Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (17): 2631-2637.doi: 10.3969/j.issn.2095-4344.2017.17.002
Previous Articles Next Articles
Optimization of repeated freeze-thaw and ultrasonication for collection of lysate of adipose-derived stem cells
Wang Jun-yi1, Jin Yin-peng2, Li Hong-chao1, Meng Ling-yu2, Li Li2, Wang Xiao-jin2, Zhou Rong2, Chen Cheng-wei2, Fu Qing-chun2, Cheng Ming-liang3
- 1Clinidal Medical College of Guizhou Medical University, Gujyang 550004, Guizhou Province, China; 2Shanghai Liver Diseases Research Center, the 85th Hospital of PLA, Shanghai 200235, China; 3Department of Infection, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China
-
Revised:2017-05-03Online:2017-06-18Published:2017-06-29 -
Contact:Fu Qing-chun, Master, Chief physician, Shanghai Liver Diseases Research Center, the 85th Hospital of PLA, Shanghai 200235, China; Cheng Ming-liang, Chief physician, Department of Infection, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou Province, China -
About author:Wang Jun-yi, Studying for master’s degree, Clinical Medical College of Guizhou Medical University, Guiyang 550004, Guizhou Province, China Jin Yin-peng, Master, Shanghai Liver Diseases Research Center, the 85th Hospital of PLA, Shanghai 200235, China Wang Jun-yi and Jin Yin-peng contributed equally to this work. -
Supported by:the Medical Innovation Project of the Nanjing Military Region, No. 14ZX01; China Hepatitis Prevention and Treatment Foundation-Tian Qing Liver Research Fund Project, No. TQGB20150104
CLC Number:
Cite this article
Wang Jun-yi, Jin Yin-peng, Li Hong-chao, Meng Ling-yu, Li Li, Wang Xiao-jin, Zhou Rong, Chen Cheng-wei, Fu Qing-chun, Cheng Ming-liang. Optimization of repeated freeze-thaw and ultrasonication for collection of lysate of adipose-derived stem cells[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(17): 2631-2637.
share this article
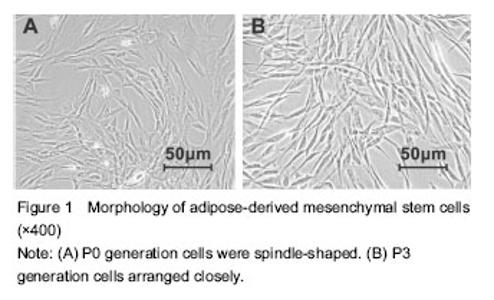
Morphology and identification of ADMSCs In the process of cultivation and amplification of primary ADMSCs, P0 generation cells grew in whirling or radial pattern from initial fibroid form to P3 generation with uniform size and close arrangement (Figure 1). Flow cytometry results showed CD73 (100%), CD44 (100%), CD90 (100%) and CD105 (99.7%) were positive over 99% cell surface, while cells were negative for CD34, CD45 and CD19 accounting for 96%."
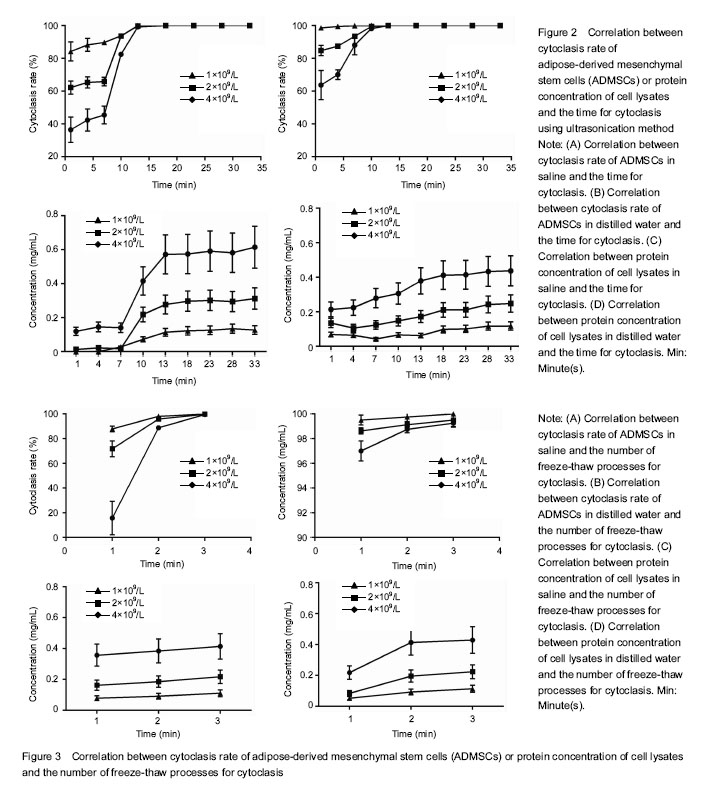
Optimization of the lysate collection from ADMSCs Ultrasonication method The cytoclasis curve of ultrasonication demonstrated that in distilled water groups (Figure 2A), the cytoclasis rate continuously increased with the time for cytoclasis in the cells with three different densities, with over 95% at 1 minute for the cells with density c, and 98.25% in 10 minutes for those with the density a. Similar results were also observed in the saline groups (Figure 2B). These findings indicate that in the same solute, a higher cell density is associated with longer cytoclasis time and lower time-cytoclasis rate, and the cytoclasis rate is significantly increased with the time for cytoclasis. Comparison between groups with different solutes showed a quicker cytoclasis process in distilled water groups than in the saline groups for all the three density cells. For example, it took approximately 10 minutes for the cells with density b to be completely disrupted (> 98%) in distilled water, in contrast to 13 minutes in saline. This shows that the cytoclasis rate of ultrosonication method is higher in distilled water than in saline for the cells with a same density. The protein concentration curve obtained by BCA method (Figure 2C, D) revealed that the protein concentration of the lysate in both of the two solutes showed an elevated trend with the time of ultrasonication, with a significantly higher protein concentration measured in saline groups than in distilled water groups at each time point. The cells had a good protein activity when a complete cytoclasis was just achieved. Meanwhile, the protein concentrations were also compared in the cells with different densities. Shown on the cytoclasis curve, the protein concentration in saline groups at the time point of cytoclasis was respectively 0.573, 0.277 and 0.114 mg/mL for the cells with three different densities, all of which were higher than those in distilled water groups. Repeated freeze-thaw method The cytoclasis curve with repeated freeze-thaw method (Figure 3A, B) showed that in distilled water, a cytoclasis rate of at least 95% was achieved after the first freeze-thaw for all the cells with three densities, and a complete disruption was obtained after the second freeze-thaw process. In contrast, in saline groups only a cytoclasis rate of 14% was obtained after the first freeze-thaw for cells with density a, and 87.88% for cells with the density c. The cytoclasis rate gradually increased with the progression of the freeze-thaw process. These results imply that repeated freeze-thaw method shares a same principle with ultrasonication for cytoclasis. Comparison between different solute groups revealed that the cytoclasis rate in freeze-thaw method was significantly higher in distilled water than in saline. Protein concentration curve derived from BCA method (Figure 3C, D) showed that the number of freeze-thawing processes was associated with the increased protein concentration of lysate in both of the solutes. Comparison between the solutes revealed the protein concentration at the timing point of complete cell disruption was higher in saline groups than in distilled water groups."
| [1] Chen G, Jin Y, Shi X, et al. Adipose-derived stem cell-based treatment for acute liver failure. Stem Cell Res Ther. 2015;6:40. [2] Christ B, Brückner S, Winkler S. The therapeutic promise of mesenchymal stem cells for liver restoration. Trends Mol Med. 2015;21(11):673-686. [3] Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125.[4] DeLany JP, Floyd ZE, Zvonic S, et al. Proteomic analysis of primary cultures of human adipose-derived stem cells: modulation by adipogenesis. Mol Cell Proteomics. 2005; 4(6): 731-740. [5] Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.[6] Fong CY, Biswas A, Subramanian A, et al. Human keloid cell characterization and inhibition of growth with human Wharton’s jelly stem cell extracts. J Cell Biochem. 2014; 115(5):826-838. [7] Gauthaman K, Fong CY, Arularasu S, et al. Human Wharton’s jelly stem cell conditioned medium and cell-free lysate inhibit human osteosarcoma and mammary carcinoma cell growth in vitro and in xenograft mice. J Cell Biochem. 2013;114(2):366-377. [8] Griffin MD, Ryan AE, Alagesan S, et al. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol. 2013; 91(1):40-51. [9] Heng BC, Toh WS, Pereira BP, et al. An autologous cell lysate extract from human embryonic stem cell (hESC) derived osteoblasts can enhance osteogenesis of hESC. Tissue Cell. 2008;40(3):219-228. [10] Baertschiger RM, Serre-Beinier V, Morel P, et al. Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One. 2009;4(8):e6657. [11] Casiraghi F, Remuzzi G, Abbate M, et al. Multipotent mesenchymal stromal cell therapy and risk of malignancies. Stem Cell Rev. 2013;9(1):65-79. [12] Forbes SJ, Russo FP, Rey V, et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126(4):955-963.[13] Li C, Kong Y, Wang H, et al. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J Hepatol. 2009; 50(6):1174-1183. [14] Sundin M, Orvell C, Rasmusson I, et al. Mesenchymal stem cells are susceptible to human herpesviruses, but viral DNA cannot be detected in the healthy seropositive individual. Bone Marrow Transplant. 2006;37(11):1051-1059.[15] Sundin M, Lindblom A, Orvell C, et al. Persistence of human parvovirus B19 in multipotent mesenchymal stromal cells expressing the erythrocyte P antigen: implications for transplantation. Biol Blood Marrow Transplant. 2008;14(10): 1172-1179. [16] Meier RP, Müller YD, Morel P, et al. Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem Cell Res. 2013;11(3): 1348-1364.[17] Herencia C, Almadén Y, Martínez-Moreno JM, et al. Human mesenchymal stromal cell lysates as a novel strategy to recover liver function. Regen Med. 2015;10(1):25-38.[18] Itaba N, Matsumi Y, Okinaka K, et al. Human mesenchymal stem cell-engineered hepatic cell sheets accelerate liver regeneration in mice. Sci Rep. 2015;5:16169. [19] Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13-27. [20] Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82-88. [21] Liang X, Ding Y, Zhang Y, et al. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045-1059. [22] Liu Z, Meng F, Li C, et al. Human umbilical cord mesenchymal stromal cells rescue mice from acetaminophen-induced acute liver failure. Cytotherapy. 2014;16(9):1207-1219. [23] Lucchini G, Introna M, Dander E, et al. Platelet-lysate-expanded mesenchymal stromal cells as a salvage therapy for severe resistant graft-versus-host disease in a pediatric population. Biol Blood Marrow Transplant. 2010;16(9):1293-1301. [24] Mishra PJ, Mishra PJ, Banerjee D. Cell-free derivatives from mesenchymal stem cells are effective in wound therapy. World J Stem Cells. 2012;4(5):35-43.[25] Motavaf M, Pakravan K, Babashah S, et al. Therapeutic application of mesenchymal stem cell-derived exosomes: A promising cell-free therapeutic strategy in regenerative medicine. Cell Mol Biol (Noisy-le-grand). 2016;62(7):74[26] Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71-74.[27] Shi R, Jin Y, Cao C, et al. Localization of human adipose-derived stem cells and their effect in repair of diabetic foot ulcers in rats. Stem Cell Res Ther. 2016; 7(1):155.[28] Tyagi D, Perez JB, Nand A, et al. Detection of embryonic stem cell lysate biomarkers by surface plasmon resonance with reduced nonspecific adsorption. Anal Biochem. 2015; 471:29-37. [29] Valina C, Pinkernell K, Song YH, et al. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28(21):2667-2677. [30] Volarevic V, Nurkovic J, Arsenijevic N, et al. Concise review: Therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells. 2014;32(11):2818-2823.[31] Zhao MM, Cui JZ, Cui Y, et al. Therapeutic effect of exogenous bone marrow?derived mesenchymal stem cell transplantation on silicosis via paracrine mechanisms in rats. Mol Med Rep. 2013;8(3):741-746. [32] Zhu J, Liu Q, Jiang Y, et al. Enhanced angiogenesis promoted by human umbilical mesenchymal stem cell transplantation in stroked mouse is Notch1 signaling associated. Neuroscience. 2015;290:288-299. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [13] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| [14] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [15] | Li Xuan, Sun Yimin, Li Longbiao, Wang Zhenming, Yang Jing, Wang Chenglin, Ye Ling. Manufacturing of nano-modified polycaprolactone microspheres and its biological effects in dental pulp cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1530-1536. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||