Yanjie, Lin Xiao, Yang Huilin, Zhou Huan, Liu Huiling, Yang Lei. Application of strontium polyphosphate with both radiopaque and osteogenic functions in calcium phosphate cement[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(22): 3539-3547.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
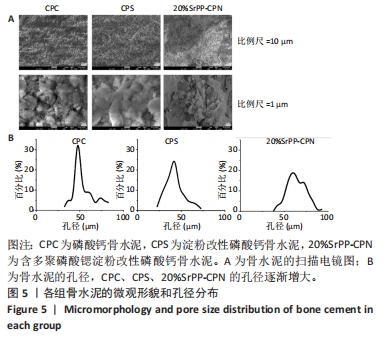
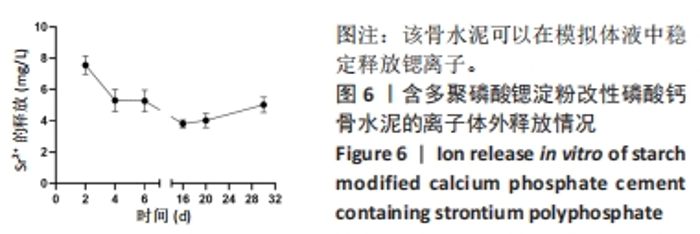
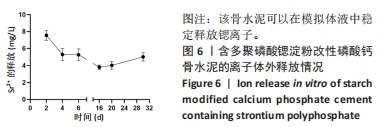
2.1 SrPP及骨水泥的显影性能 从图2A,B可以看出,皮质骨与SrPP的亮度接近,均显著强于多聚磷酸钠;对于骨水泥而言,CPC亮度较低,CPS的亮度进一步降低,而20%SrPP-CPN的亮度明显提高,与20%BaSO4-CPN的亮度接近,表明20%SrPP-CPN的显影性能较强,且显影能力与添加20%BaSO4骨水泥相似。显影灰度值定量分析显示,皮质骨、SrPP、多聚磷酸钠、CPC、CPS、20%SrPP-CPN、20%BaSO4-CPN的对比值分别为0.74±0.01,0.79±0.01,0.24±0.04,0.78±0.01,0.75±0.01,0.85±0.01,0.84±0.01,见图2C,D。结果证实,20%SrPP-CPN的显影性能可以满足临床应用需求。"
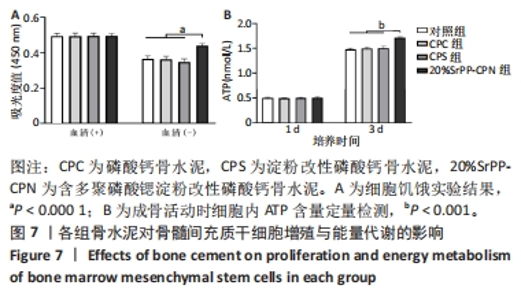
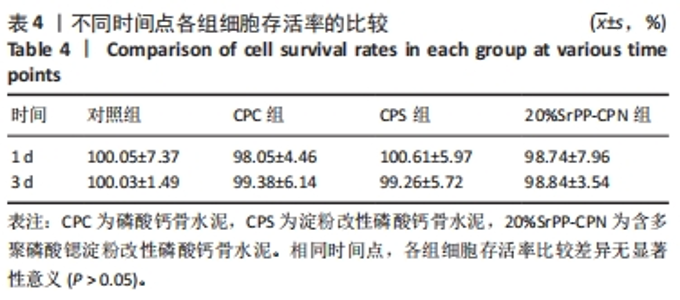
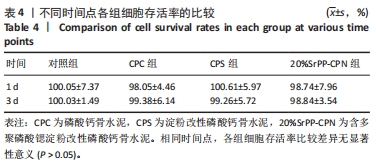
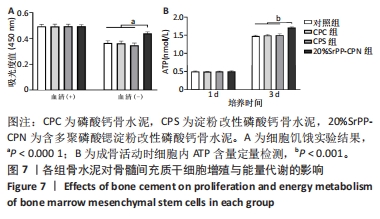
通过饥饿实验进一步验证20%SrPP-CPN浸提液对细胞增殖的影响。培养48 h后,添加胎牛血清时,各组细胞吸光度值比较差异无显著性意义(P > 0.05),表明体积分数10%胎牛血清的供能效果可以满足细胞正常生长;未添加胎牛血清时,各组细胞吸光度值均低于添加胎牛血清时对应组别,并且20%SrPP-CPN组细胞吸光度值高于其他3组(P < 0.000 1),见图7A。实验结果证明,20%SrPP-CPN浸提液能够促进骨髓间充质干细胞的增殖。 2.3.2 SrPP对骨水泥调节细胞能量代谢的影响 培养第1天,对照组、CPC组、CPS组、20%SrPP-CPN组细胞内ATP浓度分别为(0.50±0.02),(0.50±0.01),(0.51±0.01),(0.51±0.02) nmol/L,4组间比较差异无显著性意义(P > 0.05);培养第3天,对照组、CPC组、CPS组、20%SrPP-CPN组细胞内ATP浓度分别为(1.48±0.02),(1.50±0.02),(1.50±0.04),(1.71±0.02) nmol/L,20%SrPP-CPN组高于其他3组(P < 0.001),见图7B。"
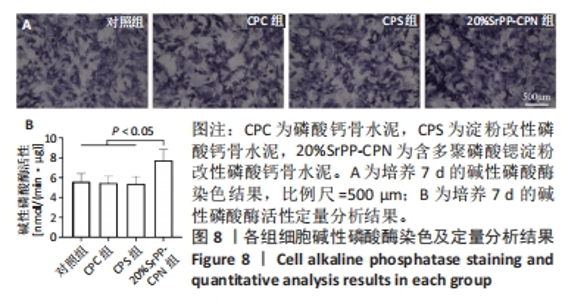
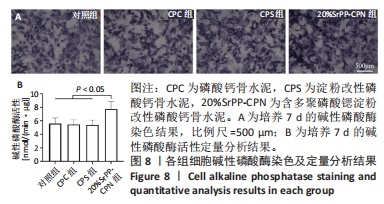
2.4 SrPP对骨水泥体外促进成骨分化的作用 2.4.1 碱性磷酸酶染色结果 从图8A可以观察到各组细胞均呈现阳性染色,证明成骨诱导培养基产生了成骨效能,CPC组、CPS组细胞阳性染色相较于对照组增强的原因可能是浸提液中Ca2+和PO43-浓度升高的作用[23],20%SrPP-CPN组细胞阳性染色增强更加明显,表明SrPP的加入增强了骨水泥促进骨髓间充质干细胞碱性磷酸酶表达的能力。 2.4.2 碱性磷酸酶活性检测结果 成骨诱导培养7 d时,对照组、CPC组、CPS组、20%SrPP-CPN组碱性磷酸酶活性分别为(5.60±0.82),(5.46±0.68),(5.40±0.70),(7.74±1.11) nmol/(min?μg),20%SrPP-CPN组高于其他3组(P < 0.05),见图8B。上述结果表明,SrPP的加入增强了CPC对骨髓间充质干细胞成骨蛋白碱性磷酸酶的诱导表达能力。"
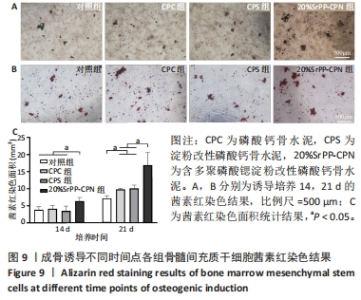
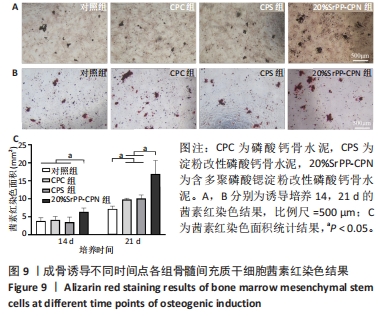
2.4.3 茜素红染色结果 如图9A,B所示,成骨诱导14 d,各组细胞外基质中均观察到茜素红染色;成骨诱导21 d,各组茜素红染色逐渐增多,相较于未添加SrPP组,20%SrPP-CPN组成骨诱导14,21 d的茜素红染色更加明显。Image J统计分析结果显示,成骨诱导14 d,对照组、CPC组、CPS组、20%SrPP-CPN组茜素红染色面积分别为(3.86±0.86),(4.17±0.93),(3.53±1.38),(6.38±1.05) mm2,20%SrPP-CPN组大于其他3组(P < 0.05);成骨诱导21 d,对照组、CPC组、CPS组、20%SrPP-CPN组茜素红染色面积分别为(7.18±0.78),(9.83±0.35),(10.11±0.95),(16.90±3.75) mm2,对照组小于CPC组、CPS组(P < 0.05),CPC组、CPS组小于20%SrPP-CPN组(P < 0.05),见图9C。表明SrPP的添加进一步增强了细胞外基质中的钙沉积。"
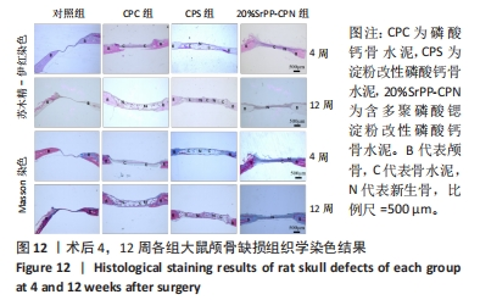
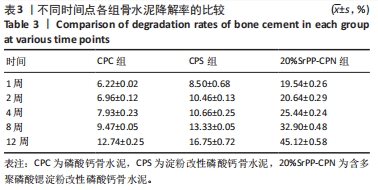
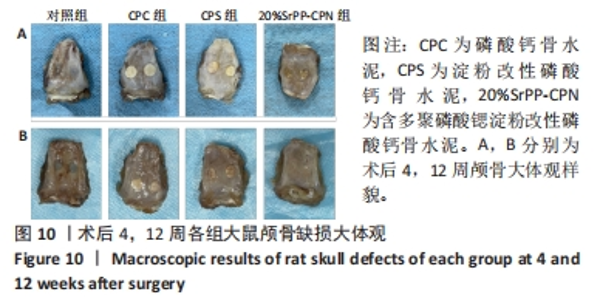
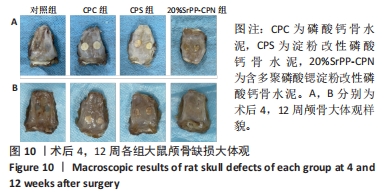
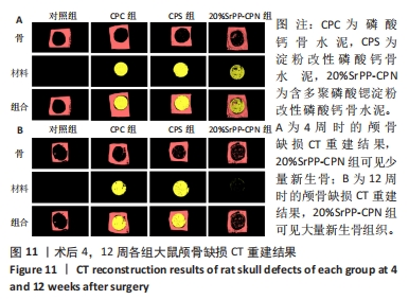
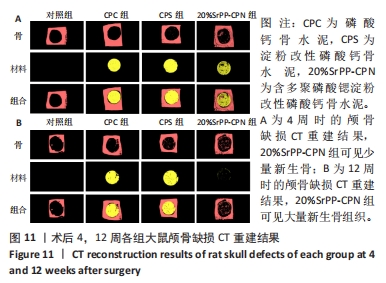
术后4周,对照组缺损处未观察到明显变化,CPC组与CPS组材料未发生明显降解,未见到明显新生骨;20%SrPP-CPN材料出现明显降解,缺损边缘有少量新生骨,并沿着骨水泥降解产生的孔隙长入骨水泥内部。术后12周,对照组缺损处仍未发生明显改变,证明了颅骨5 mm缺损为颅骨的临界缺损;CPC组材料少量降解,仅在缺损边缘产生少量的新生骨;CPS组材料降解较CPC组略微增强,在缺损边缘和降解的骨水泥内部均可观察到少量新生骨;20%SrPP-CPN组材料显著降解,降解率约为80%,新生骨相较于其他组更多,并且散在均匀分布于缺损边缘和降解的骨水泥中。 2.5.4 各组大鼠颅骨标本组织学观察结果 各组大鼠颅骨标本苏木精-伊红与Masson染色见图12。"
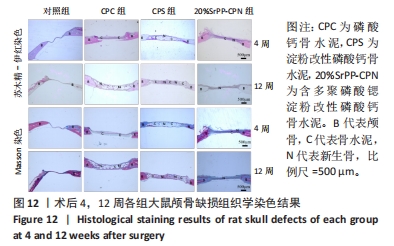
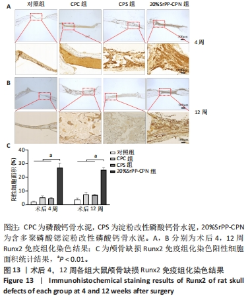
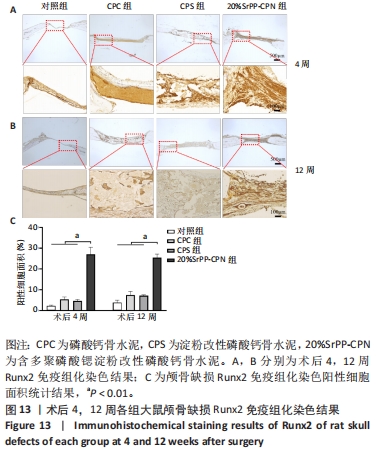
苏木精-伊红染色:术后4周,对照组骨缺损处未发生显著改变,仅有少量纤维组织连接;CPC组骨水泥未见明显变化;CPS组骨水泥发生了少量降解;20%SrPP-CPN组骨水泥降解较为明显,纤维组织均匀分散于降解的骨水泥中。术后12周,对照组骨缺损处仍未发生明显改变;CPC组骨水泥发生部分溃散,较多新生纤维包裹了骨水泥;CPS组骨水泥中存在新生纤维均匀长入;20%SrPP-CPN组中骨水泥降解明显,可观察到致密的纤维组织而未能观察到明显骨水泥存在。 Masson染色:对照组全程未观察到新生骨组织形态,仅观察到新生的纤维组织。术后4周,CPC组由于材料降解缓慢且生物活性较低,因而未能观察到明显新生骨;CPS组骨水泥发生少量降解,极少量新生骨分布于降解的骨水泥中;20%SrPP-CPN组可以观察到少量新生骨分布于增生的纤维组织中。术后12周,CPC组缺损边缘溃散部位产生了少量新生骨,CPS组中少量新生骨散在分布于骨水泥和纤维组织中,20%SrPP-CPN组中有较多新生骨分布于降解的骨水泥中。 各组大鼠颅骨标本Runx2免疫组化染色结果见图13。术后4周,对照组未观察到阳性染色;CPC组骨水泥被染料浸染,未观察到明显的阳性染色细胞;CPS组骨水泥降解部位散在分布少量的阳性染色细胞;20%SrPP-CPN组缺损部位细胞出现较强的阳性染色,均匀分布于新生纤维组织与骨水泥间隙中。术后12周,对照组阳性染色无明显变化;CPC组骨水泥边缘降解部位分布有少量的阳性染色细胞;CPS组骨水泥中均匀分布着纤维组织,少量阳性染色细胞分布其中,且染色程度较浅;20%SrPP-CPN组缺损部位由阳性染色细胞覆盖,阳性染色细胞分布均匀,细胞染色程度较深。Image J统计分析结果显示,术后4周,对照组、CPC组、CPS组、20%SrPP-CPN组Runx2阳性细胞染色面积分别为(2.23±0.39)%,(5.45±1.07)%,(4.66±0.64)%,(27.11±3.36)%,20%SrPP-CPN组大于其他3组(P < 0.01);术后12周,对照组、CPC组、CPS组、20%SrPP-CPN组Runx2阳性细胞染色面积分别为(3.87±1.18)%,(7.53±1.67)%,(7.19±0.39)%,(25.44±1.76)%,20%SrPP-CPN组大于其他3组(P < 0.01)。证实20%SrPP-CPN组表达了更多的成骨相关蛋白。"
| [1] WU T, YANG S, SHI H, et al. Preparation and cytocompatibility of a novel bismuth aluminate/calcium phosphate cement with high radiopacity. J Mater Sci Mater Med. 2018;29(9):149. [2] SNEHA KR, SAILAJA GS. Intrinsically radiopaque biomaterial assortments: a short review on the physical principles, X-ray imageability, and state-of-the-art developments. J Mater Chem B. 2021;9(41):8569-8593. [3] SAS A, HELGASON B, FERGUSON SJ, et al. Mechanical and morphological characterization of PMMA/bone composites in human femoral heads. J Mech Behav Biomed Mater. 2021;115:104247. [4] DEMIR-OĞUZ Ö, BOCCACCINI AR, LOCA D. Injectable bone cements: What benefits the combination of calcium phosphates and bioactive glasses could bring? Bioact Mater. 2023;19:217-236. [5] ZHANG R, LIU H, ZHOU H, et al. Pregelatinized starch as a cohesion promoter improves mechanical property and surgical performance of calcium phosphate bone cement: the effect of starch type. Mater Technol. 2022;37(14):3110-3121. [6] LIU H, ZHANG Z, GAO C, et al. Enhancing effects of radiopaque agent BaSO(4) on mechanical and biocompatibility properties of injectable calcium phosphate composite cement. Mater Sci Eng C Mater Biol Appl. 2020;116:110904. [7] ABERG J, HENRIKSSON HB, ENGQVIST H, et al. Biocompatibility and resorption of a radiopaque premixed calcium phosphate cement. J Biomed Mater Res A. 2012;100(5):1269-1278. [8] SABOKBAR A, FUJIKAWA Y, MURRAY DW, et al. Radio-opaque agents in bone cement increase bone resorption. J Bone Joint Surg Br. 1997;79(1):129-134. [9] LE FERREC M, MELLIER C, BOUKHECHBA F, et al. Design and properties of a novel radiopaque injectable apatitic calcium phosphate cement, suitable for image-guided implantation. J Biomed Mater Res B Appl Biomater. 2018;106(8):2786-2795. [10] HARRISON CJ, HATTON PV, GENTILE P, et al. Nanoscale Strontium-Substituted Hydroxyapatite Pastes and Gels for Bone Tissue Regeneration. Nanomaterials (Basel). 2021;11(6):1161-1180. [11] DAI J, FU Y, CHEN D, et al. A novel and injectable strontium-containing hydroxyapatite bone cement for bone substitution: A systematic evaluation. Mater Sci Eng C Mater Biol Appl. 2021;124:112052. [12] DUMRONGVUTE K, ADEL S, WADA T, et al. Distrontium Cerate as a Radiopaque Component of Hydraulic Endodontic Cement. Materials (Basel). 2021;15(1): 284-294. [13] ARDESHIR, YLAJIMI A, GOLCHIN A, et al. Increased osteogenic differentiation potential of MSCs cultured on nanofibrous structure through activation of Wnt/β-catenin signalling by inorganic polyphosphate. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S943-S949. [14] WANG X, SCHRÖDER HC, MÜLLER WEG. Amorphous polyphosphate, a smart bioinspired nano-/bio-material for bone and cartilage regeneration: towards a new paradigm in tissue engineering. J Mater Chem B. 2018;6(16):2385-2412. [15] LEE NH, KANG MS, KIM TH, et al. Dual actions of osteoclastic-inhibition and osteogenic-stimulation through strontium-releasing bioactive nanoscale cement imply biomaterial-enabled osteoporosis therapy. Biomaterials. 2021;276:121025. [16] CHEN Y, CANELI G, ALMOUSA R, et al. A novel antibacterial zirconia-containing PMMA bone cement. J Mech Behav Biomed Mater. 2022;129:105135. [17] TIAN Y, LIU H, HE L, et al. Calcium phosphate-based composite cement: Impact of starch type and starch pregelatinization on its physicochemical properties and performance in the vertebral fracture surgical models in vitro. J Biomed Mater Res B Appl Biomater. 2021;109(12):2068-2078. [18] LIU H, LIU B, GAO C, et al. Injectable, biomechanically robust, biodegradable and osseointegrative bone cement for percutaneous kyphoplasty and vertebroplasty. Int Orthop. 2018;42(1):125-132. [19] CHEN F, LIU C, MAO Y. Bismuth-doped injectable calcium phosphate cement with improved radiopacity and potent antimicrobial activity for root canal filling. Acta Biomater. 2010;6(8):3199-3207. [20] LU M, ZHANG XS, CHANG L, et al. Preparation, performance and characterization of bioactive bone materials with plasticity. Chin J Tissue Eng Res. 2015;19(21):3323. [21] WU X, TANG Z, WU K, et al. Strontium-calcium phosphate hybrid cement with enhanced osteogenic and angiogenic properties for vascularised bone regeneration. J Mater Chem B. 2021;9(30):5982-5997. [22] CUI X, ZHANG Y, WANG J, et al. Strontium modulates osteogenic activity of bone cement composed of bioactive borosilicate glass particles by activating Wnt/β-catenin signaling pathway. Bioact Mater. 2020;5(2):334-347. [23] HUANG H, LAN LQ, WU JQ, et al.[Effect of bone marrow mesenchymal stem cells on paraquat-induced pulmonary fibrosis in rats]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2020;38(5):332-338. [24] WU K, YANG Q, ZHANG L, et al. An injectable curcumin-releasing organohydrogel with non-drying property and high mechanical stability at low-temperature for expedited skin wound care. J Mater Sci Technol. 2023;133:123-134. [25] SHIBA T, NISHIMURA D, KAWAZOE Y, et al. Modulation of mitogenic activity of fibroblast growth factors by inorganic polyphosphate. J Biol Chem. 2003;278(29): 26788-26792. [26] LI R, LIN S, ZHU M, et al. Synthetic presentation of noncanonical Wnt5a motif promotes mechanosensing-dependent differentiation of stem cells and regeneration. Sci Adv. 2019;5(10):eaaw3896. [27] LUTOLF MP, WEBER FE, SCHMOEKEL HG, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003; 21(5):513-518. [28] OUYANG Y, ZHANG R, CHEN H, et al. Novel, degradable, and cytoactive bone cements based on magnesium polyphosphate and calcium citrate. New J Chem. 2022;46(27):13137-13148. [29] TADDEI P, DI FOGGIA M, ZAMPARINI F, et al. The Influence of the Matrix on the Apatite-Forming Ability of Calcium Containing Polydimethylsiloxane-Based Cements for Endodontics. Molecules. 2022;27(18):5750-5768. [30] WU T, YANG S, LU T, et al. Strontium ranelate simultaneously improves the radiopacity and osteogenesis of calcium phosphate cement. Biomed Mater. 2019;14(3):035005. [31] WON S, KO KH, PARK CJ, et al. Effect of barium silicate filler content on mechanical properties of resin nanoceramics for additive manufacturing. J Adv Prosthodont. 2022;14(5):315-323. [32] MÜLLER WEG, TOLBA E, SCHRÖDER HC, et al. Amorphous polyphosphate-hydroxyapatite: A morphogenetically active substrate for bone-related SaOS-2 cells in vitro. Acta Biomater. 2016;31:358-367. [33] KOBAYASHI K, ANADA T, HANDA T, et al. Osteoconductive property of a mechanical mixture of octacalcium phosphate and amorphous calcium phosphate. ACS Appl Mater Interfaces. 2014;6(24):22602-22611. [34] MÜLLER WE, TOLBA E, FENG Q, et al. Amorphous Ca²⁺ polyphosphate nanoparticles regulate the ATP level in bone-like SaOS-2 cells. J Cell Sci. 2015; 128(11):2202-2207. [35] QUERIDO W, ROSSI AL, FARINA M. The effects of strontium on bone mineral: A review on current knowledge and microanalytical approaches. Micron. 2016;80: 122-134. [36] PING H, WAGERMAIER W, HORBELT N, et al. Mineralization generates megapascal contractile stresses in collagen fibrils. Science. 2022;376(6589):188-192. [37] O’DONNELL MD, HILL RG. Influence of strontium and the importance of glass chemistry and structure when designing bioactive glasses for bone regeneration. Acta Biomater. 2010;6(7):2382-2385. [38] MÜLLER WEG, TOLBA E, SCHRÖDER HC, et al. A new polyphosphate calcium material with morphogenetic activity. Mater Lett. 2015;148:163-166. [39] MÜLLER WEG, TOLBA E, ACKERMANN M, et al. Fabrication of amorphous strontium polyphosphate microparticles that induce mineralization of bone cells in vitro and in vivo. Acta Biomater. 2017;50:89-101. [40] MÜLLER WEG, SCHRÖDER HC, WANG X. Inorganic Polyphosphates As Storage for and Generator of Metabolic Energy in the Extracellular Matrix. Chem Rev. 2019;119(24):12337-12374. [41] WANG X, SCHRÖDER HC, DIEHL-SEIFERT B, et al. Dual effect of inorganic polymeric phosphate/polyphosphate on osteoblasts and osteoclasts in vitro. J Tissue Eng Regen Med. 2013;7(10):767-776. [42] WANG X, SCHRÖDER HC, SCHLOßMACHER U, et al. Inorganic polyphosphates: biologically active biopolymers for biomedical applications. Prog Mol Subcell Biol. 2013;54:261-294. [43] MÜLLER WE, TOLBA E, SCHRÖDER HC, et al. Polyphosphate: A Morphogenetically Active Implant Material Serving as Metabolic Fuel for Bone Regeneration. Macromol Biosci. 2015;15(9):1182-1197. [44] TSAI CH, LIN RM, JU CP, et al. Bioresorption behavior of tetracalcium phosphate-derived calcium phosphate cement implanted in femur of rabbits. Biomaterials. 2008;29(8):984-993. |
| [1] | Liu Zhaohui, Han Xiaoqian, Duan Xin, Guo Pengda, Zhang Yuntao. Salidroside promotes osteogenic differentiation of MC3T3-E1 cells: an in vitro experiment [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(2): 231-237. |
| [2] | Zhou Shijie, Li Muzhe, Yun Li, Zhang Tianchi, Niu Yuanyuan, Zhu Yihua, Zhou Qinfeng, Guo Yang, Ma Yong, Wang Lining. Effect of Wenshen Tongluo Zhitong formula on mouse H-type bone microvascular endothelial cell/bone marrow mesenchymal stem cell co-culture system [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 8-15. |
| [3] | Zheng Qian, Liu Pingping, Gu Yujie, Xie Lei. Effect of ursolic acid on osteogenic differentiation of human periodontal ligament stem cells#br# [J]. Chinese Journal of Tissue Engineering Research, 2025, 29(1): 80-86. |
| [4] | Yang Yufang, Yang Zhishan, Duan Mianmian, Liu Yiheng, Tang Zhenglong, Wang Yu. Application and prospects of erythropoietin in bone tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1443-1449. |
| [5] | Liu Anhong, Cai Mengmeng, Han Xiao, Wang Zhanhui. Research status on element selection of medical magnesium alloy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 777-782. |
| [6] | Lan Weiwei, Yu Yaodong, Huang Di, Chen Weiyi. In vitro degradation behavior of Mg-Zn-Ca alloys [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 717-723. |
| [7] | Li Jiaqi, Huang Yuanli, Li Yan, Wang Chunren, Han Qianqian. Mechanism and influencing factors in molecular weight degradation of non-cross-linked hyaluronic acid [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(5): 747-752. |
| [8] | Yang Cheng, Li Yusheng, Jiao Hongzhuo, Shang Man, Liu Qi, Li Linzhen, Fan Fangyang, Zhang Chenglong, Zhang Xiaoyu, Zhang Juntao. Establishment and validation of the Sprague-Dawley rat model of osteoarthritis with kidney deficiency and blood stagnation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(27): 4273-4280. |
| [9] | Ye Zhikui, Zhang Zhimin, Cui Linna, Jiang Xiaowen. Mechanism by which alendronate promotes rapid mandibular distraction osteogenesis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(23): 3642-3648. |
| [10] | Chu Fuchao, Wang Zhenxin, Zhang Dazhen, Yuan Feng. Osteogenic properties of polyacrylamide-modified gelatin methacryloyl grafted titanium alloy scaffold [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(22): 3472-3477. |
| [11] | An Ran, Shao Guo, Zhang Chunyang. Effect of dura mater on enhancement of cranial osteogenesis in rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(22): 3478-3483. |
| [12] | Zheng Heishu, Zhang Yingjuan, Wei Yanhua, Huang Hui, Ma Xiangyu, Liao Hongbing. Antibacterial performance of cerium oxide nanoenzyme against Escherichia coli [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(22): 3496-3501. |
| [13] | Chen Mingxue, Niu Jianhua, Lin Haiyan, Wu Gang, Wan Ben. Physicochemical properties and cytocompatibility of biomimetically precipitated nanocrystalline calcium phosphate granules [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(22): 3502-3508. |
| [14] | Tian Ai, Li Li, Xiao Tianjiao, Kang Jiabing, Zhan Jifan, Wei Yan, Chen Helin. Deferoxamine mesylate improves the repair of jaw bone defects in an ovariectomized rat model of osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(20): 3143-3149. |
| [15] | Lin Tianye, Wu Zhiming, Zhang Wensheng, He Xiaoming, He Mincong, Zhang Qingwen, He Wei, Wei Qiushi, Li Ziqi. Mechanism of compound Shengmai Chenggu capsule in the repair of steroid-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 200-207. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||