Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (17): 2766-2775.doi: 10.3969/j.issn.2095-4344.2017.17.023
Previous Articles Next Articles
Mesenchymal stem cells in the treatment of myocardial infarction: how long is the way from bench to bedside?
Zhao Qi-ming, Sheng Kai, Zhang Xuan-fen
- The Second Hospital of Lanzhou University, Lanzhou 730030, Gansu Province, China
-
Revised:2017-04-11Online:2017-06-18Published:2017-06-29 -
About author:Zhao Qi-ming, M.D., Associate professor, Associate chief physician, the Second Hospital of Lanzhou University, Lanzhou 730030, Gansu Province, China -
Supported by:the Health Industry Scientific Research Plan of Guansu Province in 2012, No. GSWST2012-02
CLC Number:
Cite this article
Zhao Qi-ming, Sheng Kai, Zhang Xuan-fen. Mesenchymal stem cells in the treatment of myocardial infarction: how long is the way from bench to bedside?[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(17): 2766-2775.
share this article
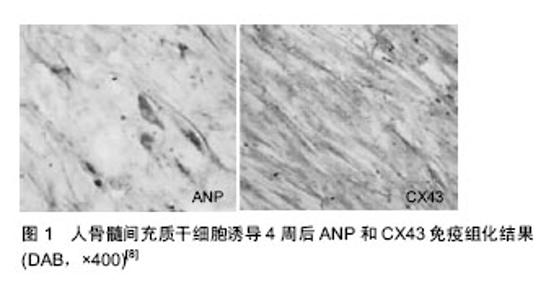
2.1 间充质干细胞来源 2.1.1 骨髓 骨髓间充质干细胞为非造血干细胞,在骨髓中含量极低,为0.001%-0.01%。研究发现,骨髓间充质干细胞与造血干细胞不同,其不表达典型造血干细胞的表面标志CD34和CD45,能特征性地表达CD29、CD44、CD71、CD90、CD105、CD106、CD120a、CD124等[7]。人间充质干细胞在体外经纯化、扩增、诱导后可表现心肌样细胞的生物学特性,可为人间充质干细胞临床移植治疗提供可靠细胞来源[8-9](图1),且因其强大的增殖能力,骨髓间充质干细胞在心肌修复中具有很明显的潜在优势[10-11]。Rota等[12]研究表明,心肌梗死后移植的干细胞不仅分化出了心肌细胞,而且局部移植的骨髓干细胞能够产生包括有心肌细胞和冠脉血管的新生心肌。应用骨髓间充质干细胞可明显减少心肌梗死面积[13],并可通过改变坏死心肌的局部微环境而上调血管内皮生长因子的表达[14]。因此,利用骨髓间充质干细胞作为种子细胞取代受损的心肌细胞具有很好的应用前景。此外,骨髓间充质干细胞移植后还具有自动归巢到梗死区的特性,可能与趋化蛋白基质细胞衍生因子1(SDF-1)及其受体CXCR4作用有关[15]。"
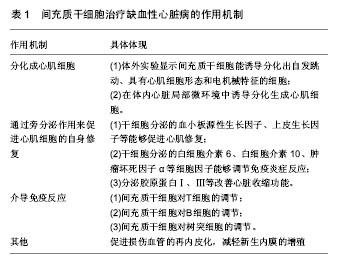
2.1.2 脐血[16-17] 脐血间充质干细胞介于成体干细胞、胚胎干细胞之间,显得更为原始,具有亚全能分化潜能,能分化为心脏、肝、肾等重要器官组织细胞。2000年,Erices等报道脐血中有很丰富的造血干细胞和间充质干细胞,提取出的干细胞也被多位研究者们证实具有良好的心肌修复功能。Lee等[18]发现从脐血中提取的间充质干细胞可分化为中胚层细胞,推测心肌作为中胚层组织亦可由其分化得到。另外有研究发现向兔心肌梗死模型中联合移植脐血间充质干细胞和脐血CD34+,可明显降低胶原蛋白沉积,并提高心肌功能[19]。并由于全球人类出生率高,脐血也能够通过脐血库很好的收集与保存,脐血间充质干细胞已被越来越多的研究者们重视,但因需在孕期采脐血,脐血间充质干细胞的提取仍存在争议[20]。 2.1.3 脐带 脐带组织,包括其动脉、静脉、华氏胶组织(Wharton’s jelly)、脐带内膜等,是间充质干细胞的可靠来源。脐带间充质干细胞具有获得过程无需有创操作,可分化为3个胚层细胞、易在组织受损或炎症反应部位聚集、可促进组织修复并调节炎症反应等明显优势[21]。另外,与骨髓间充质干细胞相比,脐带间充质干细胞自我更新快且不易形成畸胎瘤[22]。来源于Wharton’s jelly的间充质干细胞因其对心肌组织具有特异性趋化以及可分泌血管生成因子诱导心肌干细胞募集而受到关注[23-24]。Wharton’s jelly间充质干细胞从胚胎外胚层组织中提取,已被证实具有人胚胎干细胞和成体干细胞的特性,因而可作为干细胞移植的来源,另外,它还被证实具有阻隔免疫排斥、肿瘤发生、畸胎瘤形成等作用[23,25]。经冠状动脉注射Wharton’s jelly间充质干细胞,可明显提高左室射血分数,减少心肌梗死相关事件的发生,因而有望替代骨髓间充质干细胞[26]。 2.1.4 其他 大隐静脉、滑膜是近几年发现的较有潜力的间充质干细胞来源组织[27-28]。 2.2 间充质干细胞的安全性和有效性 Fazel等[29]认为间充质干细胞体外扩增的过程是安全的,且癌变的概率很小。但Furlani等[30]认为间充质干细胞长期在体外扩增过程中会产生异常的细胞群落,不但没有治疗心肌梗死的疗效,甚至会发生癌变。 更有趣的是,Khan等[31]于2011年的实验中发现间充质干细胞的供体年龄会影响移植后的疗效。实验中,Khan等分别从2月龄和18月龄大鼠中提取出间充质干细胞移植入18月龄心肌梗死模型大鼠体内。移植后观察大鼠体内衰老主导基因p16的表达,发现来自年老大鼠的间充质干细胞表达更加活跃,并由此推断间充质干细胞的活跃程度并不随年龄的增长而降低。除此之外,胰岛素样生长因子1、纤维母细胞生长因子、血管内皮生长因子、肝细胞生长因子较年轻大鼠减少,而各项心功能指标也有所不及。这项研究结果表明了间充质干细胞移植需要年轻的供体,而临床上治疗年老者心肌梗死时自体间充质干细胞移植可能并不适用。 2.3 作用机制 2.3.1 间充质干细胞分化成心肌细胞 通过实验发现,小鼠间充质干细胞在体外经过5-氮胞苷处理后,能诱导分化出自发跳动、具有心肌细胞形态和电机械特征的细胞[9,32]。研究进一步证明了骨髓间充质干细胞可以在体内心脏局部微环境中诱导分化生成心肌细胞[33-34]。胰岛素样生长因子1、干细胞生长因子、骨形态发生蛋白2等细胞因子通过与其受体结合,激活PI3-K/Akt信号转导途径,从而介导干细胞在移植微环境中的存活[35]。 2.3.2 旁分泌 以往研究者认为干细胞移植改善肌细胞的作用是由干细胞分化替代原损伤的心肌细胞或者血管生成来完成[36-37],但越来越多的研究证明干细胞可通过旁分泌来促进心肌细胞的自身修复[38-42]。 Ma等[43]于2009年尝试使用分化功能被抑制的间充质干细胞进行移植实验,发现其也能改善实验动物的心脏功能,且其改善程度与分化功能未被抑制的间充质干细胞无统计学差异。同年,Deuse等[44]做了有关间充质干细胞移植治疗鼠心肌梗死的动物实验,证明了生长因子等对间充质干细胞移植疗效的积极作用。这一结果证实细胞因子对心肌修复的作用大于心肌再生。随后的研究中,研究者通过基因重组技术培养出高表达肝细胞生长因子和血管内皮生长因子的间充质干细胞,不管是体外研究还是体内研究,均明显增加了间充质干细胞的增殖。移植了高表达肝细胞生长因子和血管内皮生长因子的间充质干细胞的动物心肌梗死面积更小,梗死区周围血管密度更高,左室功能更好。更详细的分析发现动物心肌组织乳酸脱氢酶分泌减少,心肌细胞凋亡减少,而Akt的活性增加。Gnecchi等[45]在随后的实验中阐述了Akt通路在心肌修复中的具体机制。动物实验中,Akt通路激活程度增加的大鼠心肌细胞摄入2-去氧葡萄糖(2-DG)明显减少,胞浆的pH值也在正常范围内。2-DG是天然的抗代谢类抗生素,能够抑制细胞生长。故此推测Akt通路激活能够保护细胞正常的能量代谢,维持正常的胞内pH值。 除此以外,大量的实验也已证实干细胞分泌的其他生长因子(如血小板源性生长因子、上皮生长因子等)能够促进心肌修复,分泌的白细胞介素6、白细胞介素10、肿瘤坏死因子α等细胞因子能够调节免疫炎症反应,可激活细胞内包括STAT3、PI3K-Akt、ERK1/2等在内的信号通路,促进细胞增殖[46],分泌胶原蛋白Ⅰ、Ⅲ等改善心脏收缩功能。还有研究认为,骨髓间充质干细胞可以通过诱导心脏成纤维细胞转化为肌纤维母细胞,再通过旁分泌作用修复梗死心肌[47],但Bader等[48]的体外实验发现成纤维母细胞对缺血心肌的保护作用却不及间充质干细胞好。而Li等[49]将间充质干细胞与心肌细胞共培养2周后得到经预处理分化的骨髓间充质干细胞。随后的动物实验发现预处理分化的骨髓间充质干细胞在恢复心功能方面比间充质干细胞更加有效,推测也是利用旁分泌的作用促进了间充质干细胞移植后的疗效。 2.3.3 介导免疫反应 间充质干细胞具有强大的免疫调节作用,将成为心肌梗死后平衡炎症反应和达到心肌修复的重要手段。Ben-Mordechai等[50]发现间充质干细胞对损伤心肌的保护作用是巨噬细胞介导的。 间充质干细胞对T细胞的调节:心肌梗死发生后,T细胞识别特异性抗原内源性心肌肌球蛋白和辅肌动蛋白,导致辅助T细胞(Th细胞)和细胞毒T细胞(Tc细胞)对心肌产生持续性损害[51]。间充质干细胞与T细胞共培养下有2条主要通路抑制T细胞增殖:间充质干细胞上调色氨酸的代谢酶IDO使色氨酸代谢产物犬尿氨酸堆积,抑制T细胞增殖[52];环氧合酶2的表达使PGE2释放增加也产生对T细胞增殖抑制的作用[53]。 间充质干细胞对B细胞的调节:间充质干细胞有抑制NK细胞增殖的能力,同时也能降低其细胞毒性和减少促炎因子的释放[54]。NK细胞是通过IDO途径和PGE2途径迅速降低其增殖能力,且这两条途径还可能存在协同作用[55]。间充质干细胞也可通过下调NK细胞活化受体NKp30、NKp44 和NKG2D,从而减少NK细胞的活化,使其细胞毒性降低和促炎因子释放减少[56]。 间充质干细胞对树突细胞的调节:树突细胞是体内最重要的抗原递呈细胞,心肌梗死后递呈坏死心肌组织,从而激活免疫应答。间充质干细胞可以通过阻断树突细胞的成熟,导致树突细胞表面抗原表达和共刺激分子CD40、CD80和CD86的表达下调,从而抑制T细胞的激活[55]。 2.3.4 其他 此外,有研究表明,间充质干细胞能够促进损伤血管的再内皮化,减轻新生内膜的增殖,有助于减轻损伤血管的再狭窄[57]。 间充质干细胞治疗缺血性心脏病的作用机制见表1。"
| [1] Reinhardt D, Sigusch HH, Hensse J, et al. Cardiac remodelling in end stage heart failure: upregulation of matrix metalloproteinase (MMP) irrespective of the underlying disease, and evidence for a direct inhibitory effect of ACE inhibitors on MMP. Heart. 2002;88(5):525-530.[2] Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344(23):1750-1757.[3] Herlitz J, Dellborg M, Karlson BW, et al. Prognosis after acute myocardial infarction continues to improve in the reperfusion era in the community of Göteborg. Am Heart J. 2002;144(1):89-94.[4] Henning RJ. Stem cells in cardiac repair. Future Cardiol. 2011; 7(1):99-117.[5] Bhaskar B, Mekala NK, Baadhe RR, et al. Role of signaling pathways in mesenchymal stem cell differentiation. Curr Stem Cell Res Ther. 2014;9(6):508-512.[6] 马莎. 骨髓间充质干细胞治疗缺血性心脏病的研究进展[J].医学综述, 2012, 18(16):2557-2559.[7] Zhu X, Du J, Liu G. The comparison of multilineage differentiation of bone marrow and adipose-derived mesenchymal stem cells. Clin Lab. 2012;58(9-10):897-903.[8] 唐海沁,马健,翟志敏,等.人骨髓间充质干细胞体外定向分化过程中心肌细胞特征表达及膜电位变化[J]. 临床心血管病杂志, 2007, 23(6):437-441.[9] 汤天军,杨波,杨汉东,等.人脐带血间充质干细胞体外诱导分化为心肌细胞的实验研究[J].实用医学杂志,2005,21(21):2412- 2414.[10] Wen Z, Zheng S, Zhou C, et al. Repair mechanisms of bone marrow mesenchymal stem cells in myocardial infarction. J Cell Mol Med. 2011;15(5):1032-1043.[11] Lin X, Peng P, Cheng L, et al. A natural compound induced cardiogenic differentiation of endogenous MSCs for repair of infarcted heart. Differentiation. 2012;83(1):1-9.[12] Rota M, Padin-Iruegas ME, Misao Y, et al. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103(1):107-116.[13] Zhao JJ, Liu XC, Kong F, et al. Bone marrow mesenchymal stem cells improve myocardial function in a swine model of acute myocardial infarction. Mol Med Rep. 2014;10(3): 1448-1454.[14] Rahbarghazi R, Nassiri SM, Ahmadi SH, et al. Dynamic induction of pro-angiogenic milieu after transplantation of marrow-derived mesenchymal stem cells in experimental myocardial infarction. Int J Cardiol. 2014;173(3):453-466.[15] Won YW, Patel AN, Bull DA. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials. 2014;35(21):5627-5635.[16] Ma N, Ladilov Y, Moebius JM, et al. Intramyocardial delivery of human CD133+ cells in a SCID mouse cryoinjury model: Bone marrow vs. cord blood-derived cells. Cardiovasc Res. 2006;71(1):158-169.[17] Ma N, Stamm C, Kaminski A, et al. Human cord blood cells induce angiogenesis following myocardial infarction in NOD/scid-mice. Cardiovasc Res. 2005;66(1):45-54.[18] Lee OK, Kuo TK, Chen WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669-1675.[19] Li T, Ma Q, Ning M, et al. Cotransplantation of human umbilical cord-derived mesenchymal stem cells and umbilical cord blood-derived CD34? cells in a rabbit model of myocardial infarction. Mol Cell Biochem. 2014;387(1-2): 91-100.[20] Mareschi K, Biasin E, Piacibello W, et al. Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica. 2001;86(10):1099-1100.[21] Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells. 2014;6(2):195-202.[22] Fong CY, Chak LL, Biswas A, et al. Human Wharton's jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev. 2011;7(1):1-16.[23] Musialek P, Mazurek A, Jarocha D, et al. Myocardial regeneration strategy using Wharton's jelly mesenchymal stem cells as an off-the-shelf 'unlimited' therapeutic agent: results from the Acute Myocardial Infarction First-in-Man Study. Postepy Kardiol Interwencyjnej. 2015;11(2):100-107.[24] Zhang W, Liu XC, Yang L, et al. Wharton's jelly-derived mesenchymal stem cells promote myocardial regeneration and cardiac repair after miniswine acute myocardial infarction. Coron Artery Dis. 2013;24(7):549-558.[25] Gao LR, Zhang NK, Ding QA, et al. Common expression of stemness molecular markers and early cardiac transcription factors in human Wharton's jelly-derived mesenchymal stem cells and embryonic stem cells. Cell Transplant. 2013;22(10): 1883-1900.[26] Gao LR, Chen Y, Zhang NK, et al. Intracoronary infusion of Wharton's jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med. 2015;13:162.[27] Covas DT, Piccinato CE, Orellana MD, et al. Mesenchymal stem cells can be obtained from the human saphena vein. Exp Cell Res. 2005;309(2):340-344.[28] Imanishi Y, Miyagawa S, Kitagawa-Sakakida S, et al. Impact of synovial membrane-derived stem cell transplantation in a rat model of myocardial infarction. J Artif Organs. 2009;12(3): 187-193.[29] Fazel SS, Angoulvant D, Butany J, et al. Mesenchymal stem cells engineered to overexpress stem cell factor improve cardiac function but have malignant potential. J Thorac Cardiovasc Surg. 2008;136(5):1388-1389.[30] Furlani D, Li W, Pittermann E, et al. A transformed cell population derived from cultured mesenchymal stem cells has no functional effect after transplantation into the injured heart. Cell Transplant. 2009;18(3):319-331.[31] Khan M, Mohsin S, Khan SN, et al. Repair of senescent myocardium by mesenchymal stem cells is dependent on the age of donor mice. J Cell Mol Med. 2011;15(7):1515-1527.[32] 许丹,尚小明,姜玉如.经冠状动脉自体骨髓单个核细胞移植治疗急性前壁心肌梗死的研究[J].中国心血管杂志,2006,11(4): 261-265.[33] 王伟民,孙宁玲,刘健,等.经冠状动脉注入自体骨髓单个核细胞的临床研究[J].中华心血管病杂志,2006,34(2):103-106.[34] 栾云,刘晓程,张兆华,等.骨髓间充质干细胞移植治疗小型猪急性心肌梗死[J].基础医学与临床,2011,31(10):1156-1158.[35] 国海东,谭玉珍, 王海杰. 细胞因子在干细胞移植存活与分化中的作用[J]. 国际心血管病杂志,2007,34(1):23-26.[36] Tang J, Xie Q, Pan G, et al. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg. 2006;30(2):353-361.[37] Wu Y, Chen L, Scott PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648-2659.[38] Mirotsou M, Jayawardena TM, Schmeckpeper J, et al. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50(2):280-289.[39] Chen L, Tredget EE, Wu PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008; 3(4):e1886.[40] Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316(14):2213-2219.[41] Uemura R, Xu M, Ahmad N, et al. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98(11):1414-1421.[42] Li Z, Guo J, Chang Q, et al. Paracrine role for mesenchymal stem cells in acute myocardial infarction. Biol Pharm Bull. 2009;32(8):1343-1346.[43] Ma XH, Gao CQ, Li BJ, et al. The empirical study in transplantation of bone marrow mesenchymal stem cells for acute myocardial infarction. Zhonghua Yi Xue Za Zhi. 2009; 89(15):1067-1070.[44] Deuse T, Peter C, Fedak PW, et al. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation. 2009;120(11 Suppl): S247-254.[45] Gnecchi M, He H, Melo LG, et al. Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells. 2009;27(4):971-979.[46] 乔建晶.骨髓间充质干细胞移植调节心肌梗死后炎症反应的研究现状[J].心血管病学进展,2010,31(5):729-732.[47] 杜优优,姚瑞,蒲实,等.骨髓间充质干细胞治疗增加心脏梗死区肌纤维母细胞聚集的观察[J].中华心血管病杂志, 2012, 40(12): 1045-1050.[48] Bader AM, Brodarac A, Klose K, et al. Mechanisms of paracrine cardioprotection by cord blood mesenchymal stromal cells. Eur J Cardiothorac Surg. 2014;45(6):983- 992.[49] Li XH, Fu YH, Liu ZY, et al. Efficacy comparison between transplanting microenvironmental induced and non-induced bone marrow mesenchymal stem cells in ischemic rat hearts. Zhonghua Xin Xue Guan Bing Za Zhi. 2009;37(8):680-684.[50] Ben-Mordechai T, Holbova R, Landa-Rouben N, et al. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J Am Coll Cardiol. 2013; 62(20):1890-1901.[51] Yan X, Shichita T, Katsumata Y, et al. Deleterious effect of the IL-23/IL-17A axis and γδT cells on left ventricular remodeling after myocardial infarction. J Am Heart Assoc. 2012;1(5): e004408.[52] Mándi Y, Vécsei L. The kynurenine system and immunoregulation. J Neural Transm (Vienna). 2012;119(2): 197-209.[53] François M, Romieu-Mourez R, Li M, et al. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187-195.[54] Pradier A, Passweg J, Villard J, et al. Human bone marrow stromal cells and skin fibroblasts inhibit natural killer cell proliferation and cytotoxic activity. Cell Transplant. 2011; 20(5):681-691.[55] Abumaree M, Al Jumah M, Pace RA, et al. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012;8(2):375-392.[56] Spaggiari GM, Capobianco A, Abdelrazik H, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111(3): 1327-1333.[57] 石蓓,刘志江,赵然尊,等.骨髓间充质干细胞移植对心肌梗死后心脏功能及损伤血管再狭窄的影响[J].中华医学杂志,2011,91(32): 2269-2273.[58] Assis AC, Carvalho JL, Jacoby BA, et al. Time-dependent migration of systemically delivered bone marrow mesenchymal stem cells to the infarcted heart. Cell Transplant. 2010;19(2):219-230.[59] Gyöngyösi M, Hemetsberger R, Posa A, et al. Hypoxia-inducible factor 1-alpha release after intracoronary versus intramyocardial stem cell therapy in myocardial infarction. J Cardiovasc Transl Res. 2010;3(2):114-121.[60] Dai W, Hale SL, Kay GL, et al. Delivering stem cells to the heart in a collagen matrix reduces relocation of cells to other organs as assessed by nanoparticle technology. Regen Med. 2009;4(3):387-395.[61] Tay CY, Yu H, Pal M, et al. Micropatterned matrix directs differentiation of human mesenchymal stem cells towards myocardial lineage. Exp Cell Res. 2010;316(7):1159-1168.[62] Yang Z, Zhang F, Ma W, et al. A novel approach to transplanting bone marrow stem cells to repair human myocardial infarction: delivery via a noninfarct-relative artery. Cardiovasc Ther. 2010;28(6):380-385.[63] Vulliet PR, Greeley M, Halloran SM, et al. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363(9411):783-784.[64] Grieve SM, Bhindi R, Seow J, et al. Microvascular obstruction by intracoronary delivery of mesenchymal stem cells and quantification of resulting myocardial infarction by cardiac magnetic resonance. Circ Heart Fail. 2010;3(3):e5-6.[65] Gyöngyösi M, Hemetsberger R, Wolbank S, et al. Delayed recovery of myocardial blood flow after intracoronary stem cell administration. Stem Cell Rev. 2011;7(3):616-623.[66] Llano R, Epstein S, Zhou R, et al. Intracoronary delivery of mesenchymal stem cells at high flow rates after myocardial infarction improves distal coronary blood flow and decreases mortality in pigs. Catheter Cardiovasc Interv. 2009;73(2): 251-257.[67] Schmuck EG, Mulligan JD, Ertel RL, et al. Cardiac fibroblast-derived 3D extracellular matrix seeded with mesenchymal stem cells as a novel device to transfer cells to the ischemic myocardium. Cardiovasc Eng Technol. 2014; 5(1):119-131.[68] Ghanem A, Steingen C, Brenig F, et al. Focused ultrasound- induced stimulation of microbubbles augments site-targeted engraftment of mesenchymal stem cells after acute myocardial infarction. J Mol Cell Cardiol. 2009;47(3):411-418.[69] Li L, Wu S, Liu Z, et al. Ultrasound-Targeted Microbubble Destruction Improves the Migration and Homing of Mesenchymal Stem Cells after Myocardial Infarction by Upregulating SDF-1/CXCR4: A Pilot Study. Stem Cells Int. 2015;2015:691310.[70] Tano N, Narita T, Kaneko M, et al. Epicardial placement of mesenchymal stromal cell-sheets for the treatment of ischemic cardiomyopathy; in vivo proof-of-concept study. Mol Ther. 2014;22(10):1864-1871.[71] Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009; 54(24):2277-2286.[72] Kan CD, Lee HL, Yang YJ. Cell transplantation for myocardial injury: a preliminary comparative study. Cytotherapy. 2010; 12(5): 692-700.[73] Mathieu M, Bartunek J, El Oumeiri B, et al. Cell therapy with autologous bone marrow mononuclear stem cells is associated with superior cardiac recovery compared with use of nonmodified mesenchymal stem cells in a canine model of chronic myocardial infarction. J Thorac Cardiovasc Surg. 2009; 138(3):646-653.[74] Mazo M, Gavira JJ, Abizanda G, et al. Transplantation of mesenchymal stem cells exerts a greater long-term effect than bone marrow mononuclear cells in a chronic myocardial infarction model in rat. Cell Transplant. 2010;19(3):313-328.[75] Armiñán A, Gandía C, García-Verdugo JM, et al. Mesenchymal stem cells provide better results than hematopoietic precursors for the treatment of myocardial infarction. J Am Coll Cardiol. 2010;55(20):2244-2253.[76] Li Q, Turdi S, Thomas DP, et al. Intra-myocardial delivery of mesenchymal stem cells ameliorates left ventricular and cardiomyocyte contractile dysfunction following myocardial infarction. Toxicol Lett. 2010;195(2-3):119-126.[77] 费宇行,裘毅钢,李晶.骨髓干细胞移植改善心肌梗死患者左室重构的Meta分析[J].中国循证心血管医学杂志, 2011,3(4):255-259.[78] 王德国,曹克将.干细胞治疗与室性心律失常[J].国际心血管病杂志,2008,35(3):129-132.[79] Costa AR, Panda NC, Yong S, et al. Optical mapping of cryoinjured rat myocardium grafted with mesenchymal stem cells. Am J Physiol Heart Circ Physiol. 2012;302(1):H270-277.[80] Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102(32):11474-11479.[81] 伍徐娴,何志旭,周中军.骨髓间充质干细胞移植治疗大鼠实验性心肌梗死[J].贵阳医学院学报,2010,35(4):344-348.[82] 宋晓蓉,翟志敏,章仁品,等.兔骨髓单个核细胞自体移植对缺血心肌修复重建及心功能的改善[J].中国组织工程研究,2006, 10(37):27-29.[83] Fan L, Lin C, Zhuo S, et al. Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. Eur J Heart Fail. 2009;11(11):1023-1030.[84] Chang W, Song BW, Lim S, et al. Mesenchymal stem cells pretreated with delivered Hph-1-Hsp70 protein are protected from hypoxia-mediated cell death and rescue heart functions from myocardial injury. Stem Cells. 2009;27(9):2283-2292.[85] Huang J, Zhang Z, Guo J, et al. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res. 2010;106(11):1753-1762.[86] Alfaro MP, Vincent A, Saraswati S, et al. sFRP2 suppression of bone morphogenic protein (BMP) and Wnt signaling mediates mesenchymal stem cell (MSC) self-renewal promoting engraftment and myocardial repair. J Biol Chem. 2010;285(46):35645-35653.[87] Eun LY, Song BW, Cha MJ, et al. Overexpression of phosphoinositide-3-kinase class II alpha enhances mesenchymal stem cell survival in infarcted myocardium. Biochem Biophys Res Commun. 2010;402(2):272-279.[88] Kim HW, Haider HK, Jiang S, et al. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009; 284(48):33161-33168.[89] Krausgrill B, Vantler M, Burst V, et al. Influence of cell treatment with PDGF-BB and reperfusion on cardiac persistence of mononuclear and mesenchymal bone marrow cells after transplantation into acute myocardial infarction in rats. Cell Transplant. 2009;18(8):847-853.[90] Cui X, Wang H, Guo H, et al. Transplantation of mesenchymal stem cells preconditioned with diazoxide, a mitochondrial ATP-sensitive potassium channel opener, promotes repair of myocardial infarction in rats. Tohoku J Exp Med. 2010;220(2): 139-147.[91] Numasawa Y, Kimura T, Miyoshi S, et al. Treatment of human mesenchymal stem cells with angiotensin receptor blocker improved efficiency of cardiomyogenic transdifferentiation and improved cardiac function via angiogenesis. Stem Cells. 2011;29(9):1405-1414.[92] Ikhapoh IA, Pelham CJ, Agrawal DK. Synergistic effect of angiotensin II on vascular endothelial growth factor-A-mediated differentiation of bone marrow-derived mesenchymal stem cells into endothelial cells. Stem Cell Res Ther. 2015;6:4.[93] Akomolafe SF, Oboh G, Akindahunsi AA, et al. Tetracarpidium conophorum (Mull.Arg) Hutch & Dalziel inhibits FeSO4-induced lipid peroxidation in rat's genitals. BMC Complement Altern Med. 2015;15:57.[94] Khan M, Meduru S, Mohan IK, et al. Hyperbaric oxygenation enhances transplanted cell graft and functional recovery in the infarct heart. J Mol Cell Cardiol. 2009;47(2):275-287.[95] Enoki C, Otani H, Sato D, et al. Enhanced mesenchymal cell engraftment by IGF-1 improves left ventricular function in rats undergoing myocardial infarction. Int J Cardiol. 2010;138(1): 9-18.[96] Eun LY, Song H, Choi E, et al. Implanted bone marrow-derived mesenchymal stem cells fail to metabolically stabilize or recover electromechanical function in infarcted hearts. Tissue Cell. 2011;43(4):238-245.[97] Fang J, Chen L, Fan L, et al. Enhanced therapeutic effects of mesenchymal stem cells on myocardial infarction by ischemic postconditioning through paracrine mechanisms in rats. J Mol Cell Cardiol. 2011;51(5):839-847.[98] Mias C, Lairez O, Trouche E, et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27(11):2734-2743.[99] Chen JJ, Zhou SH. Mesenchymal stem cells overexpressing MiR-126 enhance ischemic angiogenesis via the AKT/ERK-related pathway. Cardiol J. 2011;18(6):675-681.[100] Cho J, Zhai P, Maejima Y, et al. Myocardial injection with GSK-3β-overexpressing bone marrow-derived mesenchymal stem cells attenuates cardiac dysfunction after myocardial infarction. Circ Res. 2011;108(4):478-489.[101] Gao XR, Tan YZ, Wang HJ. Overexpression of Csx/Nkx2.5 and GATA-4 enhances the efficacy of mesenchymal stem cell transplantation after myocardial infarction. Circ J. 2011;75(11): 2683-2691.[102] Jin J, Jeong SI, Shin YM, et al. Transplantation of mesenchymal stem cells within a poly(lactide-co-epsilon-caprolactone) scaffold improves cardiac function in a rat myocardial infarction model. Eur J Heart Fail. 2009;11(2):147-153.[103] Cui XJ, Xie H, Wang HJ, et al. Transplantation of mesenchymal stem cells with self-assembling polypeptide scaffolds is conducive to treating myocardial infarction in rats. Tohoku J Exp Med. 2010;222(4):281-289.[104] Wang Y, Liu XC, Zhang GW, et al. A new transmyocardial degradable stent combined with growth factor, heparin, and stem cells in acute myocardial infarction. Cardiovasc Res. 2009;84(3):461-469.[105] Luan Y, Liu XC, Zhang GW, et al. Mid-term effect of stem cells combined with transmyocardial degradable stent on swine model of acute myocardial infarction. Coron Artery Dis. 2010; 21(4):233-243.[106] Afanas'ev SA, Tsapko LP, Rogovskaya YV, et al. Radiofrequency ablation as a possible method for preparing pathologically altered myocardium for intramyocardial cell transplantation. Bull Exp Biol Med. 2012;152(4):513-515. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | Yin Tingting, Du Dayong, Jiang Zhixin, Liu Yang, Liu Qilin, Li Yuntian. Granulocyte colony-stimulating factors improve myocardial fibrosis in rats with myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 730-735. |
| [5] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [6] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [7] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [8] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [9] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [10] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [11] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [12] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [13] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [14] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [15] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||