Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (14): 2227-2233.doi: 10.3969/j.issn.2095-4344.2017.14.016
Previous Articles Next Articles
Preparation and properties of collagen/chitosan composite sponge from forest frog skin
Lu Jing1, Wang Yu-jia1, Ling Wei-shi1, Chen Min-xiao1, Li Chang-hong1, Guan Shuang1, Deng Xu-ming2
- 1 College of Food Science and Engineering, 2 College of Veterinary Medicine, Jilin University, Changchun 130062, Jilin Province, China
-
Received:2017-01-23Online:2017-05-18Published:2017-06-10 -
Contact:Deng Xu-ming, Professor, Doctoral supervisor, College of Veterinary Medicine, Jilin University, Changchun 130062, Jilin Province, China -
About author:Lu Jing, College of Food Science and Engineering, Jilin University, Changchun 130062, Jilin Province, China -
Supported by:the National Science & Technology Pillar Program during the Twelfth Five-Year Plan Period, No. 2013BAD16B09
CLC Number:
Cite this article
Lu Jing, Wang Yu-jia, Ling Wei-shi, Chen Min-xiao, Li Chang-hong, Guan Shuang, Deng Xu-ming. Preparation and properties of collagen/chitosan composite sponge from forest frog skin[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(14): 2227-2233.
share this article
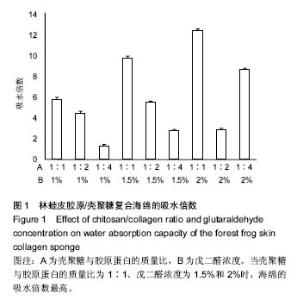
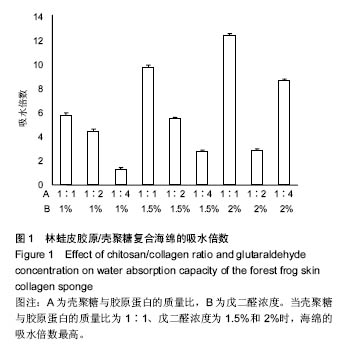
2.1 胶原海绵的吸水倍数 海绵的吸水性能够侧面反映海绵的预期止血效果,较高的吸水能力为海绵的止血奠定了初步基础,是海绵止血性能优劣的一个重要指标;此外吸水能力可以在一定程度上反映胶原海绵的品质,比如海绵孔径大小和杂质如盐分含量的多少[19]。 由图1可以看出,胶原蛋白的量和海绵的交联程度直接影响胶原海绵的吸水倍数。胶原蛋白含量越多,海绵融水越快,吸水倍数越低。戊二醛的浓度越高,交联越好,吸水倍数越高。当壳聚糖与胶原蛋白的质量比为1∶1、戊二醛浓度为1.5%和2%时,海绵的吸水倍数最高。与此次研究结果相似,有研究发现壳聚糖与胶原蛋白的比例为1∶1时胶原海绵吸水性好,并且戊二醛的浓度(0.1-0.5%)越高,胶原海绵吸水性越好[20]。也有研究发现聚糖与胶原蛋白的质量比为3∶7胶原海绵吸水性好,且在一定范围内,随着戊二醛浓度的增加,吸水倍数呈先增加后下降的趋势,在戊二醛浓度为 0.015%时吸水倍数最大[14]。因此,壳聚糖与胶原蛋白比例及戊二醛的浓度均与吸水倍数密切相关,不同研究之所以获得差异结果可能与胶原蛋白种类有关。 "
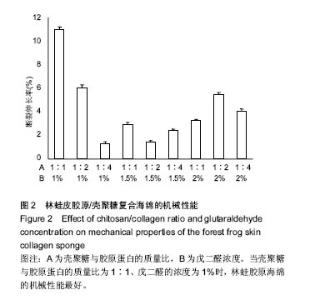
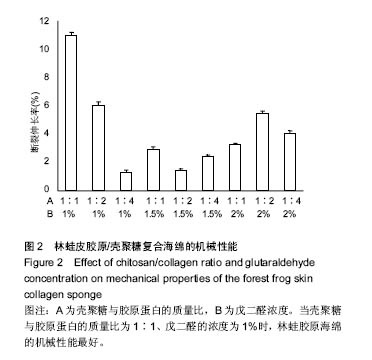
2.2 胶原海绵的机械性能 材料的力学性能与材料的内部结构密切相关,是胶原海绵材料的重要性能指标。胶原海绵是疏松多孔的结构,并且交联程度不同的海绵其内部的空隙不同,因此,海绵的机械性能与海绵的交联度有很大的关系。研究以断裂伸长率为指标考察海绵的机械性能。研究认为戊二醛交联能显著提升胶原材料的拉伸强度和断裂伸长率[21],海绵的断裂伸长率随戊二酸浓度的增加先增加后降低,并在戊二醛浓度为1.5%时达到最大值[20],与此次研究结果相似。此外,胶原蛋白的含量和种类也影响胶原海绵的机械性能[22],断裂伸长率随胶原蛋白比例的增加及壳聚糖比例的减少而降低[20]。由图2可以看出当壳聚糖与胶原蛋白的质量比为1∶1、戊二醛的浓度为1%时,林蛙胶原海绵的机械性能最好。"
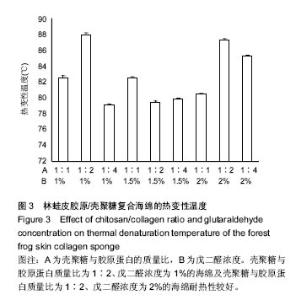
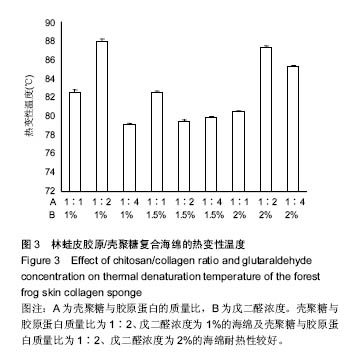
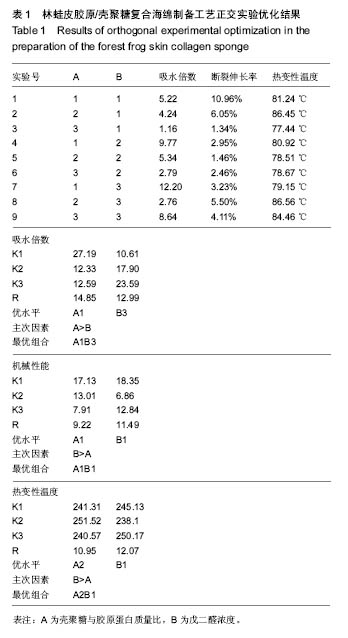
2.4 胶原海绵制备工艺优化 由表1可以看出,壳聚糖/胶原蛋白质量比对海绵吸水倍数的影响大于戊二醛的浓度,当壳聚糖/胶原蛋白质量比为1∶1、戊二醛的浓度为2%时,海绵的吸水倍数最大。戊二醛的浓度对海绵机械性能的影响大于壳聚糖/胶原蛋白质量比,当壳聚糖/胶原蛋白质量比为1∶1、戊二醛的浓度为1%时,海绵的机械性能最好。戊二醛的浓度对海绵热变性温度的影响大于壳聚糖/胶原蛋白质量比,当壳聚糖/胶原蛋白质量比为1∶2、戊二醛的浓度为1%时,海绵的热变性温度最佳。综合考虑以上各种影响因素以及对复合海绵的指标要求,采用壳聚糖/胶原蛋白为1∶1,戊二醛添加量为1%,可获得吸水性,机械性,热变性较理想的胶原海绵。"
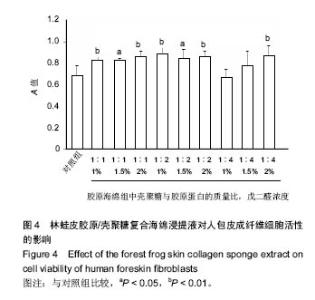
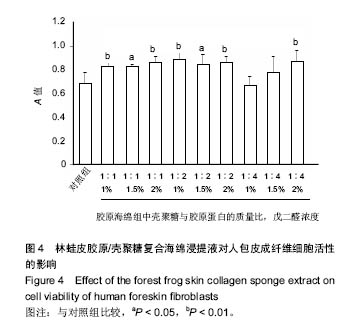
2.5 胶原海绵的细胞相容性 体外细胞相容性实验由于实验条件易于控制、能分析单一因素对细胞的影响、具有标准化、定量和可重复性等优点而成为研究生物材料相容性的主要方法[27]。MTT法检测细胞毒性是体外细胞相容性检测的首要方法[28],因此采用MTT法检测胶原海绵的体外细胞相容性。 由图4可以看出,所有的林蛙皮胶原海绵浸提液对人包皮成纤维细胞均没有抑制作用,说明胶原海绵具有良好的体外细胞相容性,并且除了壳聚糖与胶原蛋白质量比为 1∶4、戊二醛浓度为1%和5%的胶原海绵浸提液外,其他胶原海绵对对人包皮成纤维细胞的细胞活性均有促进作用,有利于细胞的活性和生长,具有良好的生物相容性,符合生物材料应用要求。"
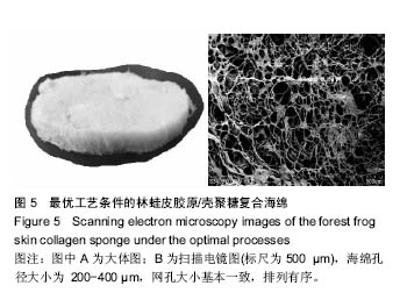
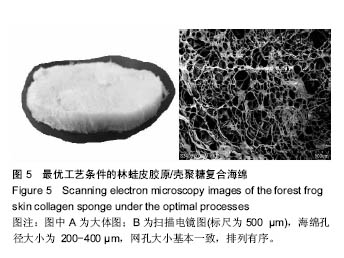
2.6 胶原海绵形貌观察 胶原的微观形貌对于有效的聚集、吸附血小板完成止血作用机制具有重要的作用,是影响材料止血性能的关键因素之一。海绵的孔径过大,则机械强度差;海绵孔径过小,影响创面初次渗血的吸收,因此,海绵孔径大小是衡量海绵质量的重要指标[29]。溶液酸度、预冻温度、溶液浓度、预冻时间、胶原浓度、无二醛交联等制备过程中的参数影响胶原海绵的微观形貌[30]。随着胶原质量的上升,林蛙皮胶原/壳聚糖复合海绵微结构中的丝状结构逐渐减少,出现了较大片状结构,同时海绵的孔径变大;随着戊二醛浓度的上升,海绵的孔结构减少。由图5可以看出优化后的海绵孔径大小为200-400 µm,网孔大小基本一致,排列有序。"
| [1]Geiger M,Li RH,Friess W.Collagen sponges for bone regeneration with rhBMP-2.Adv Drug Del Rev.2003; 55(12): 1613-1629.[2]Chattopadhyay S,Raines RT.Review collagen-based biomaterials for wound healing.Biopolymers. 2014;101(8): 821-833.[3]Ramshaw John AM.Biomedical applications of collagens.J Biomed Mater Res. 2016;104(4):665-675.[4]Silva JC,Barros AA,Aroso IM,et al.Extraction of Collagen/Gelatin from the Marine Demosponge Chondrosia reniformis (Nardo, 1847) Using Water Acidified with Carbon Dioxide - Process Optimization.Ind Eng Chem Res.2016; 55(25):6922-6930.[5]Ghodbane Salim A,Dunn Michael G.Physical and mechanical properties of cross-linked type I collagen scaffolds derived from bovine, porcine, and ovine tendons.J Biomed Mater Res A.2016;104(11):2685-2692.[6]康岚,姚霞,朱健勋,等.林蛙皮中胶原蛋白的含量测定研究[J].吉林中医药,2014,34(10):1011-1013.[7]郑淼,周亚丹,赵敏.东北林蛙皮中胶原蛋白含量的测定及提取工艺[J].东北林业大学学报,2008,36(7):81-83.[8]Zheleva DI.Review and comparative analysis of keratin biocomposites with composites based on collagen.Bulg Chem Commun.2015;47:10-15. [9]Ma L,Gao C,Mao Z,et al.Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials. 2003,24(26):4833-4841.[10]Weadock KS,Miller EJ,Bellincampi LD,et al.Physical crosslinking of collagen fibers: comparison of ultraviolet irradiation and dehydrothermal treatment.J Biomed Mater Res.1995;29(11):1373-1379.[11]Lew DH,Liu PH,Orgill DP.Optimization of UV cross-linking density for durable and nontoxic collagen GAG dermal substitute.J Biomed Mater Res B Appl Biomater.2007;82(1):51-56.[12]Martinez AW,Caves JM,Ravi S,et al.Effects of crosslinking on the mechanical properties, drug release and cytocompatibility of protein polymers.Acta Biomater. 2014;10:26-33.[13]Kishen A,Shrestha S,Shrestha A,et al.Characterizing the collagen stabilizing effect of crosslinked chitosan nanoparticles against collagenase degradation.Dent Mater. 2016;32(8):968-977.[14]黄煜.鱼鳞胶原的提取及胶原止血海绵的制备[D].福建农林大学硕士论文,2012.[15]张立彦,芮汉明,张玲.制备条件对壳聚糖-胶原蛋白海绵敷料性能的影响[J].化工进展,2011,30(2):390-395.[16]汪海婴,梁艳萍,李云雁,等.鱼源胶原蛋白海绵材料的构建及其生物学性能[J].华中科技大学学报(医学版),2012,41(6):709-715.[17]但年华,刘新华,但卫华,等.猪源I 型胶原/壳聚糖复合海绵的制备[J].皮革科学与工程,2014,24(3):17-22.[18]Nistor MT,Chiriac AP,Vasile C,et al.Synthesis of hydrogels based on poly(NIPAM) inserted into collagen sponge.Colloids Surf B Biointerfaces.2011;87(2):382-390..[19]Kim MS,Hong KD,Shin HW,et al. Preparation of porcine small intestinal submucosa sponge and their application as a wound dressing in full-thickness skin defect of rat. Int J Biol Macromol.2005;36(1-2):54-60.[20]何兰.牛骨胶原蛋白的提取及复合海绵的制备研究[D].华中农业大学硕士论文,2012.[21]汪海波,梁艳萍,李云雁,等. 交联方法对草鱼皮胶原蛋白海绵性能的影响[J].水产学报,2013,37(1):132-138.[22]Lin YK , Liu DC.Studies of novel hyaluronic acid-collagen sponge materials composed of two different species of type I collagen.J Biomater Appl.2007;21(3):265-281.[23]江颖,邓明霞,汪海婴,等.热变性对鱼胶原海绵材料性能的影响[J].功能材料,2015,46(9): 9045-9051. [24]Miles CA,Bailey AJThermal denaturation of collagen revisited.Proc Indian Acad Sci(Chem Sci).1999;111:71-80.[25]许媛媛.胶原海绵的改性及其作为活性因子载体的研究[D].中国人民解放军军事医学科学院硕士论文,2008[26]Horn MM,Martins VCA,de Guzzi Plepis AM.Interaction of anionic collagen with chitosan: effect on thermal and morphological characteristics.Carbohydr Polym.2009; 77(2): 239-243.[27]马茂.壳聚糖-类人胶原蛋白海绵的生物相容性及止血效果研究[D].西北大学博士论文,2008.[28]Sun KH,Liu Z,Liu C,et al.Evaluation of in vitro and in vivo biocompatibility of a myo-inositol hexakisphosphate gelated polyaniline hydrogel in a rat model.Sci Rep.2016;13;6:23931.[29]段志广.类人胶原蛋白止血海绵的性能研究[D].西北大学硕士论文,2008.[30]叶真铭.胶原蛋白止血海绵的研制[D].哈尔滨工业大学硕士论文,2013.[31]王慰慰.中国林蛙皮肤活性肽研究进展[J].新农业.2013;11:4-5.[32]Mahmoud Azza A,Salama Alaa H.Norfloxacin-loaded collagen/chitosan scaffolds for skin reconstruction: Preparation, evaluation and in-vivo wound healing assessment. Eur J Pharm Sci.2016;83:155-165.[33]付佳奇.鲤鱼鱼皮胶原蛋白交联物的制备研究[D].大连海洋大学硕士论文,2015.[34]郭豪.鱼胶原基复合海绵的制备及其用作缓释药膜的研究[D].福州大学硕士论文,2014.[35]Park H,Guo X,Temenoff JS,et al.Effect of swelling ratio of injectable hydrogel composites on chondrogenic differentiation of encapsulated rabbit marrow mesenchymal stem cells in vitro.Biomacromolecules.2009;10:541-546.[36]Angele P,Abke J,Kujat R,et al.Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices.Biomaterials. 2004;25:2831-2841.[37]Santhosh KB,Sahaja A,Vijaya RD.Air-dried 3D-collagen-chitosan biocomposite scaffold for tissue engineering application.Polym Compos.2012;33(11): 2029-2035.[38]Jeevithan E,Shakila RJ,Varatharajakumar A,et al. Physico-functional and mechanical properties of chitosan and calcium salts incorporated fish gelatin scaffolds. Int J Biol Macromol.2013;60:262-267.[39]Pal P,Srivas PK,Dadhich P,et al.Accelerating full thickness wound healing using collagen sponge of mrigal fish (Cirrhinus cirrhosus) scale origin.Int J Biol Macromol.2016;93(SI):1507- 1518.[40]Chandika P,Ko SC,Oh GW,et al.Fish collagen/alginate/ chitooligosaccharides integrated scaffold for skin tissue regeneration application.Int J Biol Macromol.2015;81: 504-513. [41]Muthukumar T,Prabu P,Ghosh K,et al.Fish scale collagen sponge incorporated with Macrotyloma uniflorum plant extract as a possible wound/burn dressing material.Colloids Surf B Biointerfaces.2014;113:207-212.[42]Zhou T,Wang N,Xue Y,et al.Electrospun tilapia collagen nanofibers accelerating wound healing via inducing keratinocytes proliferation and differentiation.Colloids Surf B Biointerfaces.2016;143:415-422. [43]Shekhter AB,Guller AE,Istranov LP,et al.Morphology of collagen matrices for tissue engineering (biocompatibility, biodegradation, tissue response).Arkh Patol. 2015;77(6): 29-38. [44]Venugopal B,Fernandez FB,Harikrishnan VS,et al.Post implantation fate of adipogenic induced mesenchymal stem cells on Type I collagen scaffold in a rat model.J Mater Sci Mater Med.2017;28(2):28. [45]Zhou T,Wang N,Xue Y,et al.Development of biomimetic tilapia collagen nanofibers for skin regeneration through inducing keratinocytes differentiation and collagen synthesis of dermal fibroblasts.ACS Appl Mater Interfaces.2015;7(5):3253-3262.[46]汪海波,梁艳萍,李云雁,等.交联方法对草鱼皮胶原蛋白海绵性能的影响[J].水产学报,2013,37(1):132-140. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [13] | Wang Kun, He Benxiang. Asperosaponin VI therapy for Achilles tendinopathy in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 211-217. |
| [14] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| [15] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||