Chinese Journal of Tissue Engineering Research ›› 2022, Vol. 26 ›› Issue (7): 1143-1148.doi: 10.12307/2022.158
Role of regulatory T cell subsets in liver transplantation and progress in clinical application
Xuan Juanjuan, Bai Hongtai, Zhang Jixiang, Wang Yaoquan, Chen Guoyong, Wei Sidong
- People’s Hospital, Henan University, Zhengzhou 450003, Henan Province, China
-
Received:2021-04-10Revised:2021-04-15Accepted:2021-05-27Online:2022-03-08Published:2021-10-29 -
Contact:Chen Guoyong, Chief physician, People’s Hospital, Henan University, Zhengzhou 450003, Henan Province, China Wei Sidong, Chief physician, People’s Hospital, Henan University, Zhengzhou 450003, Henan Province, China -
About author:Xuan Juanjuan, Master candidate, People’s Hospital, Henan University, Zhengzhou 450003, Henan Province, China -
Supported by:the National Natural Science Foundation of China, No. U2004124 (to CGY); the Henan Medical Science and Technology Program, No. SBGJ2018071 (to CGY); the Henan Medical Science and Technology Program, No. SB201903020 (to WSD)
CLC Number:
Cite this article
Xuan Juanjuan, Bai Hongtai, Zhang Jixiang, Wang Yaoquan, Chen Guoyong, Wei Sidong. Role of regulatory T cell subsets in liver transplantation and progress in clinical application[J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1143-1148.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
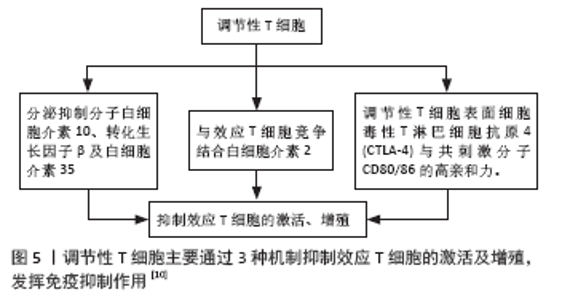
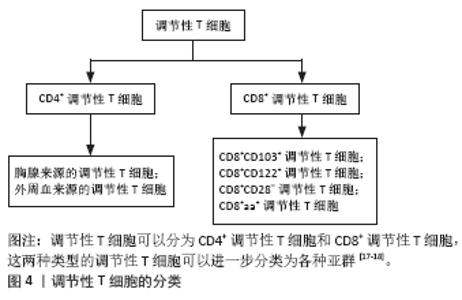
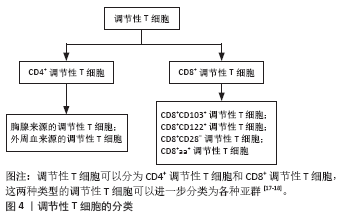
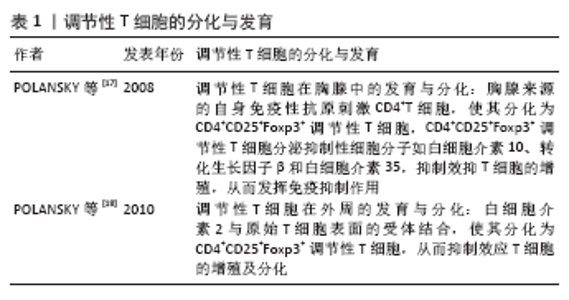
调节性T细胞主要为CD4+CD25+调节性T细胞,除表达CD4分子和CD25分子外,其特征标志为其高表达叉状头/ 翅膀状螺旋转录因子(forkhead box P3,Foxp3),叉状头转录因子是编码转录因子的基因,该转录因子调节表面受体和其他核因子的数百个基因,并与调节性T细胞的增殖、激活和自身免疫相关[11-12]。研究表明叉状头转录因子对于CD4+CD25+ 调节性T细胞的免疫抑制功能至关重要,因此被认为是在调节性T细胞中特异性表达的标记[10]。如果调节性T细胞失去Foxp3表达和免疫抑制功能,其在极端炎症条件下将转化为不稳定的效应T细胞(Teff细胞)[13-15]。CD127(也称为白细胞介素7受体亚单位α)的表达与叉状头转录因子的表达呈负相关,目前与CD25一起被用作调节性T细胞纯化的表型标志物,定义为CD4+CD25+CD127low调节性T细胞[16]。 根据调节性T细胞的来源将其分为胸腺和外周血来源的调节性T细胞,胸腺来源的调节性T细胞表现为特异性去甲基化区域完全性去甲基化,而外周血来源的调节性T细胞仅表现为特异性去甲基化区域部分去甲基化[17],调节性T细胞特异性去甲基化区域的去甲基化是其稳定性的标志。胸腺来源的调节性T细胞从胸腺中的前体单阳性CD4细胞发展而来,并在进入外周之前发展为调节性T细胞;而外周血来源的调节性T细胞是通过刺激外周血中表达叉状头转录因子的抗原从CD4+CD25-T 细胞诱导而来的[18]。根据其功能将调节性T细胞分为:Ⅰ类(P1)幼稚或静止的调节性T细胞(CD45RA+ Foxp3Lo);Ⅱ类(P2)效应调节性T细胞(CD45RA- Foxp3Hi),两者在体外均具有抑制作用;Ⅲ类(P3)是“非抑制性”分泌非调节性T细胞的细胞因子(CD45RA- Foxp3Lo)[19]。 2.3 调节性T细胞的作用机制 见图5。"
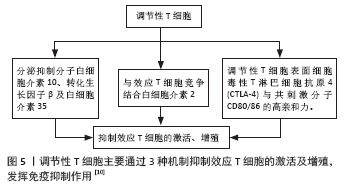
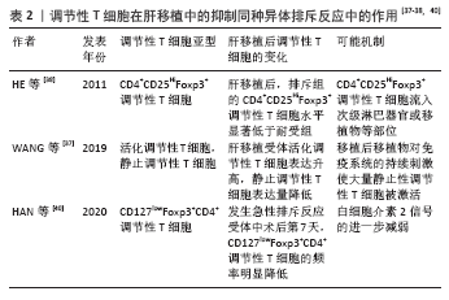
调节性T细胞利用2个不同的方式抑制免疫:旁观者抑制和感染性抑制[20-22]。旁观者抑制是T细胞受体激活的调节性T细胞可以通过抗原非特异性的方式抑制邻近CD8+T细胞,同时分泌转化生长因子β、白细胞介素10和干扰素γ等抑制性细胞因子。因此,对一种抗原具有特异性的调节性T细胞可以抑制对许多其他不同抗原特异性的效应T细胞[23-24]。感染性抑制是抑制能力能够从一个细胞群体转移到另一个细胞群体,通过抑制性细胞因子的作用产生一个新的抗原特异性的调节性T细胞群体,其抗原特异性与原始T细胞群的抗原特异性不同。这些细胞因子阻断树突状细胞的成熟和迁移,产生局部的免疫反应耐受性环境,在这种环境中,效应T细胞发生凋亡,而原始T细胞发生转化为调节性T细胞。调节性T细胞的旁观者抑制和感染性抑制使其能够在局部环境中广泛地抑制免疫反应,并创造一个耐受性环境[25-26]。调节性T细胞介导的免疫抑制通过3种机制介导实现[27]:①调节性T细胞表面表达CD25,CD25是白细胞介素2的受体α链,CD25和效应T细胞表面的白细胞介素2受体竞争白细胞介素2,剥夺效应T细胞增殖和分化所必需的白细胞介素2;②调节性T细胞分泌白细胞介素10,转化生长因子β、穿孔素和干扰素γ[28-32];③直接与抗原提呈细胞的相互作用,调节性T细胞表面分子细胞毒性T淋巴细胞抗原4(CTLA-4)与共刺激分子CD80/86的亲和力,高于传统T效应细胞上CD28与共刺激分子CD80/86的亲和力,从而阻止T效应因子的激活[33]。 2.4 调节性T细胞在肝移植抑制同种异体排斥反应中的作用 由于同种异体肝脏移植主要组织相容性抗原(MHC)不同,可诱发以淋巴细胞浸润为主的免疫排斥反应,其中1型辅助性T细胞(Th1 cells)主要通过增加移植物血管黏附性等方式促进炎症细胞的聚集,促进急性排斥反应的发生和发展,2型辅助性T细胞(Th2 cells)主要分泌白细胞介素4、白细胞介素6和白细胞介素13等[34]。肝移植后,受者受到移植肝的刺激,调节性T细胞数量及活性增加,同时转录插头因子的表达量增多[35]。调节性T细胞输注可通过抑制免疫效应细胞的功能来诱导同种抗原耐受,延长肝移植受者的存活时间[34]。在小鼠的模型中,调节性T细胞通过减弱效应T细胞反应和炎症来建立耐受,调节性T细胞不但可以限制移植物损伤,还可以引流到淋巴结以维持耐受性,抑制移植排斥反应[36]。 2.4.1 肝移植后检测调节性T细胞的变化对肝移植后的排斥反应预测具有重要意义 见表2。"
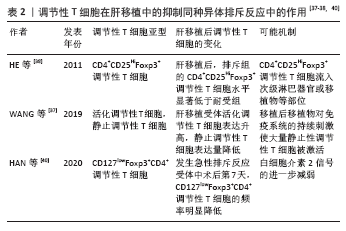
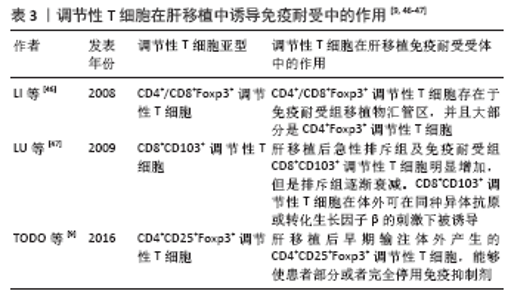
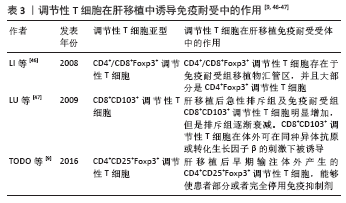
自然性调节性T细胞来源于胸腺中的前体单阳性CD4细胞,在进入外周之前发育成调节性T细胞,而诱导性调节性T细胞则是由CD4+CD25-初始T细胞在外周血抗原刺激下诱导而来的,并在被转化生长因子β激活后表达叉头状转录因子。根据CD45的表达自然性调节性T细胞被进一步分为表达CD45RA的静止调节性T细胞,或者是表达CD45RO的活化调节性T细胞。循环中的调节性T细胞主要由活化调节性T细胞组成,因为大多数静止调节性T细胞在增殖过程中会转化为活化调节性T细胞。因此,静止调节性T细胞和活化调节性T细胞可视为处于不同生长阶段的同一细胞。WANG等[37]通过流式细胞术检测 83 例肝移植后状态正常的受者中静止调节性T细胞和活化调节性T细胞、CD31的百分比,发现肝移植受者的活化调节性T细胞比例高于健康对照组,静止调节性T细胞比例低于健康对照组,推测这种差异可能与移植后新移植物对免疫系统的持续刺激有关,尤其是对静止调节性T细胞的刺激,在持续刺激下,移植后大量静止调节性T细胞转化为活化调节性T细胞,是维持免疫平衡的主要力量,这可能是长期存活组活化调节性T细胞比例较高的原因。 HE等[38]通过对2004-2009年55例接受肝移植的患者进行前瞻性分析,在移植前、移植后1年内、急性排斥反应发作时用流式细胞检测外周血CD4+CD25HiFoxp3+调节性T细胞,发现移植前后循环CD4+CD25HiFoxp3+ 调节性T细胞水平无差异,但是移植后排斥组外周血CD4+CD25HiFoxp3+调节性T细胞显著低于非排斥组,这一发现表明循环中的CD4+CD25HiFoxp3+调节性T细胞可作为预测急性排斥反应和评估肝移植受者免疫状态的替代标志物,但是排斥组外周循环T细胞减少的机制尚不完全清楚,可能与CD4+CD25HiFoxp3+调节性T细胞流动有关,如次级淋巴器官或移植物,但是目前尚且缺乏CD4+CD25HiFoxp3+调节性T细胞迁移机制的证据。同时DEMIRKIRAN等[39]的研究结果也表明在肝移植后1年,发生过急性排斥反应患者的CD4+CD25+T细胞比例明显低于未发生同种异体移植排斥反应的患者。HAN等[40]通过对128例肝移植受者外周血调节性T细胞比率及表型分析发现,在患有可疑急性排斥反应和活检证实的急性排斥反应的受者中,术后第7天总调节性T细胞和激活的调节性T细胞的频率明显降低,移植后早期调节性T细胞比率的降低与移植后急性排斥反应密切相关,移植后第7天的调节性T细胞比率可以预测急性排斥反应,并且排斥反应早期调节性T细胞频率的降低可能与白细胞介素2信号的进一步减弱有关[41-42]。 因此肝移植后检测患者外周血调节性T细胞水平可能有助于评估肝移植患者的免疫状态,有可能成为肝移植患者排斥反应诊断的敏感指标。有研究通过监测移植后1年内接受原位肝移植的患者外周血中的中央记忆调节性T细胞的频率[43],发现外周血中央记忆调节性T细胞的百分比在移植后第1周达到峰值,随后下降,并在移植后1年保持相对稳定,同时他们的研究显示在急性排斥反应发作期间,中央记忆调节性T细胞水平高于排斥前或成功的抗排斥治疗后观察到的水平,因此认为中央记忆调节性T细胞水平的升高能够在移植后的第1个月监测排斥反应的发生。经皮肝活检诊断排斥反应有发生不良事件的风险[42],通过肝移植后调节性T细胞数量的变化能够预测排斥反应的发生具有较好的前景,但是将其应用于实践仍需要大量的临床研究。 2.4.2 在肝移植前向受体输注调节性T细胞能够减轻术后的排斥反应 史留斌等[44]研究发现肝移植前7 d输注CD4+CD5+调节性T细胞的大鼠在移植后与对照组相比生存时间明显延长,并且移植肝内CD4+CD5+调节性T细胞明显增加,而浸润淋巴细胞数量、CD8+T细胞及白细胞介素2的产生明显受抑制,移植后CD4+CD5+调节性T细胞增殖的原因可能与术前输注的CD4+CD5+调节性T细胞的表面受体受到供体T淋巴细胞刺激分泌大量的转化生长因子β1,从而产生增殖扩大效应,使更多的CD4+CD5+调节性T细胞增殖,同时研究表明高水平的白细胞介素2对CD4+CD5+调节性T细胞的增殖具有抑制作用,而低水平的白细胞介素2促进CD4+CD5+调节性T细胞的增殖。PU等[45]发现在大鼠排斥模型中,输注供体同种异体抗原刺激的CD4+CD5+调节性T细胞明显延长了同种异体肝移植的存活时间(从12.1 d延长到30.2 d);然而,并不能提高肝移植物的长期生存率(> 60 d),有2个可能的原因:第一,可能有其他类型的细胞参与调节宿主对移植肝的反应;第二,肝移植后早期有大量细胞在直接异种识别途径中作为抗原提呈细胞,可能在肝移植物排斥的早期发挥关键作用,但当输注供者体外抗原刺激的CD4+CD25+调节性T细胞联合短期他克莫司,移植肝的长期生存时间明显提高,因此他们认为输注供者体外抗原刺激的CD4+CD25+调节性T细胞联合短期他克莫司治疗可能是预防肝移植后排斥反应的一种新策略。但是目前CD4+CD25+调节性T细胞在肝移植后抑制排斥反应,延长抑制肝的存活时间的具体的作用机制尚不明确,需要进一步研究证实。 2.5 调节性T细胞在肝移植后受者免疫耐受中的作用 见表3。 肝移植后的免疫耐受可能与调节性T细胞的升高有关,调节性T细胞的输注可使患者完全或者部分停用免疫抑制剂。 2.5.1 肝移植后免疫耐受组受者体内调节性T细胞升高 LI等[46]通过临床研究首次证明了免疫耐受的肝移植受者移植物中存在Foxp3+调节性T细胞,在免疫抑制药物维持免疫耐受的肝移植受者和慢性排斥肝移植受者中几乎没有Foxp3+调节性T细胞,这与啮齿动物肝移植耐受模型的研究结果一致,并且免疫组织化学和免疫荧光染色显示,CD4+Foxp3+调节性T细胞和CD8+Foxp3+调节性T细胞存在于移植物汇管区,并且大部分是CD4+Foxp3+调节性T细胞。啮齿动物和人类之间的不同之处是:在慢性排斥组的移植物中,转录插头因子信使RNA的表达与耐受功能肝移植物中的表达一样高,可能因为排斥移植物中存在大量激活的效应性T细胞,理论上,这种激活的效应T细胞可以表达插头转录因子信使RNA和蛋白,即使效应T细胞在单细胞水平上转录插头因子信使RNA的表达很低,大量效应T细胞中转录插头因子的低表达也可能导致移植物内转录插头因子信使RNA的增加。LU等[47]通过大鼠模型研究发现肝移植后急性排斥组及免疫耐受组CD8+CD103+调节性T细胞明显增加,但排斥组CD8+CD103+调节性T细胞数量随着时间的推移而减少,耐受组中CD8+CD103+调节性T细胞持续保持相当高的水平,并且CD8+CD103+调节性T细胞与CD4+CD25+调节性T细胞具有共同的功能和作用机制,CD8+CD103+调节性T细胞在体外可在同种异体抗原或转化生长因子β的刺激下被诱导,并具有免疫抑制功能,但是CD8+CD103+调节性T细胞的耗尽是否会导致耐受者的同种异体排斥反应,以及输注体外扩增的CD8+CD103+调节性T细胞是否能防止同种异体移植排斥反应,尚且未可知。并且JIANG等[48]通过对小鼠肝移植模型的研究发现,诱导肝移植后的免疫耐受中发挥重要作用的是受体肝中的调节性T细胞,而不是供体。 2.5.2 体外输注调节性T细胞能够使患者部分或者完全停用免疫抑制剂 TODO等[9]通过对10例成人患者在肝移植后早期输注体外产生的CD4+CD25+Foxp3+调节性T细胞,7例患者成功停用免疫抑制剂(停药16-33个月),其中4例患者停药超过24个月,另3例自身免疫性肝病患者在发稿时仍在停药期,并且研究表明由于肝脏是特殊的免疫特惠器官,肝脏受者的操作免疫耐受可能是肾移植后的10倍,因此体外输注CD4+CD25+Foxp3+调节性T细胞的肝移植受者耐受诱导成功。HE等[36]通过建立大鼠肝移植模型,研究发现向受体输注调节性T细胞和/或未成熟树突状细胞可以诱导同种异体抗原耐受,延长同种异体肝移植存活时间,并且联合输注调节性T细胞-未成熟树突状细胞比单独输注未成熟树突状细胞或调节性T细胞更有效,此外,输注调节性T细胞-未成熟树突状细胞组的白细胞介素10和转化生长因子β的表达明显上调,白细胞介素12的表达明显下调。"
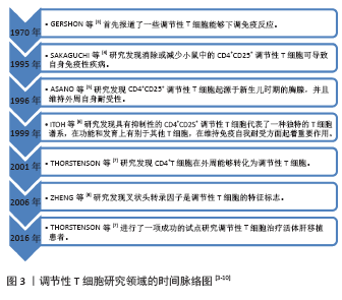
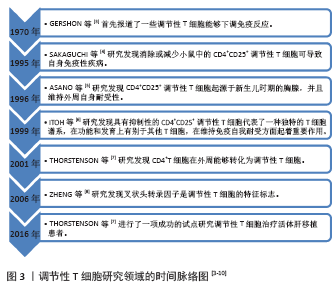
| [1] 农村立,龙腾河,郭堑.肝移植组织中调节性T淋巴细胞的表达及分布[J].中国组织工程研究,2012,16(5):903-906. [2] CALNE RY.The future of organ transplantation: from the laboratory to the clinic. Philos Trans R Soc Lond B Biol Sci. 2001;356(1409):767-771. [3] GERSHON PK, KONDO K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723-737. [4] SAKAGUCHI S, SAKAGUCHI N, ASANO M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. Immunol. 1995;155(3): 1151-1164. [5] ASANO M, TODA M, SAKAGUCHI N, et al. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387-396. [6] ITOH M, TAKAHASHI T, SAKAGUCHI N, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317-5326. [7] THORSTENSON KM, KHORUTS A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001; 167:188-195. [8] ZHENG Y, JOSEFOWICZ SZ, KAS A, et al. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007; 445:936-940. [9] TODO S, YAMASHITA K, GOTO R, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64(2):632. [10] YU J, LIU Z, LI C, et al. Regulatory T cell therapy following liver transplantation. Liver Transpl. 2021;27(2):264-280. [11] ANGERAMI MT, SUAREZ GV, VECCHIONE MB, et al. Expansion of CD25-negative forkhead box P3-positive T cells during HIV and mycobacterium tuberculosis infection. Front Immunol. 2017;8:528. [12] FERREIRA LMR, MULLER YD, BLUESTONE JA, et al. Next-generation regulatory T cell therapy. Nat Rev Drug Discov. 2019;18(10):749-769. [13] OHKURA N, KITAGAWA Y, SAKAGUCHI S, et al. Development and maintenance of regulatory T cells. Immunity. 2013;38(3):414-423. [14] KOMATSU N, OKAMOTO K, SAWA S, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20(1):62-68. [15] ZHOU X, BAILEY-BUCKTROUT SL, JEKER LT, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10(9):1000-1007. [16] LIU W, PUTNAM AL, XU-YU Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function ofhuman CD4+ T reg cells. J Exp Med. 2006;203(7):1701-1711. [17] POLANSKY JK, KRETSCHMER K, FREYER J, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38(6):1654-1663. [18] POLANSKY JK, SCHREIBER L, THELEMANN C, et al. Methylation matters: binding of Ets-1 to the demethylated Foxp3 gene contributes to the stabilization of Foxp3 expression in regulatory T cells. J Mol Med (Berl). 2010;88(10):1029-1040. [19] MIYARA M, YOSHIOKA Y, KITOH A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899-911. [20] MILLER A, LIDER O, WEINER HL, et al. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med. 1991; 174(4):791-798. [21] GERSHON PK, KONDO K. Infectious immunological tolerance. Immunology. 1971;21(6):903-914. [22] QIN S, COBBOLD SP, POPE H, et al. “Infectious” transplantation tolerance. Science. 1993;259(5097):974-977. [23] SáNCHEZ-FUEYO A, WHITEHOUSE G, GRAGEDA N, et al. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am J Transplant. 2020;20(4):1125-1136. [24] TEH WI, SEAY HR, NEWBY B, et al. Avidity and bystander suppressive capacity of human regulatory T cells expressing de novo autoreactive T-cell receptors in type 1 diabetes. Front Immunol. 2017;8:1313. [25] ESENSTEN JH, MULLER JH, MULLER YD, et al. Regulatory T-cell therapy for autoimmune and autoinflammatory diseases: the next frontier. J Allergy Clin Immunol. 2018;142(6):1710-1718. [26] DAI H, ZHENG Y, THOMSON AW, et al. Transplant tolerance induction: insights from the liver. Front Immunol. 2020;11:1044. [27] PANDIYAN P, ZHENG L, ISHIHARA S, et al. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8(12):1353-1362. [28] HARA M, KINGSLIEY CI, NIIMI M, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001; 166(6):3789-3796. [29] BELGHITH M, BLUESTONE JA, BARRIONT S, et al. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9(9): 1202-1208. [30] QIN HY, MUKHERJEE R, LEE-CHAN E, et al. A novel mechanism of regulatory T cell-mediated down-regulation of autoimmunity. Int Immunol. 2006;18(7):1001-1015. [31] SAWITZKI B, KINGSLEY CI, LIVEIRA S, et al. IFN-gamma production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005;201(12):1925-1935. [32] COOMBES JK, SIDDIQUI KR, ARANCIBIA-CáRCoombeCAMO CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757-1764. [33] CHAE WJ, EHRLICH AK, CHAN PK, et al. The Wnt antagonist dickkopf-1 promotes pathological type 2 cell-mediated inflammation. Immunity. 2016;44(2):246-258. [34] ZIMMERER JM, PHAM TA, WRIGHT CL, et al. Alloprimed CD8(+) T cells regulate alloantibody and eliminate alloprimed B cells through perforin- and FasL-dependent mechanisms. Am J Transplant. 2014;14(2): 295-304. [35] MARUYAMA T, KONKEL JE, ZAMARRON BF, et al. The molecular mechanisms of Foxp3 gene regulation. Semin Immunol. 2011;23(6): 418-423. [36] HE W, CHEN L, ZHENG L, et al. Prolonged survival effects induced by immature dendritic cells and regulatory T cells in a rat liver transplantation model. Mol Immunol. 2016;79:92-97. [37] WANG K, SONG ZL, WU B, et al. Different phenotypes of CD4+CD25+Foxp3+ regulatory T cells in recipients post liver transplantation. Int Immunopharmacol. 2019;69:194-201. [38] HE Q, FAN H, LI JQ, et al. Decreased circulating CD4+CD25highFoxp3+ T cells during acute rejection in liver transplant patients. Transplant Proc. 2011;43(5):1696-1700. [39] DEMIEKIRAN A, KOK A, KWEKKEBOOM J, et al. Low circulating regulatory T-cell levels after acute rejection in liver transplantation. Liver Transpl. 2006;12(2):277-284. [40] HAN JW, JOO DJ, KIM JH, et al. Early reduction of regulatory T cells is associated with acute rejection in liver transplantation under tacrolimus-based immunosuppression with basiliximab induction. Am J Transplant. 2020;20(8):2058-2069. [41] BOLESLAWSKI E, CONTI F, SANQUER S, et al. Defective inhibition of peripheral CD8+ T cell IL-2 production by anti-calcineurin drugs during acute liver allograft rejection. Transplantation. 2004;77(12):1815-1820. [42] KUMARS, MOHAPATRA N, BORLE DP, et al. Non invasive diagnosis of acute cellular rejection after liver transplantation - Current opinion. Transpl Immunol. 2018;47:1-9. [43] BOIX-GINER F, MILLAN O, SAN SEGUNDO D, et al. High frequency of central memory regulatory T cells allows detection of liver recipients at risk of early acute rejection within the first month after transplantation. Int Immunol. 2016;28(2):55-64. [44] 史留斌,张弘炜,彭承宏.调节性T细胞和共刺激通路阻断剂抑制大鼠肝移植急性排斥反应的研究[J].中华医学杂志,2007,87(14): 942-946. [45] PU LY, WANG XH, ZHANG F, et al. Adoptive transfusion of ex vivo donor alloantigen-stimulated CD4(+)CD25(+) regulatory T cells ameliorates rejection of DA-to-Lewis rat liver transplantation. Surgery. 2007;142(1):67-73. [46] LI Y, ZHAO X, CHENG D, et al. The presence of Foxp3 expressing T cells within grafts of tolerant human liver transplant recipients. Transplantation. 2008;86(12):1837-1843. [47] LU L, YU Y, LI G, et al. CD8(+)CD103(+) regulatory T cells in spontaneous tolerance of liver allografts. Int Immunopharmacol. 2009;9(5):546-548. [48] JIANG X, MORITA M, SUGIONKA A, et al. The importance of CD25+ CD4+ regulatory T cells in mouse hepatic allograft tolerance. Liver Transpl. 2006;12(7):1112-1118. [49] 蔡秋程.T淋巴细胞与肝移植免疫耐受[J].中国组织工程研究,2014, 18(5):791-796. |
| [1] | Wang Jianping, Zhang Xiaohui, Yu Jinwei, Wei Shaoliang, Zhang Xinmin, Xu Xingxin, Qu Haijun. Application of knee joint motion analysis in machanism based on three-dimensional image registration and coordinate transformation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(在线): 1-5. |
| [2] | Zhu Chan, Han Xuke, Yao Chengjiao, Zhang Qiang, Liu Jing, Shao Ming. Acupuncture for Parkinson’s disease: an insight into the action mechanism in animal experiments [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1330-1335. |
| [3] | Tang Wenjing, Wu Siyuan, Yang Chen, Tao Xi. Inflammatory responses in post-stroke depression [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1336-1344. |
| [4] | Zhou Ying, Zhang Huan, Liao Song, Hu Fanqi, Yi Jing, Liu Yubin, Jin Jide. Immunomodulatory effects of deferoxamine and interferon gamma on human dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1012-1019. |
| [5] | Zhang Yujie, Yang Jiandong, Cai Jun, Zhu Shoulei, Tian Yuan. Mechanism by which allicin inhibits proliferation and promotes apoptosis of rat vascular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1080-1084. |
| [6] | He Junjun, Huang Zeling, Hong Zhenqiang. Interventional effect of Yanghe Decoction on synovial inflammation in a rabbit model of early knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 694-699. |
| [7] | Zhang Xiaoyun, Li Huanan, Chen Feng, Chai Yuan, Gan Bin, Li Song, Chen Dingpeng. Potential molecular mechanism of Guizhi Shaoyao Zhimu Decoction in the treatment of gouty arthritis based on network pharmacology and molecular docking [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 245-252. |
| [8] | Zhao Xu, Mao Xin, Li Chuntian, Wang Feng. Effect of mesenchymal stem cells on myocardial ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 130-134. |
| [9] | Li Zhongkang, Zheng Jiahua, Tian Yanpeng, Huang Xianghua. Latest progress and mechanisms of mesenchymal stem cells on premature ovarian failure [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 141-147. |
| [10] | Wang Shuyun, Xie Junhui, Yu Xuefeng. Effect and mechanism of mesenchymal stem cells in the treatment of diabetic nephropathy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 148-152. |
| [11] | Jiang Xin, Qiao Liangwei, Sun Dong, Li Ming, Fang Jun, Qu Qingshan. Expression of long chain non-coding RNA PGM5-AS1 in serum of renal transplant patients and its regulation of human glomerular endothelial cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 741-745. |
| [12] | Cao Xuhan, Bai Zixing, Sun Chengyi, Yang Yanjun, Sun Weidong. Mechanism of “Ruxiang-Moyao” herbal pair in the treatment of knee osteoarthritis based on network pharmacology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 746-753. |
| [13] | Xiao Fangjun, Chen Shudong, Luan Jiyao, Hou Yu, He Kun, Lin Dingkun. An insight into the mechanism of Salvia miltiorrhiza intervention on osteoporosis based on network pharmacology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 772-778. |
| [14] | Yang Xin, Jin Zhe, Feng Xu, Lu Bing. The current situation of knowledge and attitudes towards organ, eye tissue, body donation of residents in Shenyang [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 779-784. |
| [15] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||