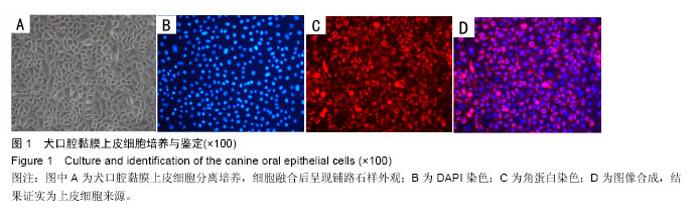
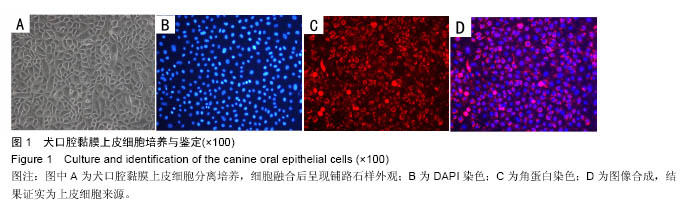
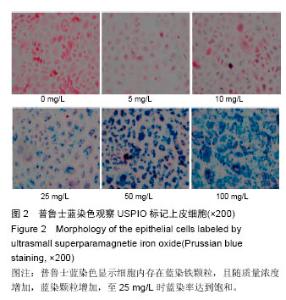
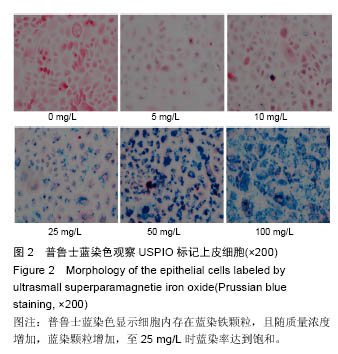
Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (52): 7796-7802.doi: 10.3969/j.issn.2095-4344.2016.52.006
Previous Articles Next Articles
Magnetic resonance imaging of canine oral epithelial cells labeled with ultrasmall superparamagnetic iron oxide
- 1Department of Urinary Surgery, 2Department of Radiology, Affiliated Sixth People’s Hospital, Shanghai Jiao Tong University, Shanghai 200233, China
-
Received:2016-09-19Online:2016-12-16Published:2016-12-16 -
Contact:Fu Qiang, Professor, Doctoral supervisor, Chief physician, Department of Urinary Surgery, Affiliated Sixth People’s Hospital, Shanghai Jiao Tong University, Shanghai 200233, China -
About author:Zhou Shu-kui, Studying for doctorate, Physician, Department of Urinary Surgery, Affiliated Sixth People’s Hospital, Shanghai Jiao Tong University, Shanghai 200233, China Yao Ting-ting, Master, Technician, Department of Radiology, Affiliated Sixth People’s Hospital, Shanghai Jiao Tong University, Shanghai 200233, China Zhou Shu-kui and Yao Ting-ting contributed equally to this work. -
Supported by:the National Natural Science Foundation of China, No. 81270780; the Subject of Shanghai Sixth People’s Hospital, No. 1711
CLC Number:
Cite this article
Zhou Shu-kui, Yao Ting-ting, Zhang Kai-le, Zou Qing-song, Fu Qiang.
share this article
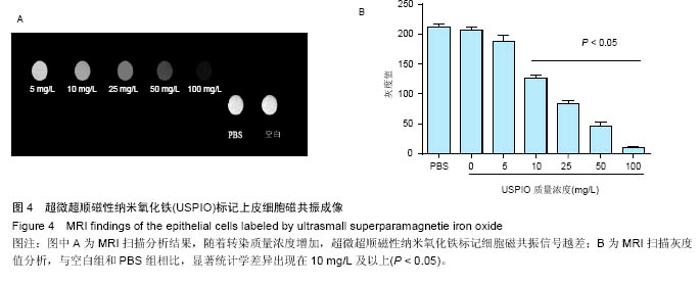
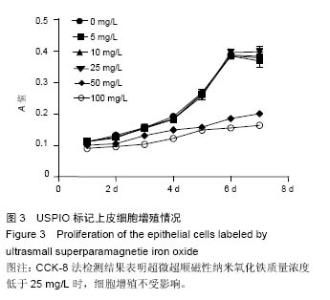
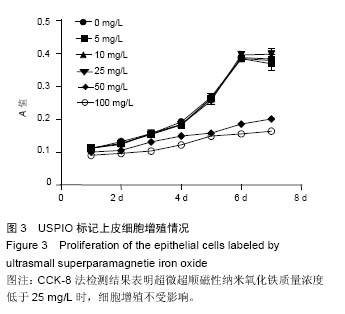
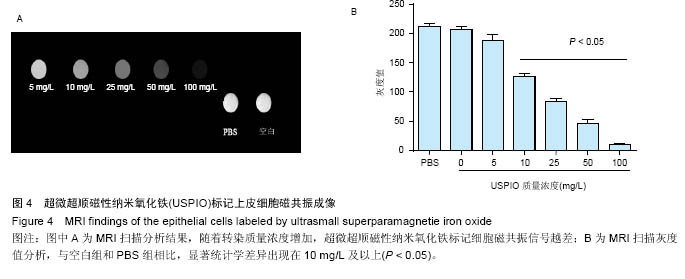
2.4 USPIO标记上皮细胞核磁显影观察 不同质量浓度USPIO标记相同数量级犬口腔黏膜上皮细胞MRI扫描结果显示,T2*WI序列均见信号降低,且随着USPIO质量浓度的增加,图像灰度值逐渐降低,呈低信号表达(图4);图像灰度分析显示,PBS组和空白组 (0 mg/L)MRI扫描图像灰度值分别为212.1±4.06,207.6±5.35;5,10,25,50,100 mg/L USPIO转染上皮细胞MRI扫描灰度值分别为189.60±9.35,127.90±4.91,81.60±4.89,48.30±6.13,9.70±1.17,相同序列下各组信号值差异有非常显著性意义(P < 0.001),通过观察发现,当USPIO质量浓度在25 mg/L时,肉眼便可对图像差异进行有效识别,因此USPIO可作为一种良好的细胞示踪剂。"
| [1]Langer R, Vacanti J. Advances in tissue engineering. J Pediatr Surg. 2016;51(1):8-12.[2]Bossù M, Pacifici A, Carbone D, et al. Today prospects for tissue engineering therapeutic approach in dentistry. Scientific World Journal. 2014;2014:151252.[3]Shokeir AA, Harraz AM, El-Din AB. Tissue engineering and stem cells: basic principles and applications in urology. Int J Urol. 2010;17(12):964-973.[4]Guo H, Sa Y, Huang J, et al. Urethral Reconstruction with Small Intestinal Submucosa Seeded with Oral Keratinocytes and TIMP-1 siRNA Transfected Fibroblasts in a Rabbit Model. Urol Int. 2016;96(2): 223-230.[5]Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17(7):484-499.[6]Tassa C, Shaw SY, Weissleder R. Dextran-coated iron oxide nanoparticles: a versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc Chem Res. 2011;44(10):842-852.[7]Azzabi F, Rottmar M, Jovaisaite V, et al. Viability, differentiation capacity, and detectability of super-paramagnetic iron oxide-labeled muscle precursor cells for magnetic-resonance imaging.Tissue Eng Part C Methods. 2015;21(2):182-191.[8]Ankamwar B, Lai TC, Huang JH, et al. Biocompatibility of Fe(3)O(4) nanoparticles evaluated by in vitro cytotoxicity assays using normal, glia and breast cancer cells. Nanotechnology. 2010; 21(7):75102.[9]Boyer C, Whittaker MR, Bulmus V, et al. The design and utility of polymer-stabilized iron-oxide nanoparticles for nanomedicine applications. NPG Asia Materials. 2010;2:23-30.[10]Singh N, Jenkins GJ, Asadi R, et al. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION).Nano Rev. 2010;1. doi: 10.3402/nano.v1i0.5358. [11]Wilkinson K, Ekstrand-Hammarström B, Ahlinder L, et al. Visualization of custom-tailored iron oxide nanoparticles chemistry, uptake, and toxicity. Nanoscale. 2012;4(23):7383-7393.[12]Arami H, Khandhar A, Liggitt D, et al. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem Soc Rev. 2015;44(23): 8576-8607.[13]Fu Q, Deng CL, Zhao RY, et al. The effect of mechanical extension stimulation combined with epithelial cell sorting on outcomes of implanted tissue-engineered muscular urethras. Biomaterials. 2014;35(1):105-112.[14]Boennelycke M, Gras S, Lose G. Tissue engineering as a potential alternative or adjunct to surgical reconstruction in treating pelvic organ prolapse. Int Urogynecol J. 2013;24(5):741-747.[15]Amiel GE, Yoo JJ, Kim BS, et al. Tissue engineered stents created from chondrocytes. J Urol. 2001;165(6 Pt 1):2091-2095.[16]Shen Y, Shao Y, He H, et al. Gadolinium(3+)-doped mesoporous silica nanoparticles as a potential magnetic resonance tracer for monitoring the migration of stem cells in vivo. Int J Nanomedicine. 2013;8:119-127.[17]Brekke C, Morgan SC, Lowe AS, et al. The in vitro effects of a bimodal contrast agent on cellular functions and relaxometry. NMR Biomed. 2007;20(2): 77-89.[18]Rojas JM, Sanz-Ortega L, Mulens-Arias V, et al. Superparamagnetic iron oxide nanoparticle uptake alters M2 macrophage phenotype, iron metabolism, migration and invasion. Nanomedicine. 2016;12(4): 1127-1138.[19]Paik SY, Kim JS, Shin SJ, et al. Characterization, Quantification, and Determination of the Toxicity of Iron Oxide Nanoparticles to the Bone Marrow Cells. Int J Mol Sci. 2015;16(9):22243-22257.[20]Guzman R, Uchida N, Bliss TM, et al. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci U S A. 2007;104(24):10211-10216.[21]Bulte JW, Douglas T, Witwer B, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19(12):1141-1147.[22]Neri M, Maderna C, Cavazzin C, et al. Efficient in vitro labeling of human neural precursor cells with superparamagnetic iron oxide particles: relevance for in vivo cell tracking. Stem Cells. 2008;26(2):505-516.[23]Shapiro EM, Sharer K, Skrtic S, et al. In vivo detection of single cells by MRI. Magn Reson Med. 2006;55(2): 242-249.[24]Farrell E, Wielopolski P, Pavljasevic P, et al. Effects of iron oxide incorporation for long term cell tracking on MSC differentiation in vitro and in vivo. Biochem Biophys Res Commun. 2008;369(4):1076-1081.[25]Jing XH, Yang L, Duan XJ, et al. In vivo MR imaging tracking of magnetic iron oxide nanoparticle labeled, engineered, autologous bone marrow mesenchymal stem cells following intra-articular injection.Joint Bone Spine. 2008; 75(4):432-438. [26]Qi CM, Ma GS, Liu NF, et al. MR imaging of injected magnetically labeled stem cells in myocardial infarction: experiment with pigs. Zhonghua Yi Xue Za Zhi. 2007; 87(22):1523-1526.[27]Zhang RP, Wang LJ, He S, et al. Effects of Magnetically Guided, SPIO-Labeled, and Neurotrophin-3 Gene-Modified Bone Mesenchymal Stem Cells in a Rat Model of Spinal Cord Injury. Stem Cells Int. 2016;2016:2018474.[28]Feng Y, Jin X, Dai G, et al. In vitro targeted magnetic delivery and tracking of superparamagnetic iron oxide particles labeled stem cells for articular cartilage defect repair. J Huazhong Univ Sci Technolog Med Sci. 2011; 31(2):204-209.[29]Roeder E, Henrionnet C, Goebel JC, et al. Dose-response of superparamagnetic iron oxide labeling on mesenchymal stem cells chondrogenic differentiation: a multi-scale in vitro study.PLoS One. 2014; 9(5):e98451. [30]Huang ZY, Ge JB, Yang S, et al. In vivo cardiac magnetic resonance imaging of superparamagnetic iron oxides-labeled mesenchymal stem cells in swines. Zhonghua Xin Xue Guan Bing Za Zhi. 2007; 35(4): 344-349.[31]Zhang C, Wängler B, Morgenstern B, et al. Silica- and alkoxysilane-coated ultrasmall superparamagnetic iron oxide particles: a promising tool to label cells for magnetic resonance imaging. Langmuir. 2007;23(3): 1427-1434.[32]Laurent S, Boutry S, Mahieu I, et al. Iron oxide based MR contrast agents: from chemistry to cell labeling. Curr Med Chem. 2009;16(35):4712-4727.[33]Babic M, Horák D, Trchová M, et al. Poly(L-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjug Chem. 2008;19(3): 740-750.[34]Mishra SK, Khushu S, Gangenahalli G. Potential stem cell labeling ability of poly-L-lysine complexed to ultrasmall iron oxide contrast agent: An optimization and relaxometry study. Exp Cell Res. 2015;339(2): 427-436.[35]Jasmin, Torres AL, Nunes HM, et al. Optimized labeling of bone marrow mesenchymal cells with superparamagnetic iron oxide nanoparticles and in vivo visualization by magnetic resonance imaging. J Nanobiotechnology. 2011;9:4. |
| [1] | Min Youjiang, Yao Haihua, Sun Jie, Zhou Xuan, Yu Hang, Sun Qianpu, Hong Ensi. Effect of “three-tong acupuncture” on brain function of patients with spinal cord injury based on magnetic resonance technology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-8. |
| [2] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [3] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [4] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [5] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [6] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [7] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [8] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [9] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [10] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [11] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [12] | Yi Meizhi, Luo Guanghua, Xiao Yawen, Hu Rong, Chen Xiaolong, Zhao Heng. MRI findings of anatomical variations of the talus [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3888-3893. |
| [13] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [14] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [15] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||